Abstract
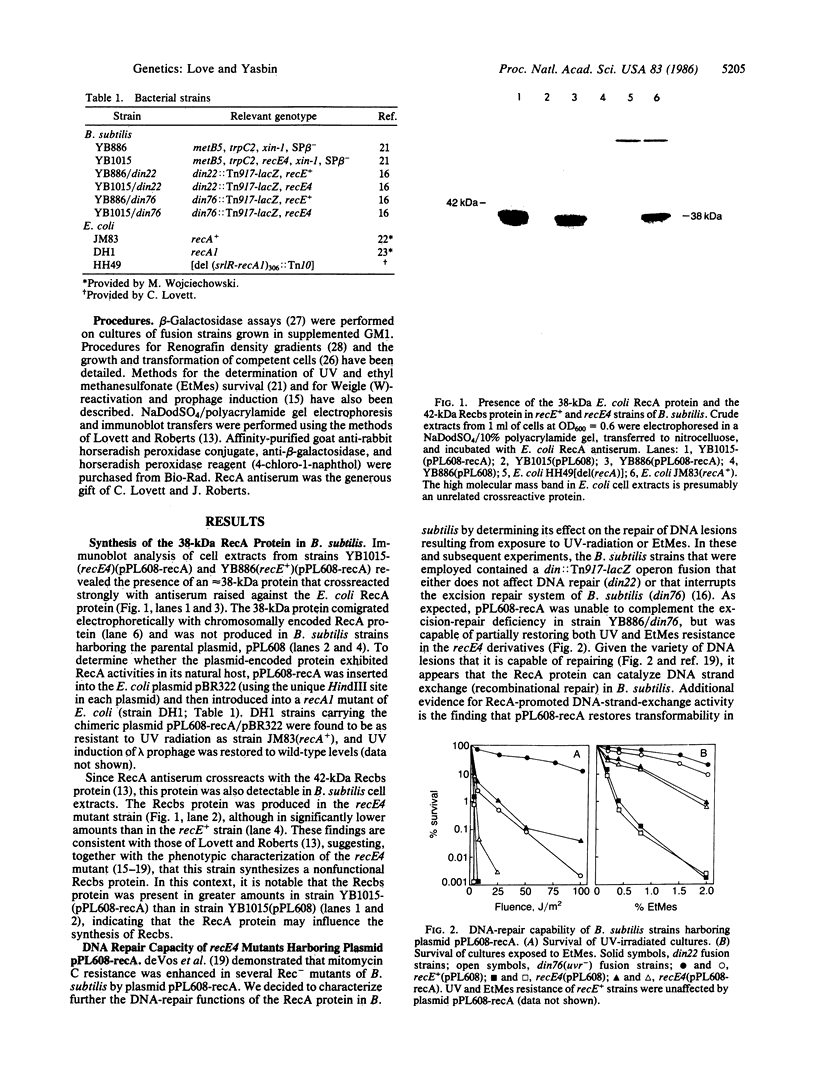
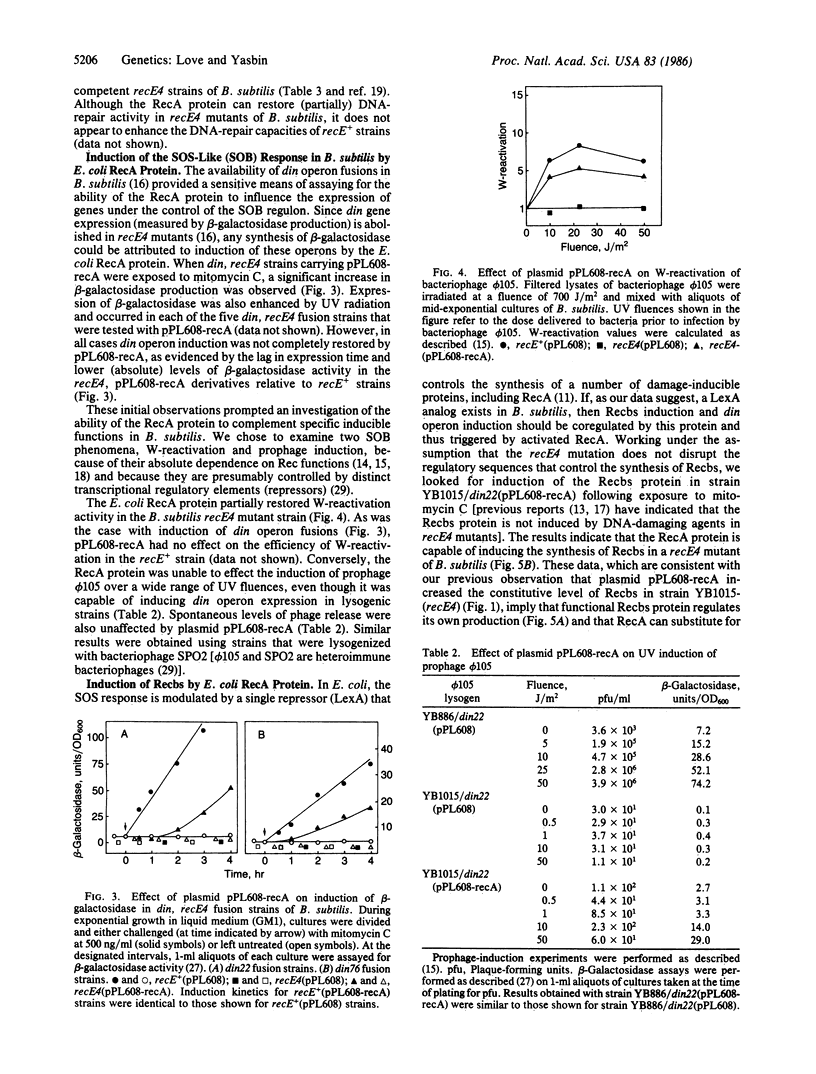
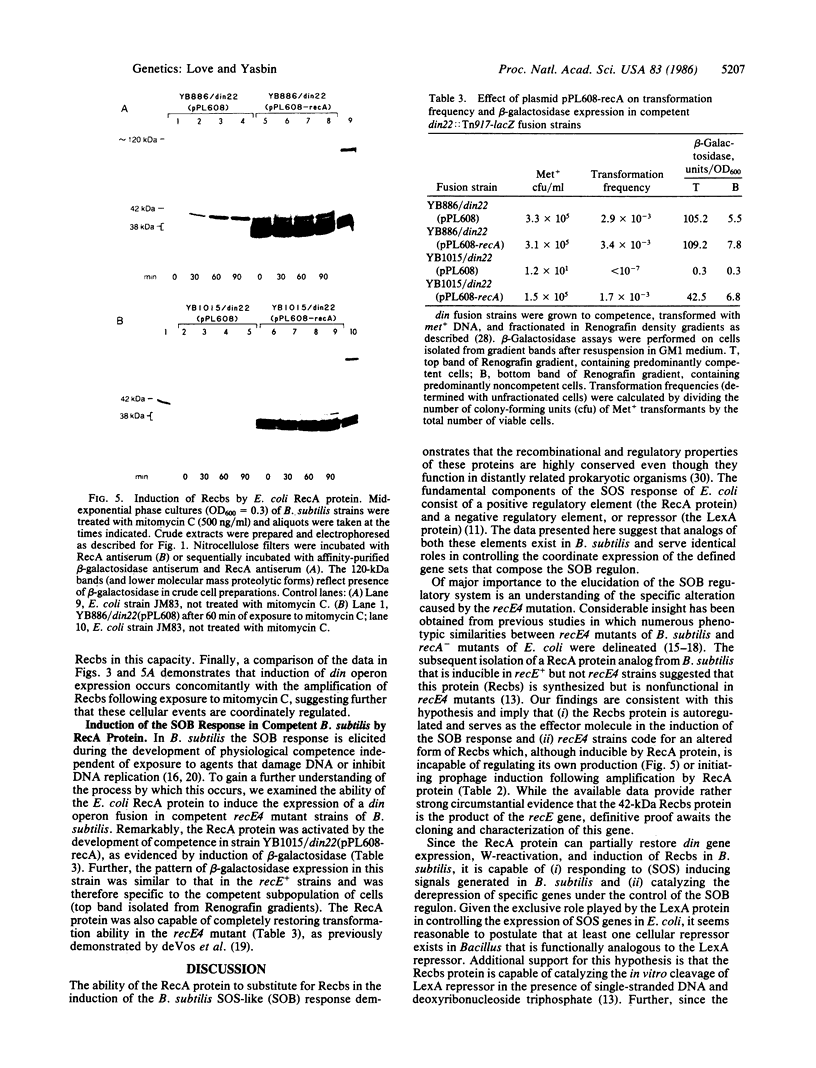
A plasmid that expresses the Escherichia coli RecA protein partially restored DNA repair and recombination capability and induction of the SOS-like (SOB) response in a recE4 mutant of Bacillus subtilis. In the presence of DNA-damaging agents, the E. coli RecA protein induced din operon expression, Weigle-reactivation activity, and synthesis of a B. subtilis recombination protein (Recbs) analogous to RecA but was unable to stimulate prophage induction. In addition, the RecA protein was capable of inducing the SOB response in competent recE4 strains of B. subtilis, independent of exposure to DNA-damaging agents. The results suggest that (i) the SOS response of E. coli and the SOB response of B. subtilis are strikingly similar from both a phenotypic and a regulatory standpoint and that RecA and LexA protein analogs exist in B. subtilis, (ii) the Recbs protein is capable of regulating its own production, and (iii) SOS-inducing (RecA-activating) signals are generated in B. subtilis following either DNA damage or the development of physiological competence.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angulo J. F., Schwencke J., Moreau P. L., Moustacchi E., Devoret R. A yeast protein analogous to Escherichia coli RecA protein whose cellular level is enhanced after UV irradiation. Mol Gen Genet. 1985;201(1):20–24. doi: 10.1007/BF00397980. [DOI] [PubMed] [Google Scholar]
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R., Scher B., Cirigliano C. Fate of transforming deoxyribonucleic acid after uptake by competent Bacillus subtilis: phenotypic characterization of radiation-sensitive recombination-deficient mutants. J Bacteriol. 1973 Apr;114(1):273–286. doi: 10.1128/jb.114.1.273-286.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitner G., Adler B., Lanzov V. A., Hofemeister J. Interspecies recA protein substitution in Escherichia coli and Proteus mirabilis. Mol Gen Genet. 1982;185(3):481–486. doi: 10.1007/BF00334144. [DOI] [PubMed] [Google Scholar]
