Abstract
The identification of human cancer-related microRNAs (miRNAs) is important for cancer biology research. Although several identification methods have achieved remarkable success, they have overlooked the functional information associated with miRNAs. We present a computational framework that can be used to prioritize human cancer miRNAs by measuring the association between cancer and miRNAs based on the functional consistency score (FCS) of the miRNA target genes and the cancer-related genes. This approach proved successful in identifying the validated cancer miRNAs for 11 common human cancers with area under ROC curve (AUC) ranging from 71.15% to 96.36%. The FCS method had a significant advantage over miRNA differential expression analysis when identifying cancer-related miRNAs with a fine regulatory mechanism, such as miR-27a in colorectal cancer. Furthermore, a case study examining thyroid cancer showed that the FCS method can uncover novel cancer-related miRNAs such as miR-27a/b, which were showed significantly upregulated in thyroid cancer samples by qRT-PCR analysis. Our method can be used on a web-based server, CMP (cancer miRNA prioritization) and is freely accessible at http://bioinfo.hrbmu.edu.cn/CMP. This time- and cost-effective computational framework can be a valuable complement to experimental studies and can assist with future studies of miRNA involvement in the pathogenesis of cancers.
INTRODUCTION
MicroRNAs (MiRNAs) are small, non-coding RNA molecules encoded in the genomes of animals. They are important regulators of cell differentiation, proliferation/growth, mobility and apoptosis in diverse cancer-related biological processes (1–4). Accumulating evidence suggests that the over-expression of several miRNAs increases tumor formation; however, other miRNAs are consistently detected at very low levels in tumors and may have tumor-suppressive effects (5–8). The identification of miRNAs linked to cancer susceptibility is useful for cancer diagnosis, prognosis, treatment and drug target discovery (9–11).
Experimental methods have been used to identify the relationship between cancers and miRNAs; methods such as microarray profiling and qRT-PCR have achieved remarkable success. Microarray profiling is a high-throughput technique that can be used to systematically detect the differential expression of miRNAs in cancer and control samples (12–15). However, the different melting temperatures of short-length miRNAs and the high sequence consistency between miRNA family members can lead to false positive microarray results; in addition, the probe design increases the cost of this technique (16–18). Therefore, the development of computational methods that use the abundant ‘omics’ data sets of miRNAs to assess their relationship with specific cancers is a valuable complement to experimental studies.
Following the recognition of the crucial regulatory functions of miRNAs, computational methods of identifying cancer-related miRNAs have been widely applied to cancer research as a powerful supplement to experimental methods. Computational methods are mostly based on the expression pattern of miRNAs in cancer (19,20) or on the regulatory effects of miRNAs on cancer susceptibility genes or protein products through pathways or functional modules (21–23). However, factors such as false positive miRNA targets, imperfect cancer miRNA profiles and miRNA interaction or coregulation cascades may reduce the efficiency of miRNA analysis. These studies suggest that it is useful to systematically prioritize potentially cancer-related miRNAs during experimental research.
Genes associated with the same or similar disorders will share common cellular and functional characteristics (24,25). The annotations in Gene Ontology (GO) reveal this functional similarity. Likewise, if miRNAs are associated with a similar regulatory pattern in the same type of cancer, their target genes may share common functional characteristics (26). Therefore, if miRNAs are associated with a cancer, the miRNA targets must have the same or a similar function as the cancer-related genes. We present a novel method for quantifying and prioritizing miRNAs related to specific cancers by using the functional consistency between miRNA target genes and cancer-related genes. This method is based on the functional consistency score (FCS), which is calculated by the semantic similarity measurement in the context of functional categories. Various applications of semantic similarity have been used for biomedical ontology (27,28) such as GO (29), Disease Ontology (DO) (30) and Human Phenotype Ontology (HPO) (31). These have been demonstrated to be powerful tools for validating biomedical results and for exploring the molecular mechanisms of human disease (32), including gene classification, gene function prediction, disease gene inference and phenotype analysis of human disease. In this article, a higher FCS revealed a high functional consistency or closer relationship between the miRNA and the cancer. We applied our method to 11 common human cancers and ranked all of the candidate miRNAs according to FCS. Our method had a significant advantage over miRNA differential expression analysis in the identification of cancer-related miRNAs with fine regulatory mechanisms. This method can be a valuable complement to experimental studies used in future studies of miRNA involvement in the pathogenesis of cancer.
MATERIALS AND METHODS
GOterm enrichment analysis
A gene product annotated on GO might be associated with or located in one or more cellular compartments (components). It is active in one or more biological processes, during which it performs one or more molecular functions. Mutant phenotypes often reflect disruptions in biological processes. Fisher's exact test was used for statistical and enrichment analysis of the GO biological process categories. The miRNA target genes and cancer genes were significantly annotated and the threshold of Fisher's P-value was selected to be 0.05. The GO annotation definitions were imported from the January 2010 monthly release (http://archive.geneontology.org/full/2010-01-01/). We implemented our analysis procedure in the Biological Process categories with all annotations (including IEA annotations).
MiRNA target gene sets and human cancer gene set
To minimize the false positives resulting from the computational prediction of miRNA targets and to build a high-confidence resource for miRNA target analysis, the strategy of integrate several miRNA target prediction programs has been widely used (33–35). We chose miRNA targets from the widely used target prediction programs miRanda, PicTar4 and TargetScan. Only target genes predicted by at least two of the programs were accepted. This miRNA target integrating method had been used before (36). We obtained a compiled miRNA–mRNA data set containing 244 miRNAs and 43 558 miRNA target pairs. All of the integrated miRNA target gene sets (MFCs) and the human common cancer-related miRNA database can be downloaded from the ‘Supplementary Data’ or from http://bioinfo.hrbmu.edu.cn/CMP.
The specific human cancer genes were downloaded from the National Cancer Institute (NCI) with a unique disease EVS ID. In this article, we only selected cancer genes with evidence ID of EV-EXP-IDA, which means they have been investigated and validated by direct experiments. These data sets can be downloaded from the ‘Supplementary Data’ or from http://bioinfo.hrbmu.edu.cn/CMP.
Calculating the FCS between miRNA and cancer
To calculate the FCS between a miRNA and cancer, we measured the semantic similarity between the MFC G1 and the cancer gene set (CFC) G2 based on their significantly enriched functional categories.
| (1) |
where 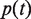 is the number of genes annotated in category
is the number of genes annotated in category  and its direct or indirect offspring is divided by the number of annotations in the GO domain.
and its direct or indirect offspring is divided by the number of annotations in the GO domain.
| (2) |
where 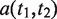 represents the set of most informative common ancestor categories of
represents the set of most informative common ancestor categories of  and
and  .
.
| (3) |
where T1 and T2 indicate the significantly enriched category sets of gene set G1 and gene set G2, respectively.
