Abstract
Successful cancer management depends on accurate diagnostics along with specific treatment protocols. Current diagnostic techniques need to be improved to provide earlier detection capabilities, and traditional chemotherapy approaches to cancer treatment are limited by lack of specificity and systemic toxicity. This review highlights advances in nanotechnology that have allowed the development of multifunctional platforms for cancer detection, therapy, and monitoring. Nanomaterials can be used as MRI, optical imaging, and photoacoustic imaging contrast agents. When used as drug carriers, nanoformulations can increase tumor exposure to therapeutic agents and result in improved treatment effects by prolonging circulation times, protecting entrapped drugs from degradation, and enhancing tumor uptake through the EPR effect as well as receptor-mediated endocytosis. Multiple therapeutic agents such as chemotherapy, antiangiogenic, or gene therapy agents can be simultaneously delivered by nanocarriers to tumor sites to enhance the effectiveness of therapy. Additionally, imaging and therapy agents can be co-delivered to provide seamless integration of diagnostics, therapy and follow-up, and different therapeutic modalities such as chemotherapy and hyperthermia can be coadministered to take advantage of synergistic effects. Liposomes, metallic nanoparticles, polymeric nanoparticles, dendrimers, carbon nanotubes, and quantum dots are examples of nanoformulations that can be used as multifunctional platforms for cancer theranostics. Nanomedicine approaches in cancer have great potential for clinically translatable advances that can positively impact the overall diagnostic and therapeutic process, and result in enhanced quality of life for cancer patients. However, a concerted scientific effort is still necessary to fully explore long-term risks, effects, and precautions for safe human use.
Keywords: nanocarriers, multifunctional nanoparticles, theranostics, cancer, nanomaterials, image-guided therapy
1. Introduction
Cancer is a complex cluster of diseases that arise from DNA mutations impacting cell growth and cell cycle processes. The fact that cancer encompasses a heterogeneous spectrum of conditions and is highly unpredictable causes numerous challenges for early diagnosis and effective treatment, and makes cancer a major public health concern worldwide in the 21st century. In the United States alone, cancer is expected to cause 569,500 deaths in 2010 [1]. Current treatment techniques for cancer include surgery, radiotherapy, chemotherapy, hyperthermia, immunotherapy, hormone therapy, stem cell therapy, and combinations thereof. In many cases, early detection is the crucial factor that directs the treatment regime and the choice of therapeutic intervention. The stage at which a tumor is detected determines whether it can be surgically resected without need for adjuvant treatment, or whether it will require a combination of approaches, which typically include surgery, radiation, and chemotherapy.
Chemotherapy is used in the treatment of many cancers, but it has important limitations including a lack of specificity that results in low concentrations of chemotherapeutic drugs/agents at tumor sites, along with numerous off-target toxic effects [2]. The concept of a "magic bullet", introduced by Paul Elrich in 1906 [3], has influenced research efforts to develop site-directed delivery strategies for chemotherapy drugs. Targeted drug delivery can improve drug concentration at the tumor site and maximize therapeutic response. Additionally, the increased selectivity of the treatment minimizes toxic side effects, and reduces the negative impact on the quality of life of patients receiving chemotherapy. In order to achieve site-directed delivery, researchers have developed many drug carrier systems that guide the administration of the drug to a specific target location [4, 5]. Some examples include liposomes [6, 7], micelles [6, 8], natural and synthetic polymer nanoparticles [9, 10], metal nanoparticles [11], microspheres [12], and direct local delivery using drug eluting patches and stents [13]. The choice of carrier system affects bioavailability, biodistribution, types of drugs that can be delivered, and the specificity and pharmacokinetics of delivery. For a given carrier, multiple factors determine the stability and fate of the delivery vehicle during storage and after administration, including size, rigidity, charge, solubility and surface modifications. Two of the most important systems in current drug delivery research include liposome-based delivery systems and polymer microparticles/nanoparticles as carrier systems.
2. Liposomal delivery systems
Liposomes are concentric, closed bilayer membranes of water insoluble polar lipids that can be used to encapsulate biomolecules and drugs for targeted delivery while protecting their bioactivity. Liposomes are a good choice for drug carrier systems because they are nature-made, biocompatible, and their size can be controlled quite precisely during the preparation process. Liposomes were first utilized as enzyme carriers in 1971 for the treatment of lysosomal storage disease [14, 15], and their use as delivery vehicles has since extended to a variety of encapsulated drugs such as antineoplastic agents, antimicrobial compounds, immunomodulators, anti-inflammatory agents, cardiovascular drugs, etc. [16–20] Currently, there are several commercially available liposomal formulations for cancer therapy, including doxorubicin (Doxil®), daunorubicin (Daunoxome®) cytarabine (Depocyt®), Myocet® and vincristine (ONCO-TCS®) [21–25]. Liposomal DOX has been investigated clinically for breast cancer, ovarian cancer, AIDS-related Kaposi’s sarcoma, head/neck cancer, and brain tumors [26–30].
