Abstract
Photochemotherapy—in which a photosensitizing drug is combined with ultraviolet or visible radiation—has proven therapeutic effectiveness. Existing approaches have drawbacks, however, and there is a clinical need to develop alternatives offering improved target cell selectivity. DNA substitution by 4-thiothymidine (S4TdR) sensitizes cells to killing by ultraviolet A (UVA) radiation. Here, we demonstrate that UVA photoactivation of DNA S4TdR does not generate reactive oxygen or cause direct DNA breakage and is only minimally mutagenic. In an organotypic human skin model, UVA penetration is sufficiently robust to kill S4TdR-photosensitized epidermal cells. We have investigated the DNA lesions responsible for toxicity. Although thymidine is the predominant UVA photoproduct of S4TdR in dilute solution, more complex lesions are formed when S4TdR-containing oligonucleotides are irradiated. One of these, a thietane/S5-(6-4)T:T, is structurally related to the (6-4) pyrimidine:pyrimidone [(6-4) Py:Py] photoproducts induced by UVB/C radiation. These lesions are detectable in DNA from S4TdR/UVA-treated cells and are excised from DNA more efficiently by keratinocytes than by leukaemia cells. UVA irradiation also induces DNA interstrand crosslinking of S4TdR-containing duplex oligonucleotides. Cells defective in repairing (6-4) Py:Py DNA adducts or processing DNA crosslinks are extremely sensitive to S4TdR/UVA indicating that these lesions contribute significantly to S4TdR/UVA cytotoxicity.
INTRODUCTION
Photochemotherapy combines ultraviolet or visible radiation with photosensitizing drugs to produce cytotoxic effects which neither drug nor radiation can achieve alone. Psoralen plus UVA (320–400 nm) radiation (PUVA), for the treatment of cutaneous T-cell lymphoma and psoriasis, and photodynamic therapy (PDT) in which tetrapyrroles are activated by light to treat external and internal malignancies (1–3) are established photochemotherapies. Although they are highly effective, these approaches have drawbacks. Long-term use of PUVA is associated with an increased risk of skin cancer (4,5). PDT is not completely selective for the tumour tissue and can be very painful. Hence, further research into alternative approaches, preferably involving lower radiation doses and/or improved selectivity is warranted.
The deliberate induction of DNA damage underlies many successful therapeutic strategies. The canonical DNA bases are damaged when they absorb ultraviolet radiation (UVR) in the UVC and UVB spectral regions (100–320 nm), but have no significant absorption at UVA wavelengths (320–400 nm). Thus, DNA is largely insensitive to direct UVA-induced photochemical damage and skin is about 1000 times less sensitive to UVA than to UVB both at the molecular and clinical levels (6). The thiopurine 6-thioguanine (6-TG) and the thiopyrimidine 4-thiothymine (S4T) are examples of base analogs that are UVA chromophores with absorbance maxima being ∼340 nm. Both are incorporated efficiently into DNA of dividing cells where they exhibit a profound synergistic cytotoxicity with UVA radiation (7–9).
4-Thiothymidine (S4TdR) is metabolized via the thymidine kinase (TK)-mediated pyrimidine nucleoside salvage pathway (8). TK is strongly up-regulated during DNA replication (10) and is more active in rapidly dividing cells (11). This property can be exploited for therapeutic advantage, as exemplified by trifluorothymidine [recently reviewed (12)]. Unlike trifluorothymidine, S4TdR itself is not detectably toxic or mutagenic in cultured human cells (8). In combination with low dose UVA, however, it causes significant toxicity in rapidly dividing cells and UVA sensitization factors of ∼100-fold are easily achievable (8,9). This effect is largely independent of p53 status (9). These two properties: selective sensitization of rapidly dividing cells and p53 independence are key properties for a treatment aimed at cancers in which p53 is often mutated or absent.
The mechanism by which DNA S4TdR increases cellular UVA sensitivity is yet to be elucidated. The UVA energy absorbed by thiobases in DNA can cause DNA damage by Type I photosensitization, or it may be transferred to molecular oxygen to generate reactive oxygen species (ROS) in a Type II photosensitization reaction. ROS cause damage to DNA and proteins, and Type II photosensitization is the predominant mechanism by which the DNA thiopurine 6-TG exerts its photochemical effects (7,13,14).
Nucleotide excision repair (NER)-defective xeroderma pigmentosum (XP) cells are particularly sensitive to the combination of S4TdR and UVA (8). This indicates that the treatment produces potentially lethal DNA lesions that are normally removed by NER. This DNA repair pathway efficiently removes the (6-4) pyrimidine:pyrimidone [(6-4) Py:Py] intrastrand DNA crosslinks induced by UVC and UVB radiation. A thietane photoproduct that is formed in a UVA-activated reaction between S4T with an adjacent thymine, and which resembles a (6-4) Py:Py DNA photoproduct, is a candidate for this potentially lethal NER substrate (15,16).
In the study reported here, UVA penetration of in vitro reconstituted skin is shown to be sufficiently robust to selectively kill dividing epidermal cells that contain DNA S4TdR. We demonstrate that DNA S4TdR photoactivation involves a predominantly Type I photosensitization and does not generate significant levels of ROS. The combination of S4TdR and UVA is only weakly mutagenic in mammalian cultured cells. DNA S4TdR photoproducts are identified and include a DNA thietane that is repaired by NER.
MATERIALS AND METHODS
Cell culture
Human HaCaT, XP12RO and MRC5VA and all Chinese hamster cell lines were grown in DMEM containing 10% fetal calf serum. The human T lymphoblast cell line CCRF-CEM from acute lymphoblastic leukaemia was grown in RPMI + 10% fetal calf serum. CHO irs1 (xrcc2 mutant) and irs1/XRCC2 (xrcc2 complemented irs1) cells (17) were provided by Dr John Thacker and CHO XRS6 (Ku80 mutant) and its Ku80+ parent K1 by Dr Mark O'Driscoll. The ERCC4(XPF) mutant CHO cell line UV41, ERCC5(XPG) mutant UV135 and their parental counterpart AA8 have been described (18,19).
Organotypic skin cultures on de-epidermalized dermis
Organotypic skin cultures were prepared as described (20). Briefly, glycerol-preserved γ-irradiated skin (Euro Skin Bank, Beverwijk, Holland) was washed extensively in PBS and incubated in antibiotic-containing PBS at 37°C for ∼10 days. The epidermis was then removed using forceps, and de-epidermalized dermis (DED) was cut into small squares and placed in culture dishes with the papillary dermal surface on the underside. Steel rings were placed on top of the dermis and normal human dermal fibroblasts were added into the rings on the reticular dermal surface. After incubation overnight, the de-epidermalized dermis was inverted, the rings were replaced and normal human keratinocytes were seeded inside the rings onto the dermis. After 2 days, the dermis was raised to the air–liquid interface in the same orientation, by placing the composites on steel grids for 14 days. The medium was replaced every 3 days. At Days 6 and 7, medium supplemented with S4TdR was added for 3 days and the rafts were irradiated. After 24 h, they were fixed in 10% formalin and embedded in paraffin. Deparaffinized sections were stained with Haematoxylin and Eosin for histologic examination.
Drug treatment
S4TdR prepared according to Xu et al. (21) was obtained from Glen Research (Sterling, VA, USA). It was dissolved in deionized water, filter-sterilized and stored at −20°C. All S4TdR treatments were for 48 h in medium containing 10% FCS which had been dialysed extensively (DFCS) through a 2 kDa exclusion membrane Spectra/Por (Spectrum Laboratories; Medicell International, Ltd.). In the mutagenicity assay, N-methly-N-nitrosourea (MNU) (Sigma Aldrich, UK) was dissolved in dimethylsulphoxide (DMSO) and added to the medium.