- Ennis D. G., Fisher B., Edmiston S., Mount D. W. Dual role for Escherichia coli RecA protein in SOS mutagenesis. Proc Natl Acad Sci U S A. 1985 May;82(10):3325–3329. doi: 10.1073/pnas.82.10.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Friedman B. M., Yasbin R. E. The genetics and specificity of the constitutive excision repair system of Bacillus subtilis. Mol Gen Genet. 1983;190(3):481–486. doi: 10.1007/BF00331080. [DOI] [PubMed] [Google Scholar]
- Hadden C., Nester E. W. Purification of competent cells in the Bacillus subtilis transformation system. J Bacteriol. 1968 Mar;95(3):876–885. doi: 10.1128/jb.95.3.876-885.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Harris W. J., Barr G. C. Structural features of DNA in competent Bacillus subtilis. Mol Gen Genet. 1971;113(4):316–330. doi: 10.1007/BF00272332. [DOI] [PubMed] [Google Scholar]
- Keener S. L., McNamee K. P., McEntee K. Cloning and characterization of recA genes froM Proteus vulgaris, Erwinia carotovora, Shigella flexneri, and Escherichia coli B/r. J Bacteriol. 1984 Oct;160(1):153–160. doi: 10.1128/jb.160.1.153-160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiec E., Holloman W. K. Homologous pairing of DNA molecules promoted by a protein from Ustilago. Cell. 1982 Jun;29(2):367–374. doi: 10.1016/0092-8674(82)90153-2. [DOI] [PubMed] [Google Scholar]
- Kokjohn T. A., Miller R. V. Molecular cloning and characterization of the recA gene of Pseudomonas aeruginosa PAO. J Bacteriol. 1985 Aug;163(2):568–572. doi: 10.1128/jb.163.2.568-572.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Edmiston S. H., Pacelli L. Z., Mount D. W. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W. The SOS regulatory system: control of its state by the level of RecA protease. J Mol Biol. 1983 Jul 15;167(4):791–808. doi: 10.1016/s0022-2836(83)80111-9. [DOI] [PubMed] [Google Scholar]