Although the field of liposomal drug delivery has shown a lot of promise, there are still some challenges to overcome, including shelf stability, unsuitability for oral administration routes, low loading efficiency, poor control of drug release, drug degradation inside the liposome, difficulty encapsulating hydrophobic drugs, and bioavailability issues in vivo, including destabilization by interaction with serum proteins as well as clearance by the reticuloendothelial system (RES) and circulating monocytes [31]. In an effort to obtain improved in vivo pharmacokinetics, researchers have developed "stealth liposomes" by attaching PEG, gangliosides, sialic acid derivatives, hydrophilic synthetic polymers, and other molecules [32–34] to the surface of the lipid bilayer. These surface decorations result in increased hydrophilicity and prolonged plasma circulation times. Further surface modifications, such as the addition of targeting moieties, can be used to make the carrier site-specific.
3. Particle carrier systems
Particles provide another option to enhance site-specific delivery and controlled delivery of chemotherapy drugs. Many systems have been designed with the common goal of enhancing drug bioavailability at target sites, protecting drugs/biomolecules from degradation, and facilitating drug absorption and diffusion across membranes. Nanoscale drug delivery systems can be targeted by molecular surface decorations specific to a given target, such as antibodies for cell surface receptors that are overexpressed in cancer cells. One of the most important factors to consider in the design of particle carriers is size, which will greatly influence the biodistribution of the resulting vehicle. There are three types of particles based on their size: (i) macroparticles (50–200 µm) (ii), microparticles (1–50 µm) and (iii), nanoparticles (10–1000 nm)
Biodegradable microparticles made of starch [12], albumin [35], or polylactic acid [36] have been used in therapeutic applications such as chemoembolisation. Since macroparticles cannot enter the capillaries, they are lodged at the arteriole level after administration, and can provide sustained and slow release of drug contents to surrounding tissue while protecting the entrapped drug from biodegradation. Release rate can be tailored by the choice of polymer pore size, swelling properties, and degradation rate. One of the main difficulties with the use of microparticles for other therapeutic applications is their rapid clearance by the RES, as well as their inability to enter capillaries that limits the possibilities for targeted tissue delivery.
Lipid, metallic, or polymeric nanoparticles provide an inherent advantage for systemic delivery because their smaller size allows decreased RES uptake, prolonged circulation times, and penetration into capillaries. Additionally, their increased surface area per unit volume allows for increased loading amounts of adsorbed drugs or biomolecules. Since the first report [37] on preparation and characterization of polymeric nanoparticles in 1976, research in this field has grown exponentially. Particle characteristics (including size distribution, surface charge, biocompatibility, biodegradation behavior, and availability of functional groups for conjugation) are crucial to the ultimate success of the delivery vehicle. Uniform sizes in the range of 110–140 nm, neutral surface properties, and high molecular weight parent polymers are desirable characteristics of polymeric nanoparticles in order to optimize biodistribution, increase circulation time, and maximize uptake by target tissues.
One strategy to impart stealth properties to nanoparticles and create "long-circulating" nanoparticles that avoid uptake by macrophages is to coat the particles with a high molecular weight dextran or with PEG, both of which decrease particle surface charge and result in prolonged circulation times [38]. Other hydrophilic ligands used for surface functionalization that can enhance accumulation at desired tumor loci include chitosan, heparin, and other polysaccharides [39, 40].
Choosing the right parent polymer is an important step in the design of carrier systems, because polymer type can determine the ultimate behavior of the delivery system in different environments. The fate of nanoparticles within the body is governed by the size (molecular weight), shape, surface charge and nature (hydrophobic or hydrophilic) of the parent polymer(s). Additionally, drug release at the target site also depends on polymer characteristics since it occurs by one of three mechanisms, namely diffusion of the drug content from hydrated particles, enzymatic degradation of the polymer network, or cleavage of the drug after hydration of the particles. Natural polymers such as albumin, gelatin, chitosan, and heparin; and synthetic polymers such as poly(amino acids), poly(alkyl-cyano acrylates) [41], poly(esters), poly(orthoesters), poly(urethanes) and poly(acrylamides) offer a plethora of chemical composition and structure combinations for nanoparticle drug carrier design and allow delivery of small drugs, oligonucleotides, DNA, and proteins [42]. Table 1 summarizes the various natural and synthetic polymers used in nanoparticle formulations.
Table 1.
Summary of number of different cancer targeting and cancer therapy approaches using natural and synthetic polymeric nanoparticle formulation. Characteristics: Size, zeta potential, imaging agent, targeting agent.