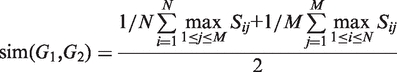 |
(4) |
The first equation describes how to measure the information content of a category. The second and third equations describe how to calculate the semantic similarity between two categories. The fourth equation describes the strategy for integrating the similarity between categories to quantify the functional consistency of two gene sets by the best-match average method. The semantic similarity score between two gene sets is the average of the best-fit column score and the best-fit row score (37).
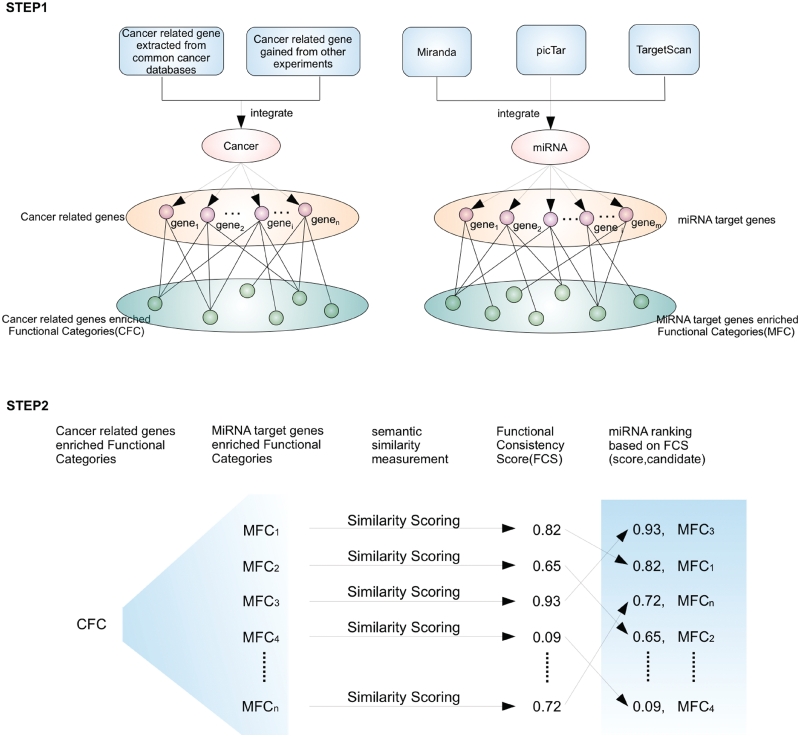
Our new approach took advantage of the term measurement of Lin's (38), and a detailed procedure chart is shown in Figure 1. We chose Lin's method because of its superiority in its normalized outputs. We used Lin's semantic similarity measurement to calculate the functional consistency between miRNAs and cancer. The final FCS scores are distributed from 0 to 1 (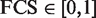 ). These normalized outputs facilitate users to identify and prioritize the direct association between a candidate miRNA and cancer. Furthermore, we cited other semantic similarity measurements of SimGIC (39), Resnik's and Jiang's, which had already proven to be effective in GO, and compare the efficiency of these measurements. We calculated FCSs between miRNA targets and colorectal cancer genes using SimGIC, Lin's, Resnik's and Jiang's, respectively. The performance of recalling known cancer miRNAs and correlation with differential expression analysis are summarized in Sheet 1 of Supplementary Table S3, more detailed information is also listed in other sheets of Supplementary Table S3.
). These normalized outputs facilitate users to identify and prioritize the direct association between a candidate miRNA and cancer. Furthermore, we cited other semantic similarity measurements of SimGIC (39), Resnik's and Jiang's, which had already proven to be effective in GO, and compare the efficiency of these measurements. We calculated FCSs between miRNA targets and colorectal cancer genes using SimGIC, Lin's, Resnik's and Jiang's, respectively. The performance of recalling known cancer miRNAs and correlation with differential expression analysis are summarized in Sheet 1 of Supplementary Table S3, more detailed information is also listed in other sheets of Supplementary Table S3.
Figure 1.
If an miRNA is involved in a specific cancer, the miRNA target genes and the cancer-related genes would be associated with the same or similar functions. The FCS can be used to quantify the association between miRNAs and a specific cancer. In the first step (STEP 1), cancer-related genes are obtained from several cancer databases or experimental results. Next, functional enrichment analyses based on GO are performed on a CFC and an MFC, and the significantly enriched functional categories of the CFC and MFC are obtained. In the second step (STEP 2), for the ith miRNA, an FCS is calculated between MFCi and CFC using a semantic similarity measurement. FCSs can be determined for all the candidate miRNAs. Higher FCS values reflect a closer relationship with the cancer.
A compendium of validated cancer-related miRNAs
Rigorous evaluation of a prediction method requires a ‘gold standard’. In this study, we used a set of validated miRNAs with known functions related to a certain cancer type. For each cancer type, the cancer-related miRNAs were drawn from the mir2Disease database (http://www.miR2Disease.org), which contains a compilation of disease-related miRNAs identified by experiment-based studies (40).
Evaluation of miRNA expression patterns
The corresponding miRNA expression profile GSE10259 with 281 human miRNAs was downloaded from GEO. This profile contained 66 samples from 49 colorectal cancer patients and one normal control; 59 of these 66 samples were cancer samples and 7 were normal samples. A Student's t-test was used to identify the differentially expressed miRNAs between the cancer and control samples in the microarray (10,15), and then each miRNA was given a significant differential P-value. The resulting list of 281 miRNAs was sorted according to P-value. Seventy miRNAs were considered to be significantly differentially expressed and had P-values lower than a threshold of 0.01.
Cell lines and tissue samples
Human colorectal cancer cell lines SW1116, SW620, HCT116, HT29 and LOVO were originally obtained from the American Type Culture Collection. The cells were maintained in Dulbecco's Modified Eagle Medium (Hyclone, USA) with 10% heat-inactivated fetal bovine serum (Hyclone) and 1% penicillin/streptomycin in a 37°C and 5% CO2 atmosphere.
The tissue samples were collected at surgery from patients who suffered from either colorectal adenocarcinoma cancer (T2N0M0 and T4N0M0) or papillary adenocarcinoma of the thyroid. Tumor tissue (0.5 × 0.5 × 0.5 cm) and normal tissue counterparts were collected as a pair from each patient, immediately flash-frozen in liquid nitrogen, and stored at −80°C. Peripheral blood mononuclear cells (PBMCs) obtained from healthy men and women were used as controls to compare selected miRNA expression with the cancer cell lines. The study was approved by the local ethics committee.
