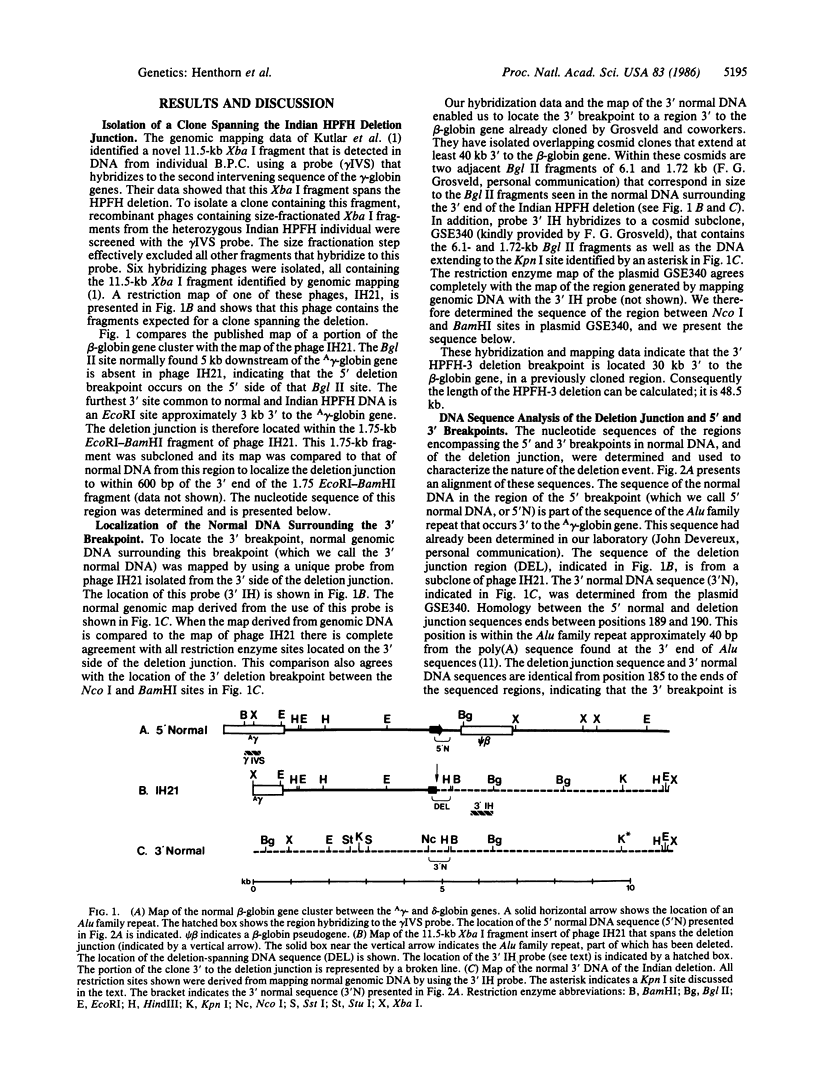
Abstract
A DNA fragment containing the deletion junction region from an Indian individual with a type of hereditary persistence of fetal hemoglobin has been cloned. Using a probe isolated from this deletion-spanning clone, we located the 3' breakpoint of the deletion in normal DNA to a region 30 kilobase pairs (kb) downstream of the beta-globin gene. The deletion removes 48.5 kb of DNA. Sequences of the deletion junction and of the normal DNA surrounding the 3' breakpoint were determined and compared to the previously determined sequence of the normal DNA surrounding the 5' breakpoint. This comparison shows that the deletion was the result of a nonhomologous recombinational event, although there is a 5-base-pair (bp) region of local homology between the normal DNAs at their breakpoints. The 5' deletion breakpoint occurs in the Alu family repeat 3' to the A gamma-globin gene. The 3' breakpoint is located within a region that contains the following: a portion of an L1 (Kpn I) repeat, a perfect 160-bp palindrome, and a set of 41-bp direct repeats that are found elsewhere in the human genome. A variation in restriction fragment lengths was observed in this region in one family.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernards R., Flavell R. A. Physical mapping of the globin gene deletion in hereditary persistence of foetal haemoglobin (HPFH). Nucleic Acids Res. 1980 Apr 11;8(7):1521–1534. doi: 10.1093/nar/8.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. S., Weissman S. M. The molecular genetics of human hemoglobin. Prog Nucleic Acid Res Mol Biol. 1984;31:315–462. doi: 10.1016/s0079-6603(08)60382-7. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman J. G., Huisman T. H. DNA sequence variation associated with elevated fetal G gamma globin production. Blood. 1985 Oct;66(4):783–787. [PubMed] [Google Scholar]
- Goodbourn S. E., Higgs D. R., Clegg J. B., Weatherall D. J. Molecular basis of length polymorphism in the human zeta-globin gene complex. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5022–5026. doi: 10.1073/pnas.80.16.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman T. H., Schroeder W. A., Efremov G. D., Duma H., Mladenovski B., Hyman C. B., Rachmilewitz E. A., Bouver N., Miller A., Brodie A. The present status of the heterogeneity of fetal hemoglobin in beta-thalassemia: an attempt to unify some observations in thalassemia and related conditions. Ann N Y Acad Sci. 1974;232(0):107–124. doi: 10.1111/j.1749-6632.1974.tb20576.x. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Karlsson S., Nienhuis A. W. Developmental regulation of human globin genes. Annu Rev Biochem. 1985;54:1071–1108. doi: 10.1146/annurev.bi.54.070185.005231. [DOI] [PubMed] [Google Scholar]
- Kutlar A., Gardiner M. B., Headlee M. G., Reese A. L., Cleek M. P., Nagle S., Sukumaran P. K., Huisman T. H. Heterogeneity in the molecular basis of three types of hereditary persistence of fetal hemoglobin and the relative synthesis of the G gamma and A gamma types of gamma chain. Biochem Genet. 1984 Feb;22(1-2):21–35. doi: 10.1007/BF00499284. [DOI] [PubMed] [Google Scholar]