| Method | Polymer | Solvent | Stabilizer | Size (nm) | Drug | Surface Modification |
Zeta Pot. |
Imaging Agent |
Multifunctional | Targeting | [ref] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SD | poly(anhydride) | Ac | - | 140–180 | Cyclodextrin | −50 | N | N | N | [73] | |
| 2step desolvation | Gelatin | Ac | glutaraldehyde | 250–300 | NeutrAvidinanti-CD3 Ab | −20 | N | N | Anti-CD3 antibody | [74] | |
| ESE | PLGA | EA | DMAB | 121 | Paclitaxel | +35to+45 | N | N | N | [67] | |
| NP | PLGA | Ac | Stabilizer free | ~156 | Taxol | **surface | ***NPQD | *I/C/H, Fe3O4 | N | [75] | |
| SESD | PLGA | ACN | PVA | ~150 | Paclitaxel | PEG-QDs | QDs | *I/C/H, Fe3O4 | PSMA | [76] | |
| NP/SD | PLGA | DMSO | Pluronic | 104–144 | N | heparin/chitosan | −20 to −50, +28 | Cy5.5 | I/C | N | [40] |
| CC | Chitosan | - | - | 237 | DNA | None | +15 | N | N | N | [77] |
| NP | PEGb-PLGA | Ac | PEG | 114–146 | Paclitaxel | PEG | −~0 | N | N | RGD peptide | [78] |
| NP | PLGA-PEG | Ac | PVA | ~149 | 9nitrocampto thecin | PEG | +1.84 | N | N | N | [79] |
| extrusion | Liposomes | N | PEG | 100 | DOX | PEG | NR | N | N | mAb 2C5 | [80] |
| self- assembled | Glycol-Chitosan-PEI | - | - | 100–300 | siRNA | +9.95 | Cy5.5 | Imaging/gene silencing | - | [81] | |
| EDE | PLGA | EA | PVA | ~166 | tamoxifen | N | ~+3.5 | N | N | N | [82] |
| EI | Chitosan-g-PEG | acetate buffer | - | ~200 | DNA | PEG | ~+15 | N | N | N | [83] |
| dialysis | Chitosan | - | - | ~260 | Paclitaxel | N | NR | Cy5.5 | I/C | N | [84] |
| w/o/w ESE | PLGA | EA | PVA | 320–360 | BSA | N | −25 | N | N | mAb | [85] |
| NP | PLGA | Ac | Pluronic | 100–125 | DOX | PEG | ~−30 | Au | *I/C/H | N | [86] |
| NP | PLGA | Ac | - | ~200 | Docetaxel | PEG | ~−26 | N | N | HER-2 | [87] |
| SESE | PLGA | MeOH/CHCl3 | PVA | ~226 | DOX | N | ~−5 | N | N | NLS | [88] |
| SD | PLA and PLGA | Ac | Tween-80 | 100, 200 | Docetaxel | −28, −7 | N | N | N | [89] | |
| Comm.available | PIHCA | - | - | - | siRNA | chitosan | NR | N | N | N | [90] |
| co-precipitation | Chitosan | Water | N | 10.5 | Fe3O4 - hyperthermia | chitosan | N | - | Fe3O4 | N | [91] |
| MESE | PLGA | DCM | PVA | 276–550 | tamoxifen | N | NR | N | N | N | [92] |
| SESD | PLGA | MeOH/ ACN | PVA | ~300 | N | N | −16.3 | ICG | N | N | [93] |
| w/o/w ESE | PLGA | DCM/AC | PVA | ~436 | DOX, pEGFP DNA | PEG-Liposome | +30.9 | Chemo/gene delivery | Folate | [94] | |
| CC | Chitosan | acetate buffer | - | ~181 | DOX, DNA. | - | NR | N | Chemo/gene delivery | Folate | [95] |
| IG | Chitosan | Water | gluteraldehyde | ~187 | methotrexate | N | +30 | N | N | N | [96] |
| NP | PLGA | Ac | PVA | ~76.2 | curcumin | N | ~0 | N | N | N | [97] |
Abbreviations: ESE=emulsion solvent evaporation, EDE =emulsion diffusion evaporation, SESE =single emulsion solvent evaporation, SD =solvent displacement, MESE =multiple emulsification (w/o/w) and solvent evaporation, SESD =spontaneous emulsion solvent diffusion, EI =electrostatic interaction, CC =complex coacervation, NP =nanoprecipitation, IG =ionic-gelation, PIHCA =polyisohexylcynoacrylate, EA = ethyl acetate, MeOH/ACN = methano/acetonitrile, DCM/AC =dichloromethane/acetone, DMSO =dimethylsulfoxide, Ac =acetone, PVA =polyvinylalcohol, PEG = polyethyleneglycol, QDs =quantum dots, N =none, NR =not reported
I/C =Imaging/Chemotherapy,
I/C/H =Imaging/chemotherapy/hyperthermia,
surface = NH2-terminal Fe3O4 and NH2-PEG-QDs,
NPQD =NH2-PEG-QDs
Chitosan, poly(lactic-glycolic acid) (PLGA), and poly(lactic acid) (PLA) in particular have been extensively investigated due to their biodegradability and biocompatibility [43]. Chitosan polymers are semi-synthetic polysaccharides with many applications in biomedicine including gene therapy, drug delivery, tissue engineering scaffolds, dressings, coatings, and sensors [44, 45]. Chitosan is used in gene therapy as a non-viral vector due to its strong polycationic properties, which allow it to form strong interaction complexes with DNA. Although transfection efficiency is still an issue, many groups have reported successful gene therapy approaches using chitosan-DNA nanoformulations [46–49]. Conjugation of chitosan with other agents, such as polyethylenimine, can enhance DNA release capability and transfection efficiency [50]. Applications of chitosan in drug/small molecule delivery have also been explored extensively in recent years. Karatas et al prepared chitosan nanoparticles loaded with a caspase-inhibitor peptide, creating a system that has potential for use in preventing apoptotic cell death. In a mouse model of neurological injury, this system was able to cross the blood-brain barrier and result in decreased infarct volume and reduced neurological deficits [51, 52]. Chitosan has also been used as a carrier for different chemotherapeutic agents, including but not limited to doxorubicin [53–55], paclitaxel [56], camptothecin [57], docetaxel [58], and 5-fluorouracil [59]. Reported multimodal formulations include combinations of chitosan with quantum dots [60], superparamagnetic iron oxide nanoparticles [61], gadolinium [62], and others, allowing for image-guided therapy. Further research is needed to investigate whether parenteral use of chitosan formulations is safe in humans because chitosan can induce coagulation [63].