- Love P. E., Lyle M. J., Yasbin R. E. DNA-damage-inducible (din) loci are transcriptionally activated in competent Bacillus subtilis. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6201–6205. doi: 10.1073/pnas.82.18.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love P. E., Yasbin R. E. Genetic characterization of the inducible SOS-like system of Bacillus subtilis. J Bacteriol. 1984 Dec;160(3):910–920. doi: 10.1128/jb.160.3.910-920.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett C. M., Jr, Roberts J. W. Purification of a RecA protein analogue from Bacillus subtilis. J Biol Chem. 1985 Mar 25;260(6):3305–3313. [PubMed] [Google Scholar]
- McClanahan T., McEntee K. Specific transcripts are elevated in Saccharomyces cerevisiae in response to DNA damage. Mol Cell Biol. 1984 Nov;4(11):2356–2363. doi: 10.1128/mcb.4.11.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phizicky E. M., Roberts J. W. Kinetics of RecA protein-directed inactivation of repressors of phage lambda and phage P22. J Mol Biol. 1980 May 25;139(3):319–328. doi: 10.1016/0022-2836(80)90133-3. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Homologous pairing and strand exchange in genetic recombination. Annu Rev Genet. 1982;16:405–437. doi: 10.1146/annurev.ge.16.120182.002201. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Craig N. L. Escherichia coli recA gene product inactivates phage lambda repressor. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4714–4718. doi: 10.1073/pnas.75.10.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer R. T., Ross M. J., Ptashne M. Cleavage of the lambda and P22 repressors by recA protein. J Biol Chem. 1982 Apr 25;257(8):4458–4462. [PubMed] [Google Scholar]