RNA isolation and quantitative real-time PCR
Total RNA was extracted from 1 × 105 cells or 0.08 g of the tissue sample with a MirVanaTM miRNA Isolation kit. Next, 0.8 µg of the RNA was reverse transcribed into cDNA with a TaqMan MiRNA Reverse Transcription kit according to the manufacturer's instructions. Then, 20 µl of the real-time PCR reaction was set up with validated TaqMan probes and specific primers including hsa-miR-20a, hsa-miR-20b, hsa-miR-27a, hsa-miR-27b, hsa-miR-106b and snRNA U6 for each miRNA. The reactions were incubated in the ABI STEPONE Real-Time PCR System (Applied Biosystems, Foster City, USA). The real-time PCR reactions were performed in duplicate and repeated three times. The threshold cycle (Ct) value was determined by the default settings. An snRNA U6 was used as an endogenous control. We calculated the relative expression of each selected miRNA (as the fold change) in a cancer cell line or tumor tissue and compared this expression to that in the PBMCs of healthy controls or relevant normal tissue counterparts with the 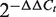 method (41). All reagents and specific primers for each miRNA were obtained from Applied Biosystems (Foster City, USA) unless otherwise indicated.
method (41). All reagents and specific primers for each miRNA were obtained from Applied Biosystems (Foster City, USA) unless otherwise indicated.
RESULTS AND DISCUSSION
The FCS procedure
Previous studies have revealed that genes associated with the same or similar disorders may participate in the same cellular pathways, molecular complexes, or functional ontologies (24,25). Within a specific cancer type, if miRNAs are associated with a similar regulatory pattern, their target genes may share common functional characteristics (22,26). We assumed that if a miRNA is involved in a specific cancer, the miRNA target genes and the cancer-related genes would be associated with the same or similar functions. Based on this assumption, we used the FCS of the miRNA target genes and cancer-related genes to quantify the association between miRNAs and a specific cancer. We calculated the FCS by using the large-scale gene product functional annotation dataset and classic semantic similarity measurements. The detailed steps are shown in the ‘Materials and Methods’ section and Figure 1. Our method can be used on the web-based server CMP (cancer miRNA prioritization), which is freely accessible at http://bioinfo.hrbmu.edu.cn/CMP.
Performance of FCS
To assess whether the FCS method reflects a biological relationship between miRNAs and cancer, we performed a validation with the known cancer miRNAs obtained from experimental data sets (see ‘Materials and Methods’ section). For a specific human cancer, each of the known miRNAs was taken as one test case. For each test case, we generated 99 negative controls, and each of the negative controls had the same target gene set size as the test case. Next, we calculated the FCSs of the case miRNA and the negative controls; we then ranked the case miRNA together with the negative controls. When the known cancer miRNA is prioritized as top 1, the empirical P < 0.01, which is widely accepted as a strict significant level. A similar performance method has been used before (42).
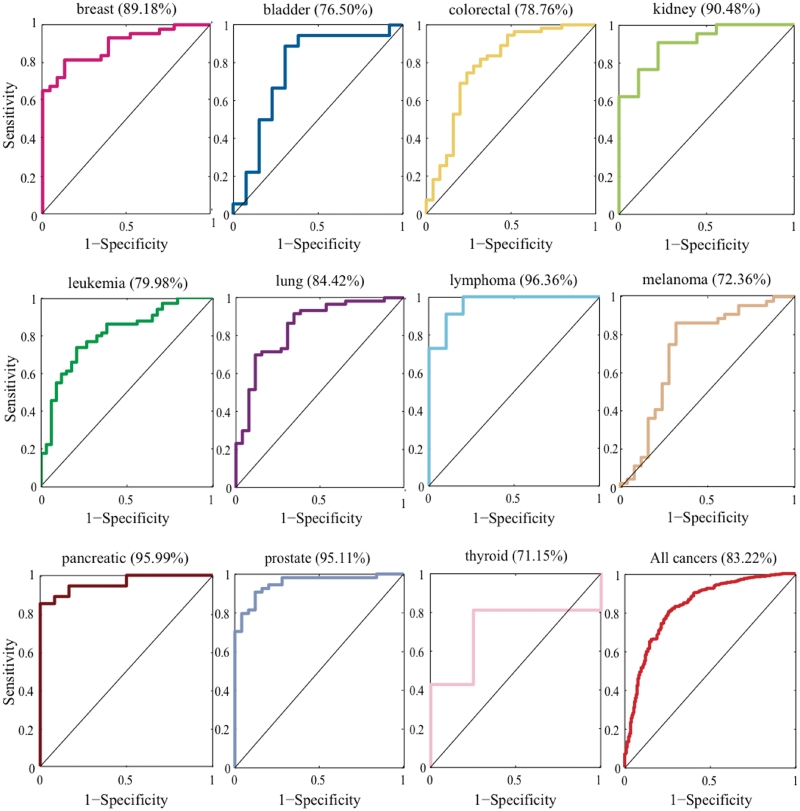
According to the 100 randomization, we examined whether known cancer miRNAs can be prioritized as top 1 to produce an ROC curve. We tested 11 human cancers and 655 miRNA–cancer associations. The highest area under ROC curve (AUC) value of 96.36% was obtained with lymphoma cancer, and the lowest AUC of 71.15% was obtained with thyroid cancer. We conducted another performance analysis and generated 999 negative controls for each test case. Then, we examined whether known cancer–miRNA can be prioritized as top 10. The AUC results of both the 100 randomization and 1000 randomization are shown in Supplementary Table S1. The results suggest that our FCS method can successfully recover known miRNA–cancer associations (Figure 2).
Figure 2.
AUC analysis of known cancer miRNAs predicted at top 1. These figures showed 1-specificity versus sensitivity when considering the miRNAs predicted at top 1 varied with the FCS threshold.
For each cancer, we tested the recall rate by analyzing the top-ranked list. If the known cancer miRNA was ranked in the top 10, the prediction was considered to be successful. The performance precision is defined as the recall rate of the top 10. Supplementary Table S1 lists all of the recall numbers of these 11 cancer miRNAs.
FCS versus miRNA differential expression analysis
To further demonstrate the advantage of the FCS method in identifying cancer miRNAs, we compared the colorectal cancer miRNA ranked lists from the FCS and differential expression analyses (DEA). The differential expression values of colorectal cancer genes were calculated using Student's t-test. Each gene was given a significant P-value and ranked by –log(P)-value. The overlap of these two lists included 216 miRNAs, and the resulting list of 244 miRNAs sorted by FCS is shown in Supplementary Table S2. We then calculated the correlation coefficient between the FCS scored list and the –log(P)-value list by DEA. We observed that miRNAs with higher FCS values tended to have lower P-values, and the correlation coefficient between the FCS and DEA was 0.1835, with a significance level of P < 0.0069 (Supplementary Table S2). In particular, among the top 10 miRNAs with the highest FCSs (Table 1), 7 miRNAs were already experimentally verified and the other 3 miRNAs were prioritized as candidate colorectal cancer miRNAs. Six of the seven known cancer miRNAs were significantly and differentially expressed with P < 0.001. In addition, hsa-miR-20b, which was not a known cancer miRNA, had a very high functional consistency with colorectal cancer (FCS = 0.83062) and was significantly downregulated with an average of 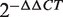 < 0.047 in five colorectal cancer cell lines as determined by qRT-PCR (Supplementary Table S3). This method has a high prediction coincidence with the expression profile analysis, and the high differentially expressed miRNAs tend to be prioritized at the top of the FCS list.