PLGA is a biodegradable synthetic polymer that has been used to prepare nanoparticles for several applications including gene therapy as well as delivery of bioactive agents such as proteins, vitamins, and pharmaceutical drugs, as shown in table 1. PLGA nanoparticle formulations can be used to entrap hydrophilic and/or hydrophobic drugs, and can be controlled for particle size and drug release rate. In the case of hydrophobic species such as doxorubicin, paclitaxel, or quercetin, which suffer from premature degradation and poor aqueous solubility in their free form, PLGA nanoparticle entrapment provides improved drug dispersibility and protects bioactive molecules allowing them to reach target/diseased sites without degrading [64]. Degradation of PLGA occurs through hydrolysis of the ester linkages throughout the matrix and through surface erosion, and the resulting byproducts (lactic acid and glycolic acid) can be naturally removed from the body. Delivery can be tuned by tailoring polymer characteristics, for example, the degradation rate of PLGA can be modified by changing the molar ratio of lactic and glycolic acid in the copolymer.
PLGA nanoparticles containing anticancer drugs have been extensively reported in the literature (Table 1), including formulations entrapping paclitaxel, doxorubicin (DOX), vincristine sulphate, dexamethasone, and cisplatin [64]. These PLGA nanoparticle formulations have significant advantages over liposomal formulations, which are still marred by issues of fast leakage and instability. Doxorubicin-loaded PLGA nanoparticles have been successfully formulated, characterized and evaluated in vitro and in vivo [65, 66]. There are also numerous examples of paclitaxel-loaded nanoparticles applied all the way from benchtop to bedside, including formulations that are suitable for oral administration [67]. A particularly successful example is nanometer-sized albumin-bound paclitaxel (Abraxane), which has been used in the clinic for the treatment of metastatic breast cancer [68], and is being evaluated in clinical trials involving many other cancers including non–small-cell lung cancer (phase II trial) [69], and advanced nonhematologic malignancies (phase I trial) [70]. At present, there are over 60 clinical trials that involve the use of nanoparticles in cancer therapy (see http://clinicaltrials.gov). An important advantage of nanoparticles when used as carriers for chemotherapy agents is their ability to overcome multidrug resistance (MDR) phenomena by bypassing the multidrug exporter pump [71, 72]. MDR is an important factor that contributes to the failure of traditional forms of chemotherapy.
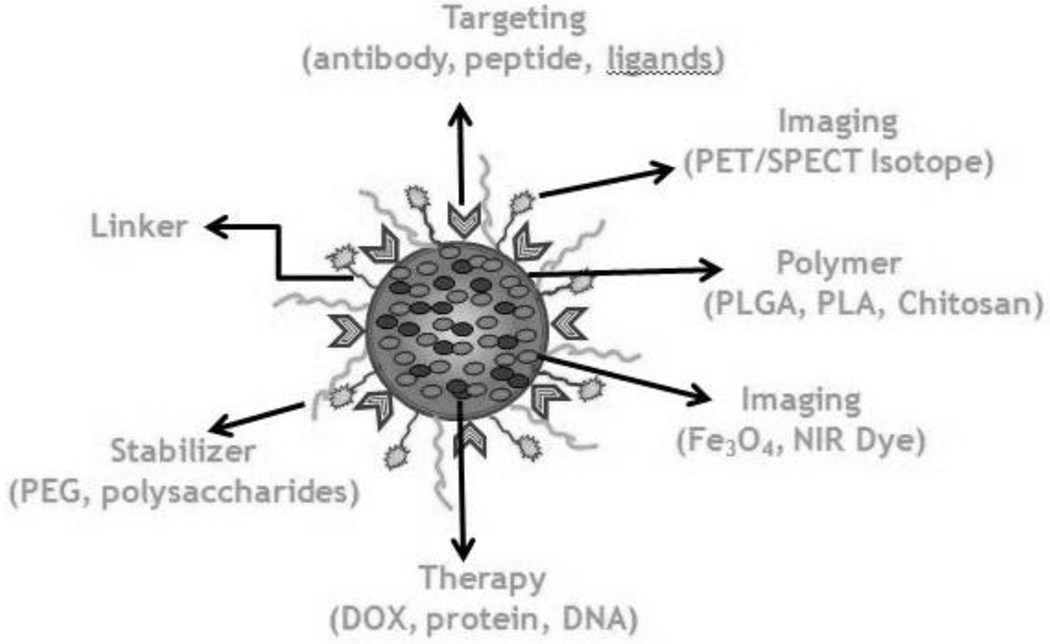
Polymeric nanoparticles offer an extensive array of functionalization options that can be used to create versatile multifunctional systems for targeted theranostic applications (figure 1). Multimodal tailored approaches to the diagnosis and treatment of cancer are more likely to result in clinically translatable advances by enhancing the efficacy and specificity of treatment regimes. Strategies can include combinations of several therapeutic molecules such as chemotherapy drugs and/or gene therapy agents, combinations of chemotherapy drugs with other modalities such as hyperthermia, and theranostic combinations in which nanoparticles are used for imaging and therapeutics.