Irradiation
UVA irradiation of oligonucleotides was at 100 W/m2 with a UVH 250 W iron bulb with a low range cut-off at 320 nm (UVLight Technologies, Birmingham, UK). For irradiation of cells, the UVA source was a UVASPOT lamp (Dr. Hönle AG UV Technology, Gräfelfing/München, Germany), emitting broadband UVA and filtered to remove UVB radiation (99.9%). The remaining 0.1% of transmitted UVB represents 10% of erythemally effective energy (EEE), which at the low UVA doses used in this study, would not contribute to any biologically significant DNA damage. Cells were irradiated in a thin layer of PBS containing Ca2+ and Mg2+ (PBS/CaMg) at an irradiance of 16.6 W/m2 and maintained on a water-cooled plate during irradiation. The UVB irradiation source was a Westinghouse FS20 SunLamp with peak emission at 313 nm. A germicidal lamp equipped with a G15T8 bulb (Sankyo Denki, Japan) was the UVC source.
Spectrally relevant UV dosimeters were from International Light Technologies Inc. (Peabody, MA, USA) calibrated against spectroradiometric measurements of the sources made by a DM150 double monochromator Bentham spectroradiometer (Bentham Instruments, Ltd., Reading, UK) calibrated against national UK standards.
Ionizing radiation (3.0 Gy/min) was from a 137Cs γ-radiation source with a Nordion GC-1000 S v2.09 cell irradiator (Ottawa, ON, Canada).
Cell survival
Irradiated cells were seeded in 48-well plates (5000 cells/well). Viability was determined 5 days later by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay. For clonal survival, cells were plated in 6-well plates (300–500 cells/well). Colonies were stained and counted 10 days later. Data are based on triplicate samples from at least two experiments.
Detection of ROS
Cells were grown in the presence or absence of S4TdR for 48 h, washed twice with PBS and incubated with 5 µM CM-H2DCFDA (Invitrogen, Paisley, Scotland) for 20 min at 37°C. After additional washing with PBS, cells were UVA irradiated in PBS/CaMg. Fluorescence was analysed by flow cytometry on a Beckton Dickinson (BD) Canto II machine.
APRT mutation assay
Mutation Frequency. The Adenine phosphoribosyltransferase (APRT) Mutation frequency was determined using CHO-D422 (22,23). Cells were maintained in HAT medium to eliminate spontaneous mutants. They were then grown for 48 h in a medium supplemented with 10% DFCS and 100 µM S4TdR and irradiated. After a further 7 days growth, 106 cells were plated per 10 cm dish in medium containing 0.4 mM 8-azaadenine. The number of resistant colonies was determined after a further 10 days’ growth. At least 1.5 × 107 cells were plated per experiment.
Immunofluorescence
HaCaT cells grown on culture slides (BD Falcon, San Jose, CA) in 100 µM S4TdR were irradiated with 10 kJ/m2 UVA. Control cells were irradiated with 10 Gy γ-rays. Irradiated cells were rinsed with PBS and returned to normal medium for 4 h. They were then fixed and processed for detection of phosphorylated γH2AX as described by Brem et al. (24). For the detection of CPD and (6-4) photoproducts, irradiated HaCaT cells were fixed immediately after UV irradiation. Photoproducts were detected using antibodies specific for thymine dimers (clone TDM-2) or (6-4) Py:Py photoproducts (clone 64 M-2) from CosmoBio Co., Ltd (Tokyo, Japan) according to the company's recommendations.
Comet assay
Alkaline comet assays were performed using the Single Cell Gel Electrophoresis Assay kit (Trevigen, Gaithersburg, MD, USA) according to the manufacturer's protocol.
Modifications with the DNA lesion-specific enzymes T4 endoV (T4N5) or recombinant human 8-oxoguanine glycosylase 1 (hOGG-1, both from New England Biolabs, Hitchin, UK) were performed as described by Cooke et al. (25). Briefly, after cell lysis, washed slides were immersed in enzyme digestion buffer (40 mM HEPES, 0.1 M KCl, 0.5 mM EDTA and 0.2 mg/ml BSA, pH 8.0), for 5 min. T4N5 (0.1 U/ml) or hOGG1 (3.2 U/ml) was added to the gels which were covered with a coverslip and incubated for 60 min (T4N5) or 45 min (hOGG1) at 37°C in a humidified atmosphere. Electrophoresis was carried out according to the manufacturer's protocol.
HPLC analysis
S4TdR
Reverse phase-high performance liquid chromatography (RP-HPLC) analysis of the irradiated nucleoside was performed using a Waters 2695 Alliance system with a Waters dC18 column (Atlantis, 3um, 150 × 2.1 mm). The column was eluted with a gradient of 0–20% methanol in 10 mM KH2PO4, pH 6.7 during the first 10 min. The methanol concentration was increased to 80% during the subsequent 10 min and to 90% in the following 3 min during which the KH2PO4 concentration was reduced to zero.
DNA
DNA was extracted using the Wizard Genomic DNA purification kit (Promega, Madison, WI, USA). DNA (50 µg) was digested with nuclease P1 (Sigma Aldrich, UK) (10 U, 1 h at 50°C) and alkaline phosphatase (2 U, 1 h at 37°C). Deoxyribonucleosides were separated by HPLC (7). Thymidine was detected and quantified by A260nm and S4TdR by A335.
Interstrand crosslink detection
The oligonucleotides complementary to S4TdR oligonucleotides were [32P] end-labelled with polynucleotide kinase. Following annealing to their complementary strand, duplex oligonucleotides were UVA irradiated, heat-denatured and analysed by denaturing PAGE followed by autoradiography (26).
RESULTS
S4TdR/UVA-induced cytotoxicity in an organotypic skin model
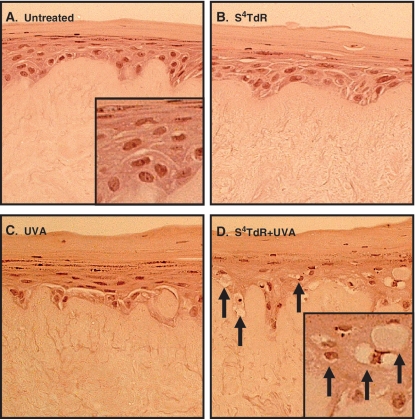
The powerful synergistic toxicity of S4TdR and UVA was tested in an organotypic 3D skin model. Reconstituted skin (raft) cultures comprising human normal primary fibroblasts and keratinocytes were treated with S4TdR by maintaining the rafts in growth medium containing the photosensitizer. The rafts were then exposed to UVA doses of ∼100 kJ/m2 (this is approximately one-fifth of a minimal erythema dose for human skin) (27) and paraffin sections were examined for the presence of apoptotic ‘sunburn’ cells (SBC). Figure 1 shows that the rafts that had received either UVA (Figure 1B) or S4TdR (Figure 1C) alone contained only apparently healthy cells and there was no sign of apoptosis. SBC were present only in rafts treated with the combination of drug and radiation (Figure 1D) and characteristic markers such as pyknosis (chromatin condensation, leading to enhanced nuclear colouration) and karyolysis (chromatin degradation leading to fading of nucleus colouration) were clearly visible in the proliferating layers down to the basement membrane at the epidermal–dermal junction. Notably, there was no sign of cell damage in the upper non-proliferating layers of the epidermis. These data demonstrate that sufficient UVA penetrates through to the proliferating target cell population to activate DNA S4TdR and that S4TdR-containing cells in the epidermis are susceptible to killing by relatively low doses of UVA.
Figure 1.
Synergistic cytotoxicity of S4TdR/UVA in organotypic skin cultures.Organotypic skin cultures were treated as indicated and fixed 24 h post-irradiation. Haematoxylin/eosin-stained sections are represented. (A) Untreated. (B) 300 µM S4TdR. (C) 100 kJ/m2 UVA. The skin/cell architecture is unaffected by treatments in B and C. (D) S4TdR/UVA combination: Apoptotic (‘sunburn’) keratinocytes displaying pyknosis (enhanced nuclear colouration) and cells showing karyolysis (loss of nuclear colouration) are shown as arrows. Note the absence of cellular damage in the upper, non-proliferating layers of the epidermis.