< 0.047 in five colorectal cancer cell lines as determined by qRT-PCR (Supplementary Table S3). This method has a high prediction coincidence with the expression profile analysis, and the high differentially expressed miRNAs tend to be prioritized at the top of the FCS list.
Table 1.
FCS ranked list of the top 10 candidate colorectal cancer miRNAs
| miRNA | FCS | Rank with FCS | P-value of DEA |
|---|---|---|---|
| hsa-miR-20a | 0.84500 | 1 | 8.59E–07 |
| hsa-miR-106b | 0.84499 | 2 | 1.69E–08 |
| hsa-miR-27a | 0.84334 | 3 | 1.80E–01 |
| hsa-miR-27b | 0.84222 | 4 | 8.44E–03 |
| hsa-miR-20b | 0.83062 | 5 | NA |
| hsa-miR-17-5p | 0.83058 | 6 | 1.27E–10 |
| hsa-miR-128a | 0.83007 | 7 | 3.67E–01 |
| hsa-miR-141 | 0.81952 | 8 | 6.02E–04 |
| hsa-miR-153 | 0.81644 | 9 | 2.89E–01 |
| hsa-miR-30a-5p | 0.81204 | 10 | 2.29E–05 |
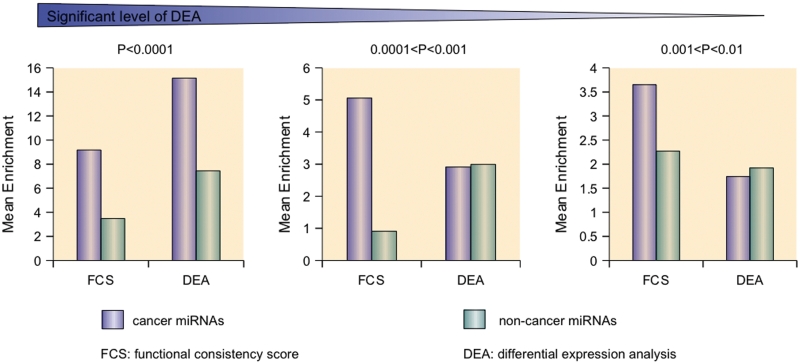
Previous studies have revealed that miRNAs may act as fine-tuning regulators and that subtle changes in miRNA expression can regulate gene functions (43,44). These important deregulating cancer miRNAs may be neglected by DEA. For example, hsa-miR-27a, which is known to be an oncogenic regulator in colorectal cancer cells, is a target for the anticancer agent CDODA-Me and regulates the zinc-finger protein ZBTB10 and the oncogenic protein Sp1. However, we discovered subtle differential expression by microarray analysis (P > 0.1) and a non-significant differential expression pattern by qRT-PCR analysis in five colorectal cancer cell lines with an average 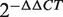 > 0.79 (Supplementary Table S3). In this case, hsa-miR-27a is neglected by DEA but can be prioritized by the high FCS of 0.84334. Enrichment analysis reviewed that FCS better distinguished cancer miRNAs and non-cancer miRNAs in different significant intervals, especially P > 0.0001 (Figure 3). Therefore, the FCS method was more efficient than DEA in identifying cancer-related miRNAs with a fine regulatory mechanism.
> 0.79 (Supplementary Table S3). In this case, hsa-miR-27a is neglected by DEA but can be prioritized by the high FCS of 0.84334. Enrichment analysis reviewed that FCS better distinguished cancer miRNAs and non-cancer miRNAs in different significant intervals, especially P > 0.0001 (Figure 3). Therefore, the FCS method was more efficient than DEA in identifying cancer-related miRNAs with a fine regulatory mechanism.
Figure 3.
Different distributions of expression significance and FCS values between cancer miRNAs and non-cancer miRNAs. The formula is enrichment = 108/(rank) for an interval of 216 miRNAs. The mean enrichment reflects the position of the cancer miRNAs in the prioritized list. FCS can distinguish cancer miRNAs and non-cancer miRNAs where cancer miRNAs are always enriched at the top positions at different expression significant levels. By contrast, expression analysis confused these two types of miRNAs.
Case study: thyroid cancer
To demonstrate the ability of FCS to uncover known cancer miRNAs and predict novel susceptibility candidates, we present a case study of thyroid cancer. Thyroid cancer mostly originates from the epidermal cells of thyroid follicles and is one of the few malignancies that is increasing in incidence (45,46). Many researchers have demonstrated that miRNAs play an important role in thyroid cancers. Here, we provide a comprehensive prediction of new thyroid cancer-related miRNAs.
First, we extracted 350 thyroid cancer-related genes from NCI (http://www.cancer.gov/) (see ‘Materials and Methods’ section). Next, we calculated the FCSs of 244 candidate miRNAs with thyroid cancer genes, and compared the known thyroid cancer miRNAs with the unknown cancer miRNAs in the FCS-ranked list.
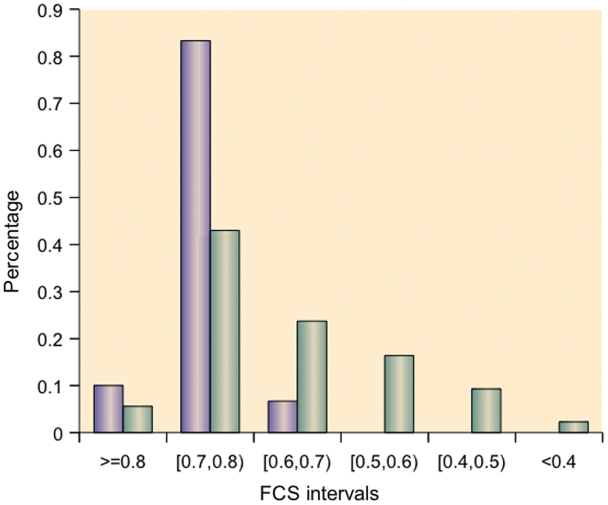
We discovered that 10% of the known thyroid cancer miRNAs have FCS values >0.8, and 83.33% of the known thyroid cancer miRNAs have FCS values > 0.7 (Figure 4). The distribution of FCSs between thyroid cancer miRNAs and unknown cancer miRNAs is significantly different (P < 3.24e–005 on the Kruskal–Wallis test).
Figure 4.
Distributions of FCSs of thyroid cancer miRNAs and other miRNAs (93.3% of known thyroid cancer miRNAs have FCSs > 0.70).