Figure 1.
Schematic representation of a multifunctional polymeric nanoparticle for image guided therapy.
Despite their numerous advantages, polymer nanoparticles also have some disadvantages, including toxicity of preparation solvents, acidity of degradation byproducts, and drug release that is frequently biphasic. Additionally, nanoparticle size is more difficult to control than for liposomes, and there are still many unknowns regarding the toxicity profile and biological effects of synthetic nanoparticles.
4. Targeted nanoformulations
4.1. Passive Targeting
Nanoparticles are passively targeted to tumor sites through the enhanced permeability and retention effect (EPR). Blood vessels supplying tumor tissues have larger pore sizes compared to those in healthy tissue, which results in preferential tumor accumulation of nanoparticle-delivered drugs, increased treatment efficacy, and reduced systemic toxicity [98, 99]. However, the EPR phenomenon depends on many different factors such as parent polymer molecular weight, particle surface charge and hydrophobicity, immunogenicity, tumor characteristics, etc [100]. This results in many challenges in the optimization of passive targeting. Current consensus is that prolonged circulation time is the most important factor in enhancing this phenomenon, and in order to obtain longer circulation times particles should be neutrally charged, have an average diameter of 10–100 nm, and molecular weights around 30 kDa, although studies have shown uptake for molecular weights up to 800 kDa [101].
4.2. Active targeting
Passive targeting alone is limited by low tumor specificity, and therapeutic concentrations can still be lower than optimal at the tumor site by simply relying on EPR-mediated accumulation. A strategy to overcome these limitations by decorating the surface of the nanoparticles with targeting moieties such as small ligands, antibodies, or biomarkers that can direct the delivery vehicle towards specific molecular targets which are overexpressed by tumor cells. This approach is called active targeting, and it results in more efficient and selective uptake of drug into the target cells. Active targeting requires careful identification of tumor biomarkers, as well as selection of specific molecules that can hone in to those markers in a selective, directed manner. Targeted particles can then be internalized by tumor cells via receptor-mediated endocytosis/phagocytosis, resulting in elevated concentrations in tumor tissue.
Although antibodies can be directly conjugated to drugs without the use of a vehicle, clinical trials have highlighted the difficulties of applying this approach [102], mostly due to potential loss of bioactivity upon conjugation, steric hindrance, and immunogenicity of the antibodies when used in their full form. Even when antibody fragments were used, drug-antibody conjugates have not shown effective results in targeted delivery of therapy to cancer because of functional changes in the conjugated components. In contrast, conjugating antibodies to the surface of a delivery vehicle does not interfere with the bioactivity or characteristics of the entrapped drug, and does not result in loss of affinity of the antibody for the target, which makes nanocarriers an excellent platform for the development of effective targeted therapies.
The applications of antibodies in targeted therapies have evolved toward the preferential use of monoclonal antibodies (mAbs), especially trying to avoid or reduce immunogenicity by using engineered chimeric or humanized forms to maximize the chances of successful clinical translation [103]. So far, several mAbs-based therapies have shown success in targeting disease processes, including formulations of trastuzumab [104], cetuximab [105], rituximab [106], and bevacizumab [107]. Another option for targeting is to use aptamers, which are oligonucleic acids with high specificity, small size, and reduced immunogenicity, albeit at high production costs [108, 109]. RNA aptamers to the VEGF isoform with 2 ′ - O -methylpurine and 2 ′ - F pyrimidines show antiangiogenic properties, including an aptamer called Pegaptanib that has been FDA-approved for the treatment of neovascular macular degeneration [110, 111]. Other aptamers that target prostate-specific membrane antigens have been conjugated to docetaxe-loaded PLGA nanoparticles which have been evaluated for efficacy in an animal model [112].