Mutagenicity
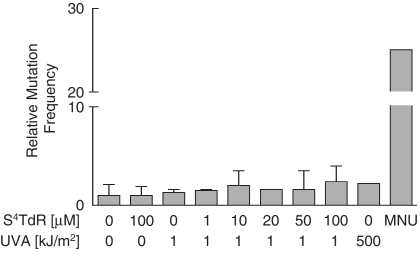
Although the toxicity of the S4TdR/UVA combination is well established (8,9), its potential mutagenicity has not been investigated in detail. We examined mutation to 8-azaadenine-resistance in the APRT hemizygous Chinese hamster ovary (CHO-D422) cell line. D442 cells incorporate S4TdR extremely efficiently (Supplementary Figure S1A) and therefore, provide a sensitive indicator of S4TdR/UVA mutagenicity. Cells were exposed to S4TdR and UVA alone or in combination and mutant colonies were scored after 10–12 days. Figure 2 shows that the most extreme condition (100 µM S4TdR plus 1 kJ/m2 UVA), which reduced cell survival to ∼1% (Supplementary Figure S1B) induced a modest 2.4-fold increase in mutation frequency. Less toxic combinations increased the mutation frequency by ≤2-fold. Neither S4TdR, nor UVA alone was measurably mutagenic at these doses. In contrast, in the absence of S4TdR treatment, a UVA dose of 500 kJ/m2 induced a similar 2- to 4-fold increase in mutation frequency. This value is comparable with published results with the same cell line (28) or other CHO clones (29). Methylnitrosourea, an acknowledged mutagen included as a positive control, induced a 25-fold increase in mutation frequency. We conclude that, the mutagenic effect of S4TdR/UVA is weak and is similar to that of high-dose UVA.
Figure 2.
Mutagenicity of S4TdR/UVA. CHO-D422 cells grown for 48 h in S4TdR as shown were washed and irradiated with UVA. Mutation to 8-azaadenine resistance was determined. Data are the mean of at least three independent determinations ± standard deviation. The mean mutation frequency in untreated cells was 2.3 × 10−5 (range: 1.2 to 3.8 × 10−5). Control cells received either 500 kJ/m2 UVA alone, 100 µM S4TdR alone or 0.5 mM MNU.
ROS
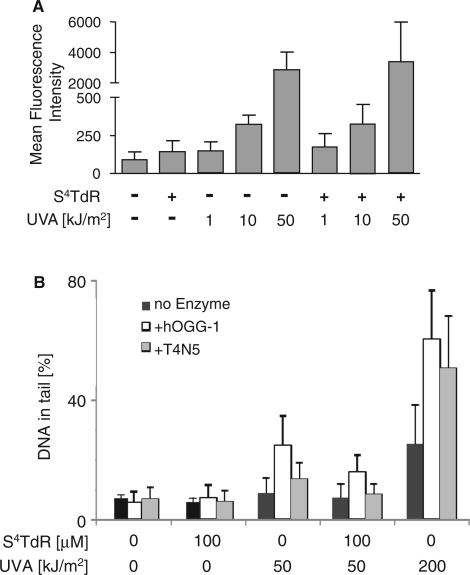
High UVA doses generate intracellular ROS. In addition, the thiopurines are Type II UVA photosensitizers and DNA 6-TG is a potent source of ROS following UVA activation (7). To investigate whether photoactivation of DNA thiopyrimidines might also produce ROS, human HaCaT keratinocytes were treated with S4TdR for 48 h to replace ∼0.1% of DNA thymidine. They were then irradiated with a range of UVA doses and ROS production was measured using the ROS-activated fluorescent probe CM-H2DCFDA. Flow cytometry analysis (summarized in Figure 3A) confirmed that UVA irradiation alone generated ROS in a dose-dependent manner. The presence of DNA S4TdR did not, however, significantly (P > 0.05) increase the levels of ROS produced by UVA indicating that it is not acting principally as a Type II UVA photosensitizer.
Figure 3.
(A) Reactive oxygen species. HaCaT cells were grown for 48 h in medium containing 100 μM S4TdR to replace ∼0.1% of DNA thymidine. PBS-washed cells were incubated with the ROS-sensitive probe CM-H2DCFDA, irradiated with UVA as indicated and ROS-induced fluorescence measured by flow cytometry. Data are presented as the mean fluorescence intensity ± standard deviation of three experiments. (B) DNA single-strand breaks/guanine oxidation/cyclobutane pyrimidine dimer (CPD) formation. HaCaT cells grown in the presence or absence of 100 µM S4TdR were washed and irradiated with 50 or 200 kJ/m2 UVA as indicated. Direct DNA single-strand breakage was analysed by the alkaline comet assay (No enzyme, black bars). The presence of DNA 8-oxoguanine was revealed by digestion with hOGG-1 (+hOGG-1, open bars) and CPDs by digestion with T4N5 (+T4N5, grey bars) as indicated. DNA damage is expressed as percentage of DNA in the comet tail.
DNA damage
Single-strand breaks, oxidized DNA guanine and cyclobutane pyrimidine dimers
The alkaline comet assay (30) was used to determine whether DNA S4TdR photoactivation leads to DNA strand breakage or oxidative DNA damage. A modest dose of UVA, 50 kJ/m2, caused just detectable DNA single-strand breakage in HaCaT cells. The presence of DNA S4TdR did not detectably influence strand breakage at this UVA dose, whereas a UVA dose of 200 kJ/m2 caused a substantial increase (Figure 3B).
The alkaline comet assay combined with recombinant human OGG-1 DNA glycosylase (hOGG-1) treatment as a probe for oxidative DNA damage indicated that UVA doses of 50 and 200 kJ/m2 generated measurable DNA 8-oxoguanine in HaCaT cells. The presence of S4TdR did not alter the hOGG-1 sensitivity of DNA following irradiation with 50 kJ/m2 UVA (Figure 3B). Thus, in agreement with the inability of DNA S4TdR to serve as a source of UVA-generated ROS, it does not detectably increase UVA-induced DNA guanine oxidation.
In parallel assays, inclusion of the cyclobutane pyrimidine dimer (CPD)-specific T4 endonuclease V (T4N5) (31) introduced significant DNA breakage in cells treated with 50 kJ/m2 or 200 kJ/m2 UVA. S4TdR treatment did not increase T4N5 susceptibility of DNA from cells exposed to 50 kJ/m2 UVA (Figure 3B). These data confirm the introduction of CPD by UVA (32) and indicate further that DNA substitution by S4TdR does not measurably increase their formation.
In summary, UVA radiation induces DNA single-strand breaks, oxidized guanine and CPD in HaCaT cells. Despite acting as a UVA sensitizer for killing by UVA, DNA S4TdR does not detectably increase the yield of any of these DNA photoproducts even under conditions that are supralethal and result in <1% cell survival (Supplementary Figure S1B). These findings are consistent with the inability of DNA S4TdR to increase the yield of UVA-induced ROS in these cells and suggest that alternative DNA lesions underlie its cytotoxic potential.
Double-strand breaks
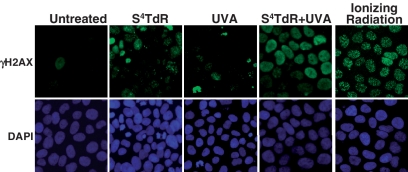
The accumulation of foci of phosphorylated histone H2AX (γH2AX) is a hallmark of the cellular response to DSB (33). HaCaT cells that had been treated with S4TdR (100 µM, 48 h) were UVA irradiated and γH2AX was detected by immunocytochemistry. After 4 h of irradiation, most S4TdR-treated cells displayed a pan-nuclear staining (Figure 4) that is consistent with inhibited replication. In contrast, following gamma irradiation (10 Gy) discrete focal γH2AX staining which reflects the presence of DSB was observed in all cells. Neither UVA nor S4TdR alone induced γH2AX staining. These findings suggest that shortly after UVA irradiation, Double-strand breaks (DSBs) are generated in, at most, a small minority of S4TdR-treated cells. They are also consistent with previous observations that DNA S4TdR photoactivation induces a severe inhibition of DNA replication (Montaner, B. and Karran, P., unpublished data).
Figure 4.
γ-H2AX staining. HaCaT cells grown in the presence of 100 µM S4TdR for 48 h were irradiated with 10 kJ/m2 UVA as shown. Control cells grown in the absence of S4TdR were treated with 10 Gy of gamma irradiation. In each case, γ-H2AX was visualized 4 h later. Note the pan-nuclear γ-H2AX staining induced by S4TdR/UVA treatment which differs from the discrete focal staining following gamma irradiation. DNA was counterstained with 4′,6-diamidino-2-phenylindole (DAPI).