The top 10 miRNAs in the FCS ranked list included miR-20a/b, miR-106b, miR-27a/b and miR-30c/e-5p, and these miRNAs were predicted to be novel thyroid cancer miRNAs (Table 2). Among these novel miRNAs, some proto-oncogenic miRNAs such as miR-20a and miR-17-5p are members of the miR-17-92 intronic miRNA cluster on chr13. Moreover, miR-106b is a member of the miR-106b-25 cluster. The miR-17-92 cluster plays an oncogenic role in anaplastic thyroid cancer cells (47). Previous research revealed that the transforming growth factor-beta (TGFβ) tumor suppressor pathway is under the inactivation control of the miR-106b-25/miR-17-92 clusters; this pathway plays a major role in the development of a variety of human tumors (48,49). For the same derived transcript, the oncogenic properties of the host gene MCM7 could be linked to the host miR-106b-25 cluster, and members of the miR-106b family have a crucial effect on the cell-cycle progression by regulating P21/CDKN1A (50,51). We also evaluated the expression level of miR-27a and miR-27b in thyroid cancer by conducting qRT-PCR experiments in two cancer samples. The fold changes were calculated by the 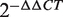 method; miR-27a and miR-27b showed a significant upregulated expression pattern in thyroid cancer tissues with average
method; miR-27a and miR-27b showed a significant upregulated expression pattern in thyroid cancer tissues with average 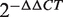 values of 2.20 and 2.15 (Supplementary Table S3), respectively. These results demonstrate that the method described in this article is powerful not only in capturing known cancer miRNAs but also in prioritizing novel cancer miRNAs not yet detected by other methods.
values of 2.20 and 2.15 (Supplementary Table S3), respectively. These results demonstrate that the method described in this article is powerful not only in capturing known cancer miRNAs but also in prioritizing novel cancer miRNAs not yet detected by other methods.
Table 2.
The top 10 prioritized thyroid cancer miRNAs in the FCS ranked list
| miRNA | FCS | Functional description | References |
|---|---|---|---|
| hsa-miR-20aa | 0.85164 | B-cell lymphoma, breast cancer, CML, HCC, lung cancer, medulloblastoma, pulmonary hypertension | Inomata M, et al. (52),Yu Z, et al. (53), Venturinin L, et al. (54), Connolly E, et al. (55), Matsubara H, et al. (56), Northcott PA, et al. (57), Brock M, et al. (58) |
| hsa-miR-106ba | 0.85073 | Alzheimer's disease, CLL, gastric cancer, HCC, multiple myeloma | Hébert SS, et al. (59), Sampath D, et al. (60), Kim YK, et al. (61), Li Y, et al. (62), Pichiorri F, et al. (63) |
| hsa-miR-17-5pb | 0.83800 | ATC, breast cancer, CML, HCC, lung cancer, MYC-rearranged lymphoma, NB, pulmonary hypertension, Sezary syndrome | Takakura S, et al. (47), Yu Z, et al. (53), Venturini L, et al. (54), Connolly E, et al. (55), Matsubara H, et al. (56), Tagawa H, et al. (64), Fontana L, et al. (65), Brock M, et al. (58), Ballabio E, et al. (66) |
| hsa-miR-20ba | 0.83752 | T-cell lymphoma | Landais S, et al. (67) |
| hsa-miR-27ac | 0.82441 | Breast cancer, gastric cancer, HCC | Guttilla IK, et al. (68), Liu T, et al. (69), Huang S, et al. (70) |
| hsa-miR-27bc | 0.82194 | ALL, AML, colorectal cancer | Mi S, et al. (71), Xi Y, et al. (72), |
| hsa-miR-30a-5pb | 0.80958 | ATC, cardiac hypertropy, colorectal cancer | Visone R, et al. (73), Sayed D, et al. (74), Arndt GM, et al. (75) |
| hsa-miR-30e-5pa | 0.80916 | Bladder cancer, DMD, HNSCC | Wang G, et al. (76), Eisenberg I, et al. (77), Hebert C, et al. (78) |
| hsa-miR-30ca | 0.80743 | Bladder cancer, cardiac hypertropy, colorectal cancer | Wang G, et al. (76), Sayed D, et al. (74), Arndt GM, et al. (75) |
| hsa-miR-30db | 0.80684 | AML, ATC, cardiac hypertrophy, CLL | Dixon-McIver A, et al. (79), Visone R, et al. (73), Marton S, et al. (80), Sayed D, et al. (74) |
aMost updated cancer-related miRNAs prioritized in the top 10.
bKnown thyroid cancer miRNAs prioritized in the top 10.
cUnknown cancer miRNAs prioritized in the top 10.
In this study, we used a systematic approach for prioritizing candidate cancer miRNAs based on the functional consistency between miRNA target genes and cancer-related genes. Our method integrated large-scale functional information from GO biological process branches and combined miRNA targets and cancer-related genes. Our approach is useful in many respects and has many advantages for research on cancer miRNAs. The FCS-based prioritized miRNA ranked list is ready for experimental verification and is a cost-effective and time-saving method; it is a powerful supplement for experimental research on miRNAs. In summary, our computational approach is a systematic biological method and is useful for cancer diagnosis, treatment, and prognosis and miRNA-related drug research in cancer pharmacology.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Funding for open access charge: National Natural Science Foundation of China (Grant Nos. 31100948, 30871394, 61073136 and 91029717); the National High Tech Development Project of China, the 863 Program (Grant Nos. 2007AA02Z329); National Science Foundation of Heilongjiang Province (Grant Nos. QC2009C23); the Science Foundation of Educational Commission of Heilongjiang Province (Grant Nos. 11551233); the Graduate Innovation Fund of Heilongjiang Province (Grant Nos. YJSCX2009-226HLJ); the Graduate Innovation Fund of Heilongjiang Province (Grant Nos. YJSCX2011-334HLJ).
Conflict of interest statement. None declared.