Many biomarkers have been identified as possible targets of antitumor drugs, including the transferrin receptor, Epidermal Growth Factor Receptor (EGFR), folate receptor, and Human Epidermal Receptor 2 (HER-2). The folate receptor is overexpressed in 80–90% of ovarian cancers, which opens the possibility of targeting this particular type of cancer in a specific manner. Folate-decorated nanocarriers have the added advantage of using a natural targeting moiety that does not cause immunological responses in human recipients [113, 114]. Anti-HER2 monoclonal antibody (transtuzumab) immunoliposomes containing doxorubicin have been shown to be superior to free doxorubicin and liposomal doxorubicin in studies with xenograft tumor models by the Park et al group [115]. Our own research has shown that DOX-loaded PLGA nanoparticles decorated with anti-HER2 moieties show significantly increased cellular uptake compared to undecorated nanoparticles in the HER-2 overexpressing ovarian cancer cell line SKOV-3 [116]. The increased uptake is specific to the HER-2 receptor-mediated process, because cells that do not overexpress HER-2 such as MES-SA and MES-SA/Dx5 do not show any differences in uptake between decorated and undecorated nanoparticles. Thus, capitalizing on the specific interactions between engineered drug delivery systems and cell surface receptors has the potential to result in customizable, tailored therapies for cancer treatment. Future challenges to overcome include drug resistance phenomena that can lead to changes in biomarker functionality, as well as the inherent variability of receptor expression within the target cell population. The multimodal combination of targeted therapies and traditional therapies is still the best approach in cancer management.
5. Combinational delivery approaches
Combining several therapeutic agents into a delivery vehicle may enhance the effectiveness of cancer interventions. This may include delivery of multiple chemotherapeutic drugs, co-delivery of chemotherapy and antiangiogenic agents, co-delivery of drugs and genes, and co-delivery of drugs and si-RNA, amongst others.
5.1. Simultaneous delivery of chemotherapeutic agents
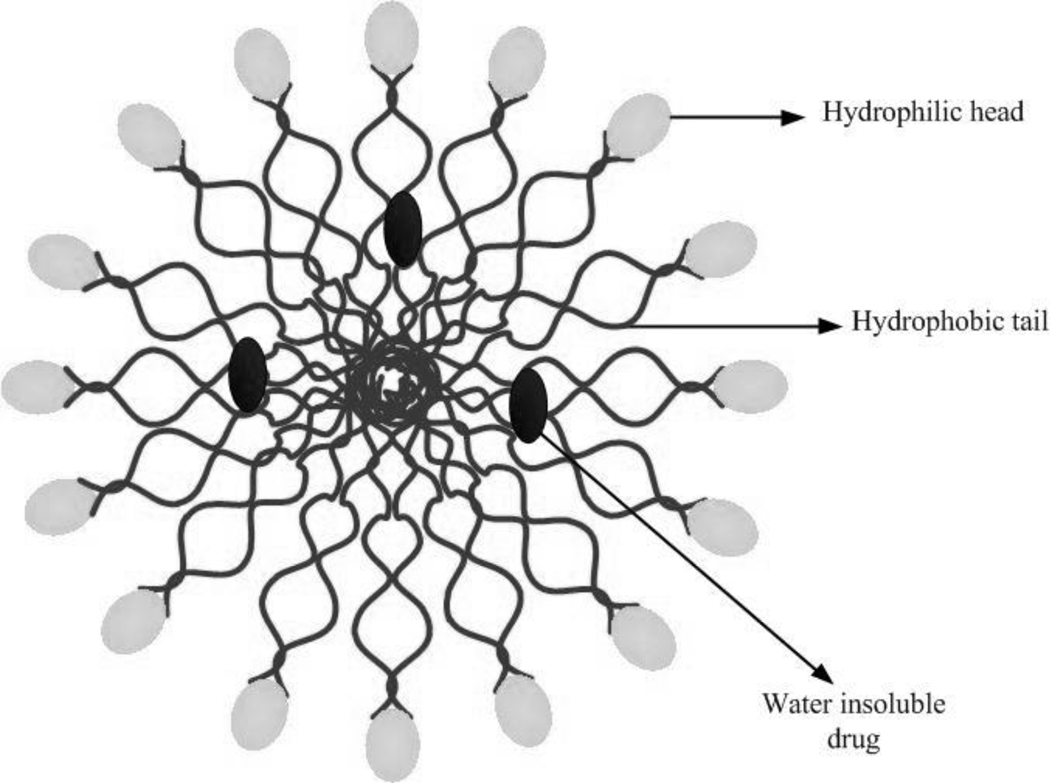
One of the first reports on multichemotherapy delivery using nanocarriers was published in 2005 by Agrawal et al, who demonstrated that dual-loaded 6-mercaptopurine/daunorubicin liposomes had increased in vitro cytotoxicity in lymphoma cells when compared to single-loaded liposome formulations of the same drugs [117]. Since then, many other formulations including synthetic (e.g., PLGA) and natural (e.g., chitosan) polymers have been used for codelivery of drugs, and have shown enhanced efficacy of treatment for multitherapy vs. monotherapy delivered to cancer cells in vitro. Some examples include PLGA nanoparticles simultaneously loaded with verapamil and vincristin on MCF-7/ADR resistant cells, BEL7402 cells, and BEL7402/5-FU human hepatocarcinoma cells [72, 118]; DOX/ cyclosporine A loaded polyalkylcynoacrylate nanoparticles [119], codelivery of PDTC and doxorubicin using multifunctional micelle nanoparticles (see figure 2 for a schematic structure of a micelle) [120], and codelivery of ICG and DOX using PLGA nanoparticles [71].