S4TdR photoproducts
S4TdR in dilute solution
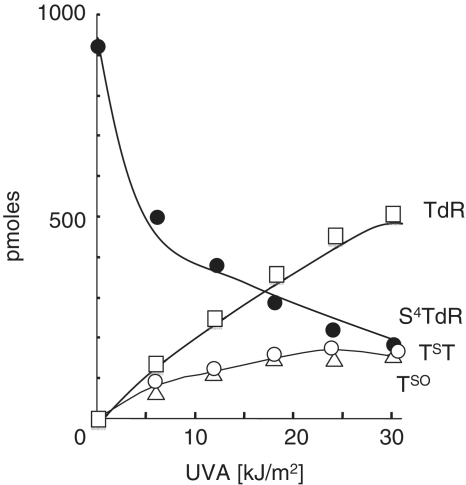
We examined the stability of the free thiodeoxynucleoside to UVA irradiation. An aqueous S4TdR solution (0.1 mM) was irradiated and the products were separated by reverse phase HPLC. UVA induced a dose-dependent disappearance of S4TdR (Figure 5) with the concurrent appearance of three photoproducts. Mass spectrometry identified thymidine as the predominant species. The remaining photoproducts comprised small amounts of dimeric S4TdR (TsT) and thymidine sulphenate (TSO) in approximately equal yields. The nature of dimeric photoproduct is not yet completely defined. Its UV absorbance spectrum has a maximum at 306 nm which is close to that of S4-methylthymidine (λmax 314 nm) and S4-hydroxyethylthiothymidine (λmax 308 nm). For this reason, we tentatively assign a TST rather than disulphide-linked (TSST) structure. The identification of thymidine sulphenate was based upon high resolution MS analysis (275.06) of the isolated peak and its unusual UV absorption (λmax 357 nm).
Figure 5.
UVA photostability of S4TdR in dilute solution. A 0.1 mM aqueous solution of S4TdR was irradiated with UVA as shown. Samples were analysed by HPLC. Products were detected by A260 or A335 and quantified. Further confirmation of TSO and TST was obtained by mass spectrometry analysis.
Thus, in aqueous solution, the most favoured reaction of photoactivated S4TdR is with water to generate thymidine. Since S4T base pairs with A, this reaction generates a canonical base pair in DNA. For this reason, it will not contribute significantly to the cytotoxicity of S4TdR/UVA. These findings suggest that reactions with the surrounding DNA bases or DNA-interacting proteins might be important in the formation of potentially lethal DNA photolesions in treated cells.
S4TdR in oligonucleotides
A series of synthetic oligonucleotides that contained a single S4TdR (Table 1) were used to examine the effects of UVA irradiation on DNA S4TdR in different sequence contexts. Oligonucleotides were irradiated in either single- or double-stranded form and digested to nucleosides which were separated by RP-HPLC. Column eluates were monitored at 335 nm (S4TdR), 260 nm (normal nucleosides) and by fluorescence (Ex 320 nm; Em 370 nm).
Table 1.
Sequences of the S4T-containing synthetic oligonucleotides used to examine the effects of UVA irradiation on DNA S4TdR in different sequence contexts
| Sequence | Code |
|---|---|
| 5′-GAATCAGCCS4TGCACAGATACGAG-3′ | CS4TG |
| GAATCAGCTS4TGCACAGATACGAG | TS4TG |
| GAATCAGCGS4TTCACAGATACGAG | GS4TT |
| GAATCAGCGS4TCCACAGATACGAG | GS4TC |
| GAATCAGCGS4TGCACAGATACGAG | GS4TG |
| GAATCAGCGS4TACACAGATACGAG | GS4TA |
S4T= 4-thiothymine.
Abbreviations used in the text for the sequences are given in the Code column.
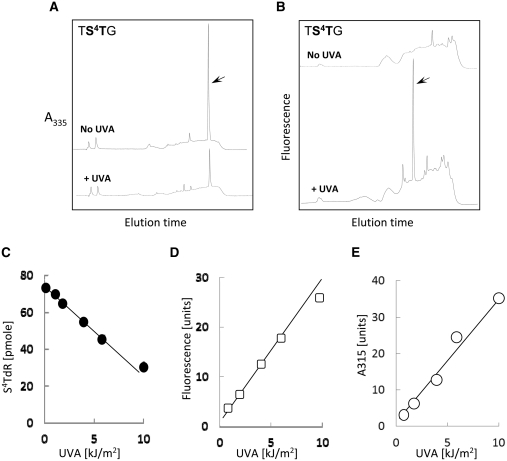
Figure 6A and B shows representative HPLC profiles for the digests of the duplex oligonucleotide in which the TS4TG oligonucleotide is annealed to a complementary strand to generate an S4T:A pair flanked by a 5′T and a 3′G (Table 1). A UVA dose of 10 kJ/m2 reduced the yield of S4TdR (elution time 26 min) by >60% and this coincided with the appearance of a fluorescent product eluting at 19 min (Figure 6B). This photoproduct also absorbed at 315 nm. The destruction of S4TdR and the formation of the fluorescent/A315 absorbing material were linearly related to UVA dose ∼10 kJ/m2 (Figure 6C–E). UVA irradiation of the single-stranded TS4TG oligonucleotide generated the same 19-min product in a similarly dose-dependent fashion in approximately similar yields (data not shown).
Figure 6.
UVA photoproducts in oligonucleotides containing S4TdR with a 5′T. The oligonucleotide TS4TG annealed to a complementary strand to place a single S4T:A base pair 3′ to T:A base pair was irradiated with 10 kJ/m2 UVA. Following digestion to nucleosides, the products were analysed by RP-HPLC and the eluate monitored by: (A) A335. Upper trace unirradiated. Lower trace 10 kJ/m2 UVA. The absorbance scale is the same for both traces. The arrow indicates S4TdR eluting at 26 min. (B) Fluorescence (Ex 320 nm; Em 370 nm). Upper trace unirradiated. Lower RP-HPLC trace 10 kJ/m2 UVA. The arrow indicates the fluorescent photoproduct eluting at 19 min. Fluorescence scale is the same for both traces. (C) UVA dose dependence of S4TdR destruction. Residual S4TdR in digests of irradiated TS4TG duplexes was quantified from A335 nm absorbance following HPLC separation. (D) UVA dose dependence of the formation of the fluorescent (D) and 315 nm absorbing (E) photoproduct eluting from HPLC at 19 min.
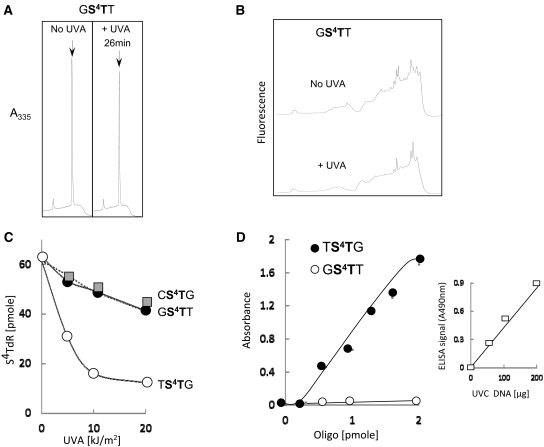
S4T was more UVA resistant in the closely related GS4TT duplex in which it is paired to A and flanked by 5′G:C and a 3′T:A pairs (Table 1). A dose of 10 kJ/m2 destroyed only ∼10% of the S4TdR (Figure 7A). Higher UVA doses were more effective and only 30% of the starting S4TdR remained after 100 kJ/m2. No photoproducts were detectable by fluorescence (Figure 7B), A335 or A260 (data not shown). In particular, there was no concomitant increase in recovery of TdR, confirming that the photochemistry of DNA S4TdR differs from that of the free thionucleoside. The photosensitivity of S4TdR in single- or double-stranded GS4TT was typical of oligonucleotides in which S4T was not immediately 3′ to T. These included CS4TG indicating that a 5′C cannot substitute for T to confer enhanced UVA sensitivity. Comparison between TS4TG and other duplex oligonucleotides (CS4TG & GS4TT) is presented in Figure 7C.