REFERENCES
- 1.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 2.Jovanovic M, Hengartner MO. miRNAs and apoptosis: RNAs to die for. Oncogene. 2006;25:6176–6187. doi: 10.1038/sj.onc.1209912. [DOI] [PubMed] [Google Scholar]
- 3.Lynam-Lennon N, Maher SG, Reynolds JV. The roles of microRNA in cancer and apoptosis. Biol. Rev. Camb. Philos. Soc. 2009;84:55–71. doi: 10.1111/j.1469-185X.2008.00061.x. [DOI] [PubMed] [Google Scholar]
- 4.Schickel R, Boyerinas B, Park SM, Peter ME. MicroRNAs: key players in the immune system, differentiation, tumorigenesis and cell death. Oncogene. 2008;27:5959–5974. doi: 10.1038/onc.2008.274. [DOI] [PubMed] [Google Scholar]
- 5.He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voorhoeve PM. MicroRNAs: Oncogenes, tumor suppressors or master regulators of cancer heterogeneity? Biochim. Biophys. Acta. 2010;1805:72–86. doi: 10.1016/j.bbcan.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev. Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 9.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat. Rev. Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 10.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl Acad. Sci. USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 12.Baskerville S, Bartel DP. Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA. 2005;11:241–247. doi: 10.1261/rna.7240905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaur A, Jewell DA, Liang Y, Ridzon D, Moore JH, Chen C, Ambros VR, Israel MA. Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Res. 2007;67:2456–2468. doi: 10.1158/0008-5472.CAN-06-2698. [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez NC, Sarasquete ME, Misiewicz-Krzeminska I, Delgado M, De Las Rivas J, Ticona FV, Ferminan E, Martin-Jimenez P, Chillon C, Risueno A, et al. Deregulation of microRNA expression in the different genetic subtypes of multiple myeloma and correlation with gene expression profiling. Leukemia. 2010;24:629–637. doi: 10.1038/leu.2009.274. [DOI] [PubMed] [Google Scholar]
- 15.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 16.Barad O, Meiri E, Avniel A, Aharonov R, Barzilai A, Bentwich I, Einav U, Gilad S, Hurban P, Karov Y, et al. MicroRNA expression detected by oligonucleotide microarrays: system establishment and expression profiling in human tissues. Genome Res. 2004;14:2486–2494. doi: 10.1101/gr.2845604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Gelfond JA, McManus LM, Shireman PK. Reproducibility of quantitative RT-PCR array in miRNA expression profiling and comparison with microarray analysis. BMC Genomics. 2009;10:407. doi: 10.1186/1471-2164-10-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saba R, Booth SA. Target labelling for the detection and profiling of microRNAs expressed in CNS tissue using microarrays. BMC Biotechnol. 2006;6:47. doi: 10.1186/1472-6750-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bandyopadhyay S, Bhattacharyya M. Analyzing miRNA co-expression networks to explore TF-miRNA regulation. BMC Bioinformatics. 2009;10:163. doi: 10.1186/1471-2105-10-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gennarino VA, Sardiello M, Avellino R, Meola N, Maselli V, Anand S, Cutillo L, Ballabio A, Banfi S. MicroRNA target prediction by expression analysis of host genes. Genome Res. 2009;19:481–490. doi: 10.1101/gr.084129.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Backes C, Meese E, Lenhof HP, Keller A. A dictionary on microRNAs and their putative target pathways. Nucleic Acids Res. 2010;38:4476–4486. doi: 10.1093/nar/gkq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bandyopadhyay S, Mitra R, Maulik U, Zhang MQ. Development of the human cancer microRNA network. Silence. 2010;1:6. doi: 10.1186/1758-907X-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen E, Diao X, Wei C, Wu Z, Zhang L, Hu B. MicroRNAs target gene and signaling pathway by bioinformatics analysis in the cardiac hypertrophy. Biochem. Biophys. Res. Commun. 2010;397:380–385. doi: 10.1016/j.bbrc.2010.05.116. [DOI] [PubMed] [Google Scholar]
- 24.Ravasz E, Somera AL, Mongru DA, Oltvai ZN, Barabasi AL. Hierarchical organization of modularity in metabolic networks. Science. 2002;297:1551–1555. doi: 10.1126/science.1073374. [DOI] [PubMed] [Google Scholar]
- 25.Goh KI, Cusick ME, Valle D, Childs B, Vidal M, Barabasi AL. The human disease network. Proc. Natl Acad. Sci. USA. 2007;104:8685–8690. doi: 10.1073/pnas.0701361104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang D, Wang J, Lu M, Song F, Cui Q. Inferring the human microRNA functional similarity and functional network based on microRNA-associated diseases. Bioinformatics. 26:1644–1650. doi: 10.1093/bioinformatics/btq241. [DOI] [PubMed] [Google Scholar]
- 27.Pesquita C, Faria D, Falcao AO, Lord P, Couto FM. Semantic similarity in biomedical ontologies. PLoS Comput. Biol. 2009;5:e1000443. doi: 10.1371/journal.pcbi.1000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hugo Bastos BT, Pesquita C, Faria D, Couto F. Application of Gene Ontology to gene identification. Methods Mol. Biol. 2011;760:141–157. doi: 10.1007/978-1-61779-176-5_9. [DOI] [PubMed] [Google Scholar]
- 29.The Gene Ontology in 2010: extensions and refinements. Nucleic Acids Res. 2010;38:D331–D335. doi: 10.1093/nar/gkp1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osborne JD, Flatow J, Holko M, Lin SM, Kibbe WA, Zhu LJ, Danila MI, Feng G, Chisholm RL. Annotating the human genome with Disease Ontology. BMC Genomics. 2009;10(Suppl. 1):S6. doi: 10.1186/1471-2164-10-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson PN, Kohler S, Bauer S, Seelow D, Horn D, Mundlos S. The Human Phenotype Ontology: a tool for annotating and analyzing human hereditary disease. Am. J. Hum. Genet. 2008;83:610–615. doi: 10.1016/j.ajhg.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Washington NL, Haendel MA, Mungall CJ, Ashburner M, Westerfield M, Lewis SE. Linking human diseases to animal models using ontology-based phenotype annotation. PLoS Biol. 2009;7:e1000247. doi: 10.1371/journal.pbio.1000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao F, Zuo Z, Cai G, Kang S, Gao X, Li T. miRecords: an integrated resource for microRNA-target interactions. Nucleic Acids Res. 2009;37:D105–D110. doi: 10.1093/nar/gkn851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tripathi R, Saini HK, Rad R, Abreu-Goodger C, van Dongen S, Enright AJ. Messenger RNA and microRNA profiling during early mouse EB formation. Gene Expr. Patterns. 