Figure 2.
Schematic representation of a micelle entrapping a water insoluble drug.
Some combinational therapy nanocarriers have reached Phase II clinical trials, including cytarabine/daunorubicin loaded liposomes (CPX-531 formulation) for the treatment of acute myeloid leukemia [121], and a CPX-1 formulation composed of irinotecan and flouridine for treatment of colorectal cancer [122]. Some in vivo studies using multitherapy carriers include codelivery of daunorubicine and tamoxifen using stealth liposomes for breast cancer [123], and RGD-based combinatorial delivery of siRNA and doxorubicin [124].
An important challenge in combination chemotherapy is tailoring the release of drugs to optimize therapeutic regimes and administration schedules, especially when the drugs entrapped in the carrier have very different hydrophilicity characteristics. The ability to control the release profiles for different agents in the same delivery vehicle so that they can be independently adjusted can be attained with a multicompartmental design. Several groups have been able to design carriers in which release profiles for different drugs can be modulated to provide better therapeutic timing. Zhang et al [125] used an aptamer–nanoparticle bioconjugate where docetaxel was entrapped and DOX was intercalated, resulting in a faster release profile for DOX than for docetaxel. Fan et al [120] created folate-conjugated chitosan micellar nanoparticles for codelivery of pyrrolidinedithiocarbamate (PDTC) and DOX in HepG-2 liver cancer cells, in which the release rate of DOX was controlled by environmental pH conditions.
5.2. Combinational chemotherapy/antiangiogenesis
An interesting field of research in cancer therapy is the potential applications of antiangiogenic factors to arrest the growth of tumors by decreasing their blood supply. Some examples of angiogenic factors that can be blocked to enhance cancer therapy include fibroblast growth factor (FGF), insulin-like growth factor, and VEGF [126]. Researchers have found that there is an enhanced treatment effect when chemotherapy and antiangiogenic agents are administered in combination, for example, the use of paclitaxel and DOX with VEGF receptor 2 inhibitor JNJ-1 7029259 [127], and the administration of bevacizumab in combination with standard chemotherapeutics (irinotecan, fluorouracil, calcium folinate and leucovorin), which has been proven to improve survival time for patients with metastatic colorectal cancer [128]. However, there are still many challenges to overcome in combinational chemotherapy/antiangiogenesis, and nanocarriers may provide a way to enhance simultaneous delivery of these agents to tumor loci.
Couzin et al first showed in 2002 that antiangiogenic agents can be delivered with a nanoparticle carrier to mice tumor blood vessels [129], and researchers have since demonstrated the potential of multitherapy using nanocarriers. In 2005, Sengupta et al [130] formulated a system called a nanocell, in which a nanoscale pegylated-phospholipid block-copolymer envelope coated a nuclear PLGA nanoparticle. The multilayer system contained DOX conjugated to the PLGA nanoparticle core, along with an antiangiogenesis agent (combretastatin-A4) in the lipid envelope. The system demonstrated a multistage release behavior based on the design of the nanocell, so that the antiangiogenic drug was released first from the envelope to suppress the tumor vasculature, and then DOX was released from the PLGA core through hydrolysis. Thus, the chemotherapeutic agent was accumulated at the site and released after the antiangiogenic molecule had exerted its effect, resulting in an enhanced therapeutic index and a decrease in off-site toxicity.
5.3. Combinational chemotherapy/gene therapy
One of the main limitations of gene therapy is the difficulty in overcoming the barriers for delivery of genes into cancer cells, such as stability in systemic delivery, targeting and penetration into cells, and nuclear translocation for gene expression. Nanocarriers can be a good strategy to enhance gene delivery to target sites, and many natural and synthetic polymeric materials such as chitosan, polyethyleneglycol (PEG), and polyethyleneimine (PEI) have been successfully employed to deliver genes and biomolecules. The versatility of polymeric nanoparticles makes them excellent candidates for multifunctional applications in which simultaneous delivery of genes and drugs to a target site can be used to enhance therapeutic results [131, 132]. An excellent example is the work of Wang et al [133], who prepared PLGA/folate coated PEGylated polymeric liposome coreshell nanoparticles (PLGA/FPL NPs) for the co-delivery of drug and genes. This delivery vehicle is composed of a hydrophobic PLGA core that can entrap hydrophobic drugs such as DOX, and a hydrophilic cationic lipid shell that can be used to bind DNA. Wang's group demonstrated that these carriers are able to simultaneously deliver drugs and genes to MDA-MB-231 breast cancer cells with high gene transfection and drug delivery efficiency.