Figure 7.
UVA photoproducts in oligonucleotides containing S4TdR with a 5′G or C (A and B) The GS4TT duplex was irradiated with 10 kJ/m2 UVA and analysed as described in the legend to Figure 6A and B. Eluted S4TdR (26 min) is shown as arrows. The A335 scales in the two panels in A and the fluorescence (Ex 320; Em 370) scales in B are the same for both traces. (C) UVA dose-dependence of S4TdR destruction in the CS4TG and GS4TT duplexes. Residual S4TdR in digests of irradiated duplexes was quantified from A335. Data for the TS4TG duplex are shown for comparison. (D) Detection by ELISA of photoproducts in UVA (10 kJ/m2) irradiated TS4TG and GS4TT duplexes. Inset: ELISA of DNA extracted from CCRF-CEM cells irradiated with 10 J/m2 UVC.
Based on these findings, we conclude that S4TdR in single- or double-stranded DNA is susceptible to destruction by UVA. It is atypically photosensitive when placed 3′- but not 5′T. In the former context, we observed a photoproduct with fluorescence and absorbance properties consistent with those reported for thio (6-4)T:T, (S5-(6-4)T:T) (16), which is analogous to the (6-4) Py:Py UVC/UVB DNA photoproduct. The likely identity of the S5-(6-4)T:T was confirmed by ELISA assays. Figure 7D shows that the UVA-irradiated TS4TG oligonucleotide was recognized by the antibody against (6-4) Py:Py and gave a strong ELISA signal. In contrast, irradiated GS4TT gave only background signals. In the absence of a 5′T, the destruction of S4TdR requires higher UVA doses and is not associated with photoproducts with detectable UVA or UVC absorbance or fluorescence under our standard conditions. Although TdR is the predominant UVA photoproduct after high-dose irradiation of S4TdR in aqueous solution, we did not see an equivalent efficient conversion to TdR in irradiated oligonucleotides.
DNA interstrand crosslinks
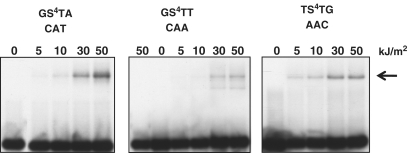
When duplex S4TdR oligonucleotides were UVA irradiated and analysed by denaturing PAGE, a radioactive photoproduct that migrated with the characteristics of a cross-linked duplex was observed. Interstrand crosslinks (ICL) formation was UVA dose-dependent. The efficiencies of crosslinking with the GS4TA, GS4TT and TS4TG duplexes were not widely different, suggesting that the generation of ICL is largely independent of the immediate sequence context of the S4TdR (Figure 8). Crosslinking was dependent on the presence of the complementary strand and UVA irradiation did not induce a change in migration of single-stranded S4TdR-containing oligonucleotides (Figure 8, middle panel).
Figure 8.
DNA interstrand photocrosslinking in S4TdR-containing duplex oligonucleotides. The GS4TA, GS4TT and TS4TG oligonucleotides were annealed to their [32P]-labelled complementary strands and irradiated with the UVA doses shown. A [32P]-labelled unannealed GS4TT strand was also irradiated with 50 kJ/m2 UVA (far left lane of central panel). Products were separated by denaturing PAGE and detected by autoradiography.
DNA repair
In order to investigate the potential toxicity of S4TdR DNA photoproducts, we examined the S4TdR/UVA sensitivity of a number of cell lines with known DNA repair defects. The previously reported hypersensitivity of NER-defective XPA cells was confirmed (Supplementary Figure S2).
DNA DSB rejoining
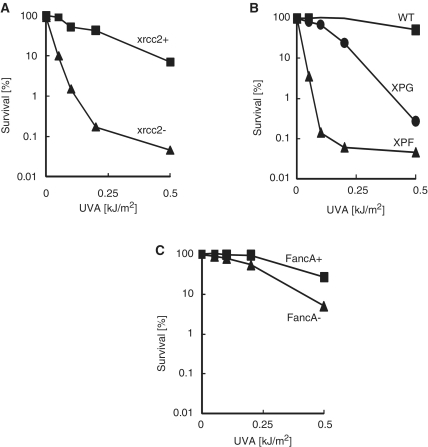
DNA S4TdR photoproducts inhibit replication (Montaner, B. and Karran, P., unpublished data). Homologous recombinational repair (HR) is one of the predominant pathways by which lesions that impair replication are processed. At comparable levels of DNA S4TdR (∼0.3% replacement of TdR), HR-defective xrcc2 hamster cells were extremely sensitive to UVA. Whereas >80% of xrcc2+ cells survived a UVA dose of 0.1 kJ/m2, the same dose reduced survival of the HR-defective cells to around 1% (Figure 9A). In the absence of S4TdR pre-treatment, neither the HR-proficient nor HR-defective cells were affected by these very low UVA doses. To address DSBs that arise outside of S phase, we examined cells defective in the non-homologous end joining (NHEJ) pathway. Neither the NHEJ-defective (Ku80 mutant) xrs6, nor the repair-proficient parental K1 hamster cells exhibited any S4TdR-related sensitivity over the UVA dose range that was extremely toxic to the HR-defective cells (data not shown). The xrs6 cells incorporated S4TdR poorly, however, and in several experiments replaced ≤0.03% of DNA TdR compared with the 0.3% for the parental CHO K1 cells. Owing to this, we are unable to draw firm conclusions about the contribution of NHEJ to S4TdR/UVA resistance. Taken together with the findings from the γH2AX immunocytochemistry, however, the data indicate that DNA lesion(s) that block replication and thereby generate DSB that require HR for repair are a major contributor to the lethal effects of S4TdR/UVA.
Figure 9.
S4TdR/UVA sensitivity in DNA repair-defective cell lines. (A) Homologous recombination. xrcc2-deficient CHO irs1 cells and their XRCC2 complemented counterparts were grown for 48 h in 100 µM S4TdR and UVA irradiated at the doses indicated. Survival was determined by clonal assay. (B) XPF and XPG. CHO UV135 (XPG) and CHO UV41 (XPF) cells were treated as in A. Survival was determined by clonal assay. (C) Fanconi A. FancA-defective and FancA-corrected MEFs were treated as in A. Survival was determined by clonal assay.
DNA crosslink processing
The HR pathway is part of the complex system that processes ICL. The XPF:ERCC1 endonuclease is also involved and XPF-deficient cells are extremely sensitive to DNA interstrand crosslinking agents. CHO UV41 cells are defective in the hamster homolog of XPF. Figure 9B shows that 0.1 kJ/m2 UVA reduced the survival of S4TdR-treated UV41 cells to ∼0.1%. At comparable levels of DNA S4TdR (0.2–0.3%), the parental AA8 cells were essentially insensitive to UVA at doses up to five times higher. The UV135 XPG mutant CHO cells exhibited an intermediate sensitivity compatible with their NER defect (Figure 9B). Mouse embryonic fibroblasts defective in the FancA protein, a member of the Fanconi anaemia (FA) pathway core complex, were also more sensitive to S4TdR/UVA than their FancA-proficient counterparts (Figure 9C). The pattern of S4TdR/UVA sensitivity among these various mutant cell lines indicates that ICL are a significant contributor to toxicity.