- Loenen W. A., Blattner F. R. Lambda Charon vectors (Ch32, 33, 34 and 35) adapted for DNA cloning in recombination-deficient hosts. Gene. 1983 Dec;26(2-3):171–179. doi: 10.1016/0378-1119(83)90187-7. [DOI] [PubMed] [Google Scholar]
- Manuelidis L., Biro P. A. Genomic representation of the Hind II 1.9 kb repeated DNA. Nucleic Acids Res. 1982 May 25;10(10):3221–3239. doi: 10.1093/nar/10.10.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Poncz M., Solowiejczyk D., Harpel B., Mory Y., Schwartz E., Surrey S. Construction of human gene libraries from small amounts of peripheral blood: analysis of beta-like globin genes. Hemoglobin. 1982;6(1):27–36. doi: 10.3109/03630268208996930. [DOI] [PubMed] [Google Scholar]
- Roth D. B., Porter T. N., Wilson J. H. Mechanisms of nonhomologous recombination in mammalian cells. Mol Cell Biol. 1985 Oct;5(10):2599–2607. doi: 10.1128/mcb.5.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C. W., Jelinek W. R. The Alu family of dispersed repetitive sequences. Science. 1982 Jun 4;216(4550):1065–1070. doi: 10.1126/science.6281889. [DOI] [PubMed] [Google Scholar]
- Schroeder W. A., Huisman T. H., Sukumaran P. K. A second type of hereditary persistence of foetal haemoglobin in India. Br J Haematol. 1973 Jul;25(1):131–135. doi: 10.1111/j.1365-2141.1973.tb01722.x. [DOI] [PubMed] [Google Scholar]
- Stoker N. G., Cheah K. S., Griffin J. R., Pope F. M., Solomon E. A highly polymorphic region 3' to the human type II collagen gene. Nucleic Acids Res. 1985 Jul 11;13(13):4613–4622. doi: 10.1093/nar/13.13.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Uhlenbeck O. C., Levine M. D. Estimation of secondary structure in ribonucleic acids. Nature. 1971 Apr 9;230(5293):362–367. doi: 10.1038/230362a0. [DOI] [PubMed] [Google Scholar]
- Tuan D., Feingold E., Newman M., Weissman S. M., Forget B. G. Different 3' end points of deletions causing delta beta-thalassemia and hereditary persistence of fetal hemoglobin: implications for the control of gamma-globin gene expression in man. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6937–6941. doi: 10.1073/pnas.80.22.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanin E. F., Henthorn P. S., Kioussis D., Grosveld F., Smithies O. Unexpected relationships between four large deletions in the human beta-globin gene cluster. Cell. 1983 Dec;35(3 Pt 2):701–709. doi: 10.1016/0092-8674(83)90103-4. [DOI] [PubMed] [Google Scholar]
- Wainscoat J. S., Old J. M., Wood W. G., Trent R. J., Weatherall D. J. Characterization of an Indian (delta beta)0 thalassaemia. Br J Haematol. 1984 Oct;58(2):353–360. doi: 10.1111/j.1365-2141.1984.tb06094.x. [DOI] [PubMed] [Google Scholar]
- Yang R., Fristensky B., Deutch A. H., Huang R. C., Tan Y. H., Narang S. A., Wu R. The nucleotide sequence of a new human repetitive DNA consists of eight tandem repeats of 66 base pairs. Gene. 1983 Nov;25(1):59–66. doi: 10.1016/0378-1119(83)90167-1. [DOI] [PubMed] [Google Scholar]