- Sedgwick S. G., Goodwin P. A. Interspecies regulation of the SOS response by the E. coli lexA+ gene. Mutat Res. 1985 May;145(3):103–106. doi: 10.1016/0167-8817(85)90015-x. [DOI] [PubMed] [Google Scholar]
- Siede W., Eckardt F. Inducibility of error-prone DNA repair in yeast? Mutat Res. 1984 Oct;129(1):3–11. doi: 10.1016/0027-5107(84)90116-7. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. C., Countryman J. K., Howard-Flanders P. Purification and properties of the recA protein of Proteus mirabilis. Comparison with Escherichia coli recA protein; specificity of interaction with single strand binding protein. J Biol Chem. 1983 Apr 10;258(7):4648–4654. [PubMed] [Google Scholar]
- West S. C., Little J. W. P. mirabilis RecA protein catalyses cleavage of E. coli LexA protein and the lambda repressor in vitro. Mol Gen Genet. 1984;194(1-2):111–113. doi: 10.1007/BF00383505. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Duvall E. J., Lovett P. S. Cloning restriction fragments that promote expression of a gene in Bacillus subtilis. J Bacteriol. 1981 Jun;146(3):1162–1165. doi: 10.1128/jb.146.3.1162-1165.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasbin R. E. DNA repair in Bacillus subtilis. I. The presence of an inducible system. Mol Gen Genet. 1977 Jun 8;153(2):211–218. [PubMed] [Google Scholar]
- Yasbin R. E. DNA repair in Bacillus subtilis. II. Activation of the inducible system in competent bacteria. Mol Gen Genet. 1977 Jun 8;153(2):219–225. [PubMed] [Google Scholar]
- Yasbin R. E., Wilson G. A., Young F. E. Transformation and transfection in lysogenic strains of Bacillus subtilis: evidence for selective induction of prophage in competent cells. J Bacteriol. 1975 Jan;121(1):296–304. doi: 10.1128/jb.121.1.296-304.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos W. M., de Vries S. C., Venema G. Cloning and expression of the Escherichia coli recA gene in Bacillus subtilis. Gene. 1983 Nov;25(2-3):301–308. doi: 10.1016/0378-1119(83)90234-2. [DOI] [PubMed] [Google Scholar]