DNA S5-(6-4)T:T and excision repair
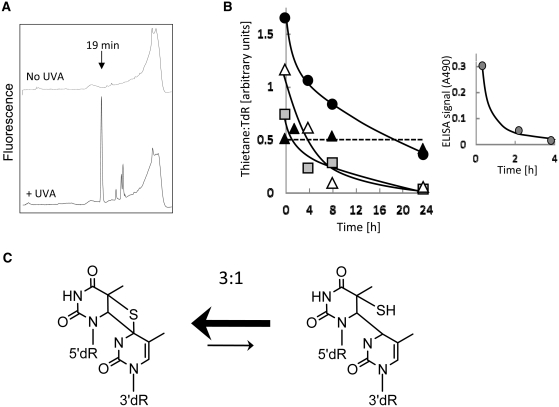
We examined whether the S5-(6-4)T:T lesion was detectable in DNA from S4TdR/UVA-treated cells and whether it was subject to excision repair. HaCaT cells in which S4TdR replaced between 0.1% and 0.2% of DNA TdR were irradiated with UVA and DNA was extracted immediately or ∼24 h after irradiation. DNA digests were analysed by HPLC with fluorescence detection. Figure 10A shows that a photoproduct with the characteristic fluorescence and elution time (19 min) of the S5-(6-4)T:T lesion was formed in cellular DNA. At 20 kJ/m2 UVA, 30–40% of the DNA S4TdR was destroyed. The fluorescent photoproduct was easily detectable at doses as low 5 kJ/m2 and formation was linear with UVA dose up to 20 kJ/m2, the highest dose examined (Supplementary Figure S3A). At similar levels of DNA S4TdR (0.2%), the UVA dose dependency of DNA S4TdR destruction and of S5-(6-4)T:T formation in human CCRF-CEM cells was comparable with that of HaCaT cells (Supplementary Figure S3B).
Figure 10.
Induction and repair of the thietane/S5-(6-4)T:T photoproduct in HaCaT cells. (A) Formation of S5-(6-4)T:T. HaCaT cells that had been grown for 48 h in 300 µM S4TdR were irradiated with 10 kJ/m2 UVA. DNA extracted immediately after irradiation was digested to nucleosides that were separated by HPLC with fluorescence (Ex 320 nm; Em 370 nm) detection. The arrow indicates the elution time of the thietane/S5-(6-4)T:T photoproduct. The fluorescence scale is the same for both traces. (B) Excision of the thietane/S5-(6-4)T:T photoproduct. HaCaT cells were treated with S4TdR (300 µM/48 h) and irradiated with UVA: 5 (filled grey square), 10 (open triangle) or 20 (filled black circle) kJ/m2. XPA cells treated with 100 µM/48 h were irradiated with 5 kJ/m2 (filled black triangle). DNA was extracted, digested to nucleosides and analysed by HPLC. The thietane/S5-(6-4)T:T photoproduct was detected by its fluorescence at 370 nm. Thietane/S5-(6-4)T:T levels are expressed by dividing the total fluorescent signal (mV) by the number of pmoles of TdR determined by A260 in the same sample. Inset: Excision of UVC-induced (6-4) Py:Py photoproducts. At the times indicated, DNA was extracted from HaCaT cells irradiated with 30 J/m2 UVC and analysed by ELISA assay. (C) The thietane/S5-(6-4)T:T photoproduct equilibrium (38,39). The thietane species with the closed four-membered ring predominates and comprises ∼75% of the total. For a normal (6-4) Py:Py photoproduct, the corresponding ring-closed oxetane structure is unstable and essentially all of this product is in the ring-open form.
HaCaT cells removed S5-(6-4)T:T lesions. Figure 10B shows that they were excised with a half-life of between 6 h and 12 h. By 24 h, the fluorescent product was barely detectable in DNA from cells treated with 5 kJ/m2 or 10 kJ/m2 UVA. The removal of UVC-induced DNA (6-4) Py:Py photoproducts was considerably faster and they were excised with a half-life of <1 h (Figure 10B, insert). S5-(6-4)T:T repair was significantly less efficient in CCRF-CEM cells and they removed around half of the initial photoproducts in 24 h (Supplementary Figure S3C). The S5-(6-4)T:T lesions persisted at unchanged levels for 24 h in XPA-defective XP12RO cells (Figure 10B) confirming that repair of this photoproduct is by NER.
The high sensitivity of the fluorescence detection renders S5-(6-4)T:T photoproducts easily detectable in HPLC eluates. These rare DNA adducts were not, however, detectable in HaCaT cells by immunocytochemical staining (Supplementary Figure S4) or by ELISA (data not shown) using antibodies against UVC-induced DNA (6-4) Py:Py lesions.
DISCUSSION
The powerful synergistic toxicity of S4TdR and UVA in cultured cells is well established (8,9). Its recently reported effectiveness in inhibiting tumour growth in a rat bladder carcinoma model (34) indicates that it is a promising photochemotherapeutic option. The most attractive application for S4TdR would be for hyperproliferative skin conditions that are readily accessible to UVA irradiation. Possible limiting factors for effectiveness in this context are penetration of UVA through the epidermis and accumulation of DNA S4TdR in the proliferating target cells. As a first step in establishing the possible efficacy of S4TdR/UVA, we used reconstituted skin raft cultures, a model which reproduces many of the three-dimensional aspects of skin (20). In this model, modest radiation doses delivered sufficient UVA to the lower levels of the epidermis to kill S4TdR-sensitized cells. Recent evidence from our laboratory shows that physiological doses of UVA induce CPD in human skin (35). Importantly, in our raft experiments, the penetrating UVA was not detectably toxic without the photosensitizer. The rat bladder carcinoma study provided indications that effective levels of DNA S4TdR can be achieved in the target cell population. In that study, systemic (intravenous) treatment resulted in significantly higher levels of DNA S4TdR in dividing tumour cells than in the adjacent normal bladder epithelium (34). It is also noteworthy that incorporation into normal skin was extremely low, suggesting that the treatment would selectively target hyperproliferative epidermal cells while sparing normal cells. Taken together, these studies indicate the potential effectiveness of S4TdR/UVA therapy. Treatment-induced mutation and the possible development of therapy-related cancer, is a potential hazard of any DNA damage-based therapy. The study reported here demonstrates another encouraging aspect of S4TdR/UVA treatment, its relatively weak mutagenicity. UVA photoactivation of DNA S4TdR combines effective cell killing with minimal potential long-term risks.
The weak mutagenicity of S4TdR/UVA is at least partially accounted for by its photosensitizing mechanism. UVA activation of DNA S4TdR does not generate measurable ROS. As a consequence, the DNA damage it produces appears to be qualitatively different to that caused by high-dose UVA alone (36). Activation of DNA S4TdR did not detectably increase DNA single-strand breakage or guanine oxidation and in this regard, it differs from the effects of DNA 6-TG (24,25). We cannot, however, entirely exclude the possible formation of these DNA lesions by S4TdR/UVA, because the simultaneous introduction of ICL reduces the sensitivity of the comet assay. In this regard, we note that 6-TG/UVA, which also causes interstrand DNA crosslinking, induces single-strand breaks that are easily measurable by the comet assay (24). It is clear that, in comparison with 6-TG/UVA treatment, single-strand breakage and DNA guanine oxidation are relatively infrequent events in cells treated with S4TdR/UVA.
The extreme sensitivity of NER-deficient cells and cells with defects in HR or in the processing of ICL indicates that the cytotoxicity of S4TdR/UVA depends on its ability to induce bulky DNA lesions that are severe impediments to DNA replication. We identified two possible candidate DNA lesions: ICL and a S5-(6-4)T:T adduct. ICL are among the most difficult DNA lesions for the cell to process. They prevent the helix unwinding, which is an essential step in DNA excision repair, replication and transcription. Their processing involves a complex interplay between some NER proteins, specialized DNA polymerases, members of the Fanconi anaemia family of proteins and those of HR (37). Defects in any of these proteins confer extreme sensitivity to ICL. The sensitivity profiles of the cells we examined, in particular the hypersensitivity of the irs1 and UV41 cell lines, are consistent with a significant contribution of ICL to the cytotoxicity of S4TdR/UVA. This conclusion is reinforced by the ease of ICL formation in duplex oligonucleotides in vitro. Crosslinking occurred at low doses of UVA, was independent of the sequences surrounding the S4TdR and involved a canonical base in the complementary DNA strand. All of these observations suggest that ICL are likely to be abundant in cells in which, as many as, 107 DNA thymines may be replaced by S4TdR. In agreement with this possibility, comet assays of S4TdR/UVA-treated bladder carcinoma cells reveal extensive DNA crosslinking that is only partially reversed by protease digestion to remove DNA-adducted proteins (34).