2010;11:334–344. doi: 10.1016/j.gep.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Nam S, Kim B, Shin S, Lee S. miRGator: an integrated system for functional annotation of microRNAs. Nucleic Acids Res. 2008;36:D159–D164. doi: 10.1093/nar/gkm829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang F, Barbacioru C, Bao S, Lee C, Nordman E, Wang X, Lao K, Surani MA. Tracing the derivation of embryonic stem cells from the inner cell mass by single-cell RNA-Seq analysis. Cell Stem Cell. 2010;6:468–478. doi: 10.1016/j.stem.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlicker A, Domingues FS, Rahnenfuhrer J, Lengauer T. A new measure for functional similarity of gene products based on Gene Ontology. BMC Bioinformatics. 2006;7:302. doi: 10.1186/1471-2105-7-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin D. An information-theoretic definition of similarity. In fifteenth International Conference on Machine Learning. 1998:296–304. [Google Scholar]
- 39.Pesquita C, Faria D, Bastos H, Ferreira AE, Falcao AO, Couto FM. Metrics for GO based protein semantic similarity: a systematic evaluation. BMC Bioinformatics. 2008;9(Suppl 5):S4. doi: 10.1186/1471-2105-9-S5-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang Q, Wang Y, Hao Y, Juan L, Teng M, Zhang X, Li M, Wang G, Liu Y. miR2Disease: a manually curated database for microRNA deregulation in human disease. Nucleic Acids Res. 2009;37:D98–D104. doi: 10.1093/nar/gkn714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 42.Wu X, Jiang R, Zhang MQ, Li S. Network-based global inference of human disease genes. Mol. Syst. Biol. 2008;4:189. doi: 10.1038/msb.2008.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller BH, Wahlestedt C. MicroRNA dysregulation in psychiatric disease. Brain Res. 2010;1338:89–99. doi: 10.1016/j.brainres.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ying SY, Lin SL. MicroRNA: fine-tunes the function of genes in zebrafish. Biochem. Biophys. Res. Commun. 2005;335:1–4. doi: 10.1016/j.bbrc.2005.06.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schroder S, Dralle H, Bay V, Bocker W. [Immunohistology and prognosis in thyroid cancer. Determination of the malignancy potential of papillary and medullary neoplasms by the detection of S-100 protein and Leu-M1 antigen] Acta Med. Austriaca. 1989;16:2–5. [PubMed] [Google Scholar]
- 46.Subramanian S, Goldstein DP, Parlea L, Thabane L, Ezzat S, Ibrahim-Zada I, Straus S, Brierley JD, Tsang RW, Gafni A, et al. Second primary malignancy risk in thyroid cancer survivors: a systematic review and meta-analysis. Thyroid. 2007;17:1277–1288. doi: 10.1089/thy.2007.0171. [DOI] [PubMed] [Google Scholar]
- 47.Takakura S, Mitsutake N, Nakashima M, Namba H, Saenko VA, Rogounovitch TI, Nakazawa Y, Hayashi T, Ohtsuru A, Yamashita S. Oncogenic role of miR-17-92 cluster in anaplastic thyroid cancer cells. Cancer Sci. 2008;99:1147–1154. doi: 10.1111/j.1349-7006.2008.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petrocca F, Vecchione A, Croce CM. Emerging role of miR-106b-25/miR-17-92 clusters in the control of transforming growth factor beta signaling. Cancer Res. 2008;68:8191–8194. doi: 10.1158/0008-5472.CAN-08-1768. [DOI] [PubMed] [Google Scholar]
- 49.Poliseno L, Salmena L, Riccardi L, Fornari A, Song MS, Hobbs RM, Sportoletti P, Varmeh S, Egia A, Fedele G, et al. Identification of the miR-106b∼25 microRNA cluster as a proto-oncogenic PTEN-targeting intron that cooperates with its host gene MCM7 in transformation. Sci. Signal. 2010;3:ra29. doi: 10.1126/scisignal.2000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ivanovska I, Ball AS, Diaz RL, Magnus JF, Kibukawa M, Schelter JM, Kobayashi SV, Lim L, Burchard J, Jackson AL, et al. MicroRNAs in the miR-106b family regulate p21/CDKN1A and promote cell cycle progression. Mol. Cell. Biol. 2008;28:2167–2174. doi: 10.1128/MCB.01977-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petrocca F, Visone R, Onelli MR, Shah MH, Nicoloso MS, de Martino I, Iliopoulos D, Pilozzi E, Liu CG, Negrini M, et al. E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell. 2008;13:272–286. doi: 10.1016/j.ccr.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 52.Inomata M, Tagawa H, Guo YM, Kameoka Y, Takahashi N, Sawada K. MicroRNA-17-92 down-regulates expression of distinct targets in different B-cell lymphoma subtypes. Blood. 2009;113:396–402. doi: 10.1182/blood-2008-07-163907. [DOI] [PubMed] [Google Scholar]
- 53.Yu Z, Wang C, Wang M, Li Z, Casimiro MC, Liu M, Wu K, Whittle J, Ju X, Hyslop T, et al. A cyclin D1/microRNA 17/20 regulatory feedback loop in control of breast cancer cell proliferation. J. Cell. Biol. 2008;182:509–517. doi: 10.1083/jcb.200801079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Venturini L, Battmer K, Castoldi M, Schultheis B, Hochhaus A, Muckenthaler MU, Ganser A, Eder M, Scherr M. Expression of the miR-17-92 polycistron in chronic myeloid leukemia (CML) CD34+ cells. Blood. 2007;109:4399–4405. doi: 10.1182/blood-2006-09-045104. [DOI] [PubMed] [Google Scholar]
- 55.Connolly E, Melegari M, Landgraf P, Tchaikovskaya T, Tennant BC, Slagle BL, Rogler LE, Zavolan M, Tuschl T, Rogler CE. Elevated expression of the miR-17-92 polycistron and miR-21 in hepadnavirus-associated hepatocellular carcinoma contributes to the malignant phenotype. Am. J. Pathol. 2008;173:856–864. doi: 10.2353/ajpath.2008.080096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsubara H, Takeuchi T, Nishikawa E, Yanagisawa K, Hayashita Y, Ebi H, Yamada H, Suzuki M, Nagino M, Nimura Y, et al. Apoptosis induction by antisense oligonucleotides against miR-17-5p and miR-20a in lung cancers overexpressing miR-17-92. Oncogene. 2007;26:6099–6105. doi: 10.1038/sj.onc.1210425. [DOI] [PubMed] [Google Scholar]
- 57.Northcott PA, Fernandez LA, Hagan JP, Ellison DW, Grajkowska W, Gillespie Y, Grundy R, Van Meter T, Rutka JT, Croce CM, et al. The miR-17/92 polycistron is up-regulated in sonic hedgehog-driven medulloblastomas and induced by N-myc in sonic hedgehog-treated cerebellar neural precursors. Cancer Res. 2009;69:3249–3255. doi: 10.1158/0008-5472.CAN-08-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brock M, Trenkmann M, Gay RE, Michel BA, Gay S, Fischler M, Ulrich S, Speich R, Huber LC. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ. Res. 2009;104:1184–1191. doi: 10.1161/CIRCRESAHA.109.197491. [DOI] [PubMed] [Google Scholar]