Our findings also confirm the particular photosensitivity of S4T when it is immediately 3′ to a normal thymine in DNA. The fluorescent product that was formed after irradiation of the TS4TG duplex has the properties reported for the S5-(6-4)T:T adduct which resembles the DNA (6-4) Py:Py lesion (38,39). The S5-(6-4)T:T adduct was also detectable in DNA from S4TdR/UVA treated cells. HaCaT keratinocytes excised these photoproducts quite efficiently. Nevertheless, they were more persistent than UVC-induced (6-4) Py:Py lesions. We observed a rapid removal of (6-4) Py:Py lesions, consistent with their reported half-life of ≤1 h in HaCaT cells (40). Removal of S5-(6-4)T:T adducts was even slower in CCRF-CEM leukaemia cells in which most of them persisted for up to 24 h.
Rapid repair of (6-4) Py:Py lesions reflects the significant DNA distortion they create. The relative persistence of S5-(6-4)T:T suggests that it may cause less DNA distortion. This, in turn, may reflect the greater stability of the ring-closed, thietane form (38,39). Despite obvious structural similarities between S5-(6-4)T:T and (6-4) T:T—they are identical except for the replacement of the single O4 atom of the 3′-thymine by S in the former—there are significant differences in behaviour (16,38,39). In particular, although, both photoproducts are formed via a four-membered closed ring intermediate, the ring-closed oxetane isomer of the (6-4) T:T is unstable and is immediately converted to the open ring form. The thietane is more stable and exists as an equilibrium mixture in which the ring-closed thietane is favoured over S5-(6-4)T:T (38,39) (Figure 10C). It is possible that, although the structural similarities between (6-4) T:T and thietane permits recognition of the latter in the (6-4) Py:Py ELISA assay, the rotational constraints imposed by the four-membered ring mitigate DNA distortion by the thietane and impede its recognition by NER. We note, however, that in order to induce detectable levels of thietane/S5-(6-4)T:T photoproducts, it was necessary to expose cells to combinations of S4TdR and UVA that cause significant cell death. It is therefore, possible that the one or more of the other DNA S4TdR photoproducts, such as ICL, interferes with the processing of thietane/S5-(6-4)T:T lesions by NER. These possibilities are currently under investigation.
This study confirmed the extreme sensitivity of XP12RO cells. These findings indicate that NER repairs potentially lethal S4TdR/UVA DNA damage. The sequence dependency of DNA thietane/S5-(6-4)T:T formation suggests that these lesions may comprise a relatively minor fraction of the total DNA photoproducts. Despite this, they contribute significantly to the lethal effects of S4TdR/UVA. The NER system is particularly efficient in keratinocytes (41,42) and the superior repair of thietane/S5-(6-4)T:T lesions by HaCaT cells is associated with a high survival. XP129, a UVC-resistant ‘revertant’ of XP12RO, which has regained a normal ability to excise (6-4) Py:Py, but not other UVC photoproducts (43,44) provides additional evidence linking thietane/S5-(6-4)T:T to toxicity. XP129 cells are only slightly (≤2-fold) more sensitive to S4TdR/UVA than NER proficient cells (45). This contrasts to the ∼100-fold sensitivity of the parental XP12RO cells and emphasizes the likely cytotoxicity of unrepaired thietane/S5-(6-4)T:T lesions.
In summary, UVA photoactivation of S4TdR—a highly efficient cytotoxic combination in cultured cells—has properties that suggest it might be an effective highly targeted treatment for hyperproliferative skin conditions. Sufficient UVA penetrates reconstituted skin and skin in vivo to generate cytotoxic DNA lesions in the lower levels of the epidermis. Its low mutagenicity suggests that the combination is a potentially effective and safe therapeutic option. The DNA lesions generated by these photosensitized reactions differ from those produced by high-dose UVA alone and thietane/S5-(6-4)T:T photoproducts and ICLs are both likely contributors to the cytotoxicity of S4TdR/UVA.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Figures S1–S4.
FUNDING
The British Skin Foundation (BSF) grant under Project No: 1040; Cancer Research UK; the UK Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy's and St Thomas’ NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust. Funding for open access charge: King's College London
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
We thank Dr John Thacker and Dr Mark O'Driscoll for providing cell lines. The assistance of Cancer Research UK Central Cell Services is gratefully acknowledged.
REFERENCES
- 1.Babilas P, Schreml S, Landthaler M, Szeimies RM. Photodynamic therapy in dermatology: state-of-the-art. Photodermatol. Photoimmunol. Photomed. 2010;26:118–132. doi: 10.1111/j.1600-0781.2010.00507.x. [DOI] [PubMed] [Google Scholar]
- 2.Pinthus JH, Bogaards A, Weersink R, Wilson BC, Trachtenberg J. Photodynamic therapy for urological malignancies: Past to current approaches. J. Urol. 2006;175:1201–1207. doi: 10.1016/S0022-5347(05)00701-9. [DOI] [PubMed] [Google Scholar]
- 3.Fayter D, Corbett M, Heirs M, Fox D, Eastwood A. A systematic review of photodynamic therapy in the treatment of pre-cancerous skin conditions, Barrett's oesophagus and cancers of the biliary tract, brain, head and neck, lung, oesophagus and skin. Health. Technol. Asses. 2010;14:1–288. doi: 10.3310/hta14370. [DOI] [PubMed] [Google Scholar]
- 4.Stern RS, Lunder EJ. Risk of squamous cell carcinoma and methoxsalen (psoralen) and UV-A radiation (PUVA) - A meta-analysis. Arch. Dermatol. 1998;134:1582–1585. doi: 10.1001/archderm.134.12.1582. [DOI] [PubMed] [Google Scholar]
- 5.Lever LR, Farr PM. Skin cancers or premalignant lesions occur in half of high-dose PUVA patients. Br. J. Dermatol. 1994;131:215–219. doi: 10.1111/j.1365-2133.1994.tb08494.x. [DOI] [PubMed] [Google Scholar]
- 6.Walker SL, Young AR. An action spectrum (290-320 nm) for TNFalpha protein in human skin in vivo suggests that basal-layer epidermal DNA is the chromophore. Proc. Natl Acad. Sci. USA. 2007;104:19051–19054. doi: 10.1073/pnas.0703385104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Donovan P, Perrett CM, Zhang X, Montaner B, Xu YZ, Harwood CA, McGregor JM, Walker SL, Hanaoka F, Karran P. Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science. 2005;309:1871–1874. doi: 10.1126/science.1114233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massey A, Xu Y-Z, Karran P. Photoactivation of DNA thiobases as a potential novel therapeutic option. Curr. Biol. 2001;11:1142–1146. doi: 10.1016/s0960-9822(01)00272-x. [DOI] [PubMed] [Google Scholar]
- 9.Reelfs O, Xu YZ, Massey A, Karran P, Storey A. Thiothymidine plus low-dose UVA kills hyperproliferative human skin cells independently of their human papilloma virus status. Mol. Cancer Ther. 2007;6:2487–2495. doi: 10.1158/1535-7163.MCT-07-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherley JL, Kelly TJ. Regulation of human thymidine kinase during the cell cycle. J. Biol. Chem. 1988;263:8350–8358. [PubMed] [Google Scholar]
- 11.Hengstschlager M, Pfeilstocker M, Wawra E. Thymidine kinase expression. A marker for malignant cells. Adv. Exp. Med. Biol. 1998;431:455–460. [PubMed] [Google Scholar]