- 59.Hebert SS, Horre K, Nicolai L, Bergmans B, Papadopoulou AS, Delacourte A, De Strooper B. MicroRNA regulation of Alzheimer's Amyloid precursor protein expression. Neurobiol. Dis. 2009;33:422–428. doi: 10.1016/j.nbd.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 60.Sampath D, Calin GA, Puduvalli VK, Gopisetty G, Taccioli C, Liu CG, Ewald B, Liu C, Keating MJ, Plunkett W. Specific activation of microRNA106b enables the p73 apoptotic response in chronic lymphocytic leukemia by targeting the ubiquitin ligase Itch for degradation. Blood. 2009;113:3744–3753. doi: 10.1182/blood-2008-09-178707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim YK, Yu J, Han TS, Park SY, Namkoong B, Kim DH, Hur K, Yoo MW, Lee HJ, Yang HK, et al. Functional links between clustered microRNAs: suppression of cell-cycle inhibitors by microRNA clusters in gastric cancer. Nucleic Acids Res. 2009;37:1672–1681. doi: 10.1093/nar/gkp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Y, Tan W, Neo TW, Aung MO, Wasser S, Lim SG, Tan TM. Role of the miR-106b-25 microRNA cluster in hepatocellular carcinoma. Cancer Sci. 2009;100:1234–1242. doi: 10.1111/j.1349-7006.2009.01164.x. [DOI] [PubMed] [Google Scholar]
- 63.Pichiorri F, Suh SS, Ladetto M, Kuehl M, Palumbo T, Drandi D, Taccioli C, Zanesi N, Alder H, Hagan JP, et al. MicroRNAs regulate critical genes associated with multiple myeloma pathogenesis. Proc. Natl. Acad. Sci. USA. 2008;105:12885–12890. doi: 10.1073/pnas.0806202105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tagawa H, Karube K, Tsuzuki S, Ohshima K, Seto M. Synergistic action of the microRNA-17 polycistron and Myc in aggressive cancer development. Cancer Sci. 2007;98:1482–1490. doi: 10.1111/j.1349-7006.2007.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fontana L, Fiori ME, Albini S, Cifaldi L, Giovinazzi S, Forloni M, Boldrini R, Donfrancesco A, Federici V, Giacomini P, et al. Antagomir-17-5p abolishes the growth of therapy-resistant neuroblastoma through p21 and BIM. PLoS One. 2008;3:e2236. doi: 10.1371/journal.pone.0002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ballabio E, Mitchell T, van Kester MS, Taylor S, Dunlop HM, Chi J, Tosi I, Vermeer MH, Tramonti D, Saunders NJ, et al. MicroRNA expression in Sezary syndrome: identification, function, and diagnostic potential. Blood. 2010;116:1105–1113. doi: 10.1182/blood-2009-12-256719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Landais S, Landry S, Legault P, Rassart E. Oncogenic potential of the miR-106-363 cluster and its implication in human T-cell leukemia. Cancer Res. 2007;67:5699–5707. doi: 10.1158/0008-5472.CAN-06-4478. [DOI] [PubMed] [Google Scholar]
- 68.Guttilla IK, White BA. Coordinate regulation of FOXO1 by miR-27a, miR-96, and miR-182 in breast cancer cells. J. Biol. Chem. 2009;284:23204–23216. doi: 10.1074/jbc.M109.031427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu T, Tang H, Lang Y, Liu M, Li X. MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by targeting prohibitin. Cancer Lett. 2009;273:233–242. doi: 10.1016/j.canlet.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 70.Huang S, He X, Ding J, Liang L, Zhao Y, Zhang Z, Yao X, Pan Z, Zhang P, Li J, et al. Upregulation of miR-23a approximately 27a approximately 24 decreases transforming growth factor-beta-induced tumor-suppressive activities in human hepatocellular carcinoma cells. Int. J. Cancer. 2008;123:972–978. doi: 10.1002/ijc.23580. [DOI] [PubMed] [Google Scholar]
- 71.Mi S, Lu J, Sun M, Li Z, Zhang H, Neilly MB, Wang Y, Qian Z, Jin J, Zhang Y, et al. MicroRNA expression signatures accurately discriminate acute lymphoblastic leukemia from acute myeloid leukemia. Proc. Natl. Acad. Sci. USA. 2007;104:19971–19976. doi: 10.1073/pnas.0709313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xi Y, Shalgi R, Fodstad O, Pilpel Y, Ju J. Differentially regulated micro-RNAs and actively translated messenger RNA transcripts by tumor suppressor p53 in colon cancer. Clin. Cancer Res. 2006;12:2014–2024. doi: 10.1158/1078-0432.CCR-05-1853. [DOI] [PubMed] [Google Scholar]
- 73.Visone R, Pallante P, Vecchione A, Cirombella R, Ferracin M, Ferraro A, Volinia S, Coluzzi S, Leone V, Borbone E, et al. Specific microRNAs are downregulated in human thyroid anaplastic carcinomas. Oncogene. 2007;26:7590–7595. doi: 10.1038/sj.onc.1210564. [DOI] [PubMed] [Google Scholar]
- 74.Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ. Res. 2007;100:416–424. doi: 10.1161/01.RES.0000257913.42552.23. [DOI] [PubMed] [Google Scholar]
- 75.Arndt GM, Dossey L, Cullen LM, Lai A, Druker R, Eisbacher M, Zhang C, Tran N, Fan H, Retzlaff K, et al. Characterization of global microRNA expression reveals oncogenic potential of miR-145 in metastatic colorectal cancer. BMC Cancer. 2009;9:374. doi: 10.1186/1471-2407-9-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang G, Zhang H, He H, Tong W, Wang B, Liao G, Chen Z, Du C. Up-regulation of microRNA in bladder tumor tissue is not common. Int. Urol. Nephrol. 2009;42:95–102. doi: 10.1007/s11255-009-9584-3. [DOI] [PubMed] [Google Scholar]
- 77.Eisenberg I, Eran A, Nishino I, Moggio M, Lamperti C, Amato AA, Lidov HG, Kang PB, North KN, Mitrani-Rosenbaum S, et al. Distinctive patterns of microRNA expression in primary muscular disorders. Proc. Natl. Acad. Sci. USA. 2007;104:17016–17021. doi: 10.1073/pnas.0708115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hebert C, Norris K, Scheper MA, Nikitakis N, Sauk JJ. High mobility group A2 is a target for miRNA-98 in head and neck squamous cell carcinoma. Mol. Cancer. 2007;6:5. doi: 10.1186/1476-4598-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dixon-McIver A, East P, Mein CA, Cazier JB, Molloy G, Chaplin T, Andrew Lister T, Young BD, Debernardi S. Distinctive patterns of microRNA expression associated with karyotype in acute myeloid leukaemia. PLoS One. 2008;3:e2141. doi: 10.1371/journal.pone.0002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marton S, Garcia MR, Robello C, Persson H, Trajtenberg F, Pritsch O, Rovira C, Naya H, Dighiero G, Cayota A. Small RNAs analysis in CLL reveals a deregulation of miRNA expression and novel miRNA candidates of putative relevance in CLL pathogenesis. Leukemia. 2008;22:330–338. doi: 10.1038/sj.leu.2405022. [DOI] [PubMed] [Google Scholar]