- 12.Temmink OH, Emura T, de Bruin M, Fukushima M, Peters GJ. Therapeutic potential of the dual-targeted TAS-102 formulation in the treatment of gastrointestinal malignancies. Cancer Sci. 2007;98:779–789. doi: 10.1111/j.1349-7006.2007.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montaner B, O'Donovan P, Reelfs O, Perrett CM, Zhang X, Xu YZ, Ren X, Macpherson P, Frith D, Karran P. Reactive oxygen-mediated damage to a human DNA replication and repair protein. EMBO Rep. 2007;8:1074–1079. doi: 10.1038/sj.embor.7401084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Jeffs G, Ren X, O'Donovan P, Montaner B, Perrett CM, Karran P, Xu YZ. Novel DNA lesions generated by the interaction between therapeutic thiopurines and UVA light. DNA Repair. 2007;6:344–354. doi: 10.1016/j.dnarep.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Favre A, Saintome C, Fourrey JL, Clivio P, Laugaa P. Thionucleobases as intrinsic photoaffinity probes of nucleic acid structure and nucleic acid-protein interactions. J. Photochem. Photobiol. B. 1998;42:109–124. doi: 10.1016/s1011-1344(97)00116-4. [DOI] [PubMed] [Google Scholar]
- 16.Warren MA, Murray JB, Connolly BA. Synthesis and characterisation of oligodeoxynucleotides containing thio analogues of (6-4) pyrimidine-pyrimidinone photo-dimers. J. Mol. Biol. 1998;279:89–100. doi: 10.1006/jmbi.1998.1719. [DOI] [PubMed] [Google Scholar]
- 17.Cartwright R, Tambini CE, Simpson PJ, Thacker J. The XRCC2 DNA repair gene from human and mouse encodes a novel member of the recA/RAD51 family. Nucleic Acids Res. 1998;26:3084–3089. doi: 10.1093/nar/26.13.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuller LF, Painter RB. A Chinese hamster ovary cell line hypersensitive to ionizing radiation and deficient in repair replication. Mutat. Res. 1988;193:109–121. doi: 10.1016/0167-8817(88)90041-7. [DOI] [PubMed] [Google Scholar]
- 19.Thompson LH, Rubin JS, Cleaver JE, Whitmore GF, Brookman K. A screening method for isolating DNA repair-deficient mutants of CHO cells. Somatic Cell Genet. 1980;6:391–405. doi: 10.1007/BF01542791. [DOI] [PubMed] [Google Scholar]
- 20.Prunieras M, Regnier M, Woodley D. Methods for cultivation of keratinocytes with an air-liquid interface. J. Invest. Dermatol. 1983;81:28s–33s. doi: 10.1111/1523-1747.ep12540324. [DOI] [PubMed] [Google Scholar]
- 21.Xu YZ, Zheng QG, Swann PF. Simple synthesis of 4-thiothymidine, 4-thiouridine and 6-thio-2′-deoxyguanosine. Tetrahedron Lett. 1991;32:2817–2820. [Google Scholar]
- 22.Goncalves O, Drobetsky E, Meuth M. Structural alterations of the aprt locus induced by deoxyribonucleoside triphosphate pool imbalances in Chinese hamster ovary cells. Mol. Cell Biol. 1984;4:1792–1799. doi: 10.1128/mcb.4.9.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phear G, Armstrong W, Meuth M. Molecular basis of spontaneous mutation at the aprt locus of hamster cells. J. Mol. Biol. 1989;209:577–582. doi: 10.1016/0022-2836(89)90595-0. [DOI] [PubMed] [Google Scholar]
- 24.Brem R, Li F, Montaner B, Reelfs O, Karran P. DNA breakage and cell cycle checkpoint abrogation induced by a therapeutic thiopurine and UVA radiation. Oncogene. 2010;29:3953–3963. doi: 10.1038/onc.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooke MS, Duarte TL, Cooper D, Chen J, Nandagopal S, Evans MD. Combination of azathioprine and UVA irradiation is a major source of cellular 8-oxo-7,8-dihydro-2′-deoxyguanosine. DNA Repair. 2008;7:1982–1989. doi: 10.1016/j.dnarep.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Brem R, Daehn I, Karran P. Efficient DNA interstrand crosslinking by 6-thioguanine and UVA radiation. DNA Repair. 2011;10:869–876. doi: 10.1016/j.dnarep.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Young AR, Chadwick CA, Harrison GI, Nikaido O, Ramsden J, Potten CS. The similarity of action spectra for thymine dimers in human epidermis and erythema suggests that DNA is the chromophore for erythema. J. Invest. Dermatol. 1998;111:982–988. doi: 10.1046/j.1523-1747.1998.00436.x. [DOI] [PubMed] [Google Scholar]
- 28.Sage E, Lamolet B, Brulay E, Moustacchi E, Châteauneuf A, Drobetsky EA. Mutagenic specificity of solar UV light in nucleotide excision repair-deficient rodent cells. Proc. Natl Acad. Sci. USA. 1996;93:176–180. doi: 10.1073/pnas.93.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drobetsky EA, Turcotte J, Chateauneuf A. A role for ultraviolet A in solar mutagenesis. Proc. Natl Acad. Sci. USA. 1995;92:2350–2354. doi: 10.1073/pnas.92.6.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 31.Yasuda S, Sekiguchi M. T4 endonuclease involved in repair of DNA. Proc. Natl Acad. Sci. USA. 1970;67:1839–1845. doi: 10.1073/pnas.67.4.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Douki T, Reynaud-Angelin A, Cadet J, Sage E. Bipyrimidine photoproducts rather than oxidative lesions are the main type of DNA damage involved in the genotoxic effect of solar UVA radiation. Biochemistry. 2003;42:9221–9226. doi: 10.1021/bi034593c. [DOI] [PubMed] [Google Scholar]
- 33.Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pridgeon SW, Heer R, Taylor GA, Newell DR, O'Toole K, Robinson M, Xu YZ, Karran P, Boddy AV. Thiothymidine combined with UVA as a potential novel therapy for bladder cancer. Br. J. Cancer. 2011;104:1869–1876. doi: 10.1038/bjc.2011.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tewari A, Sarkany R, Young AR. UVA1 induced Cyclobutane pyrimidine dimers but not 6-4 photoproducts in human skin in vivo. J. Invest. Dermatol. 2011 doi: 10.1038/jid.2011.283. In press doi: jid2011283. [DOI] [PubMed] [Google Scholar]
- 36.Cadet J, Douki T, Ravanat JL. Oxidatively generated base damage to cellular DNA. Free Radic. Biol. Med. 2010;49:9–21. doi: 10.1016/j.freeradbiomed.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 37.Deans AJ, West SC. DNA interstrand crosslink repair and cancer. Nat. Rev. Cancer. 2011;11:467–480. doi: 10.1038/nrc3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clivio P, Fourrey JL, Gasche J, Favre A. DNA photodamage mechanistic studies - Characterization of a thietane intermediate in a model reaction relevant to 6-4 lesions. J. Am. Chem. Soc. 1991;113:5481–5483. [Google Scholar]
- 39.Clivio P, Fourrey JL, Gasche J, Favre A. Novel insight into the stereochemical pathway leading to (6-4) pyrimidine-pyrimidone photoproducts in DNA. Tetrahedron Lett. 1992;33:1615–1618. [Google Scholar]
- 40.Garcin G, Douki T, Stoebner PE, Guesnet J, Guezennec A, Martinez J, Cadet J, Meunier L. Cell surface expression of melanocortin-1 receptor on HaCaT keratinocytes and alpha-melanocortin stimulation do not affect the formation and repair of UVB-induced DNA photoproducts. Photochem. Photobiol. Sci. 2007;6:585–593. doi: 10.1039/b615656h. [DOI] [PubMed] [Google Scholar]
- 41.D'Errico M, Teson M, Calcagnile A, Nardo T, De Luca N, Lazzari C, Soddu S, Zambruno G, Stefanini M, Dogliotti E. Differential role of transcription-coupled repair in UVB-induced response of human fibroblasts and keratinocytes. Cancer Res. 2005;65:432–438. [PubMed] [Google Scholar]
- 42.Pines A, Backendorf C, Alekseev S, Jansen JG, de Gruijl FR, Vrieling H, Mullenders LH. Differential activity of UV-DDB in mouse keratinocytes and fibroblasts: impact on DNA repair and UV-induced skin cancer. DNA Repair. 2009;8:153–161. doi: 10.1016/j.dnarep.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 43.Jones CJ, Cleaver JE, Wood RD. Repair of damaged DNA by extracts from a xeroderma pigmentosum complementation group A revertant and expression of a protein absent in its parental cell line. Nucleic Acids Res. 1992;20:991–995. doi: 10.1093/nar/20.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wakasugi M, Kasashima H, Fukase Y, Imura M, Imai R, Yamada S, Cleaver JE, Matsunaga T. Physical and functional interaction between DDB and XPA in nucleotide excision repair. Nucleic Acids Res. 2009;37:516–525. doi: 10.1093/nar/gkn964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Massey A. DNA repair and the cytotoxic effects of cisplatin and DNA thiobases. University of London: PhD Thesis; 2001. [Google Scholar]