5.4. Combinational chemotherapy/si-RNA therapy
The ability of siRNA to silence gene expression can be used as a form of cancer therapy, but one of the main challenges of this approach is optimizing delivery and cellular entry so that siRNA sequences can be directed to target RNA inside the cell [134]. Engineered nanosize co-delivery systems for siRNA and chemotherapeutic drugs can improve the selective delivery of the siRNA sequence to target cells, while providing the opportunity for combinational treatment effects [135–137]. Some examples include the work of Saad et al [138], who showed that a siRNA/DOX loaded liposomal delivery system was able to reverse MDR in lung cancer cells in vitro; and the micelleplex approach by Sun et al [139], who used micellar nanoparticles of a biodegradable triblock copolymer poly(ethylene glycol)-b-poly(ε-caprolactone)-b-poly(2-aminoethyl ethylene phosphate) to systemically deliver siRNA and a chemotherapeutic drug.
6. Nanotechnology-based sensing
Nanosize platforms provide an opportunity for advances in cancer diagnostics due to their versatility, size, and physicochemical characteristics. Nanomaterials can be functionalized with an array of moieties that potentially allow for customized detection of cancer-specific biomarkers, and they can be used in miniaturized detection systems [140]. Gold nanoparticles, gold nanorods, quantum dots and other nanomaterials have been used as sensors for cancer marker and cancer cell detection by conjugating them with peptides, aptamers, antibodies, and oligonucleotides [140].
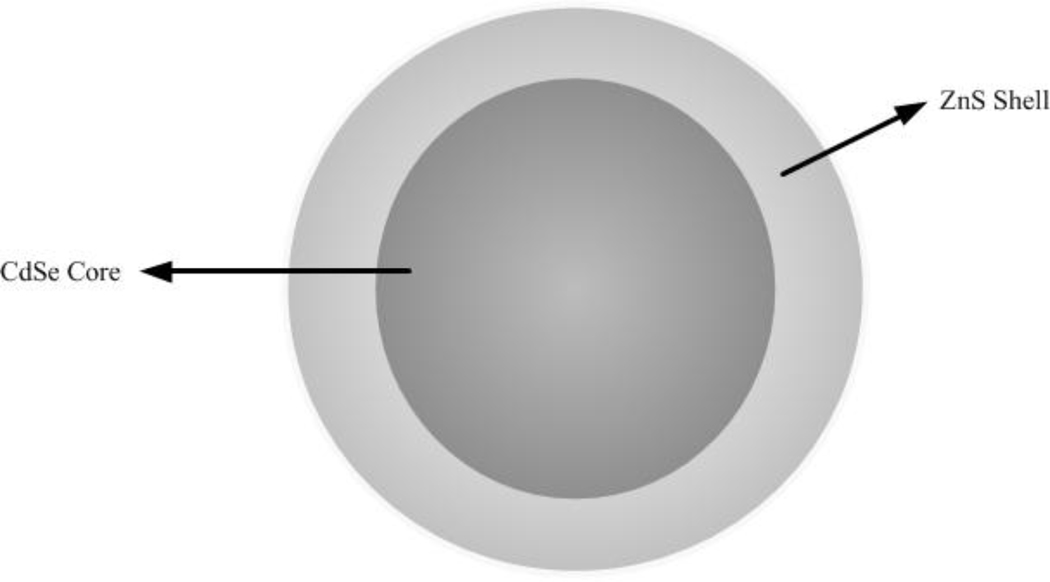
Several examples of these applications are available in recent literature. Grubisha et al were able to detect femtomolar concentrations of prostate-specific antigen (PSA) using gold nanoparticles coated with a strong surface-enhanced Raman scatterer in a sandwich assay format [141]. Gerion et al prepared a microarray containing DNA-nanocrystal conjugates with a semiconductor CdSe/ZnS core/shell. Testing of the microarray demonstrated the detection of p53 mutations within minutes, as well as the potential for multiallele detection using different crystal colors [142]. Xia et al developed a bioluminescence resonance energy transfer (BRET) - based assay using bioluminescent proteins as donors, and quantum dot nanosensors conjugated with protease substrates as acceptors. The goal of the assay was to detect matrix metalloproteinases (MMPs), which are overexpressed in many cancers, and the authors were able to detect MMP activity in the nanogram/mL range in buffer and in mouse serum [143]. Oishi et al recently designed an assay to detect protein kinase inhibitors in cell lysates using citrate-coated gold nanoparticles. In the presence of an inhibitor, gold nanoparticles aggregate and there is a colorimetric change in the solution. This method allows quick, practical screening of potential protein kinase inhibitors for use in cancer treatment [144]. Another example of novel detection methods using nanotechnology is the bio-barcode assay, which is based on double-functionalized gold nanoparticles that are decorated with both an oligonucleotide (the "barcode" that can be scanned for rapid sensing) and a target recognition element such as an antibody (for protein detection) or another oligonucleotide (for nucleic acid detection). These are combined with magnetic nanoparticle probes to capture antigens in solution [145, 146]. An example of bio-barcode application using gold nanoparticles was reported by Stoeva et al, who were able to detect low-femtomolar concentrations of cancer markers such as prostate specific antigen, human chorionic gonadotropin (HCG), and α-fetoprotein (AFP) in buffer and serum [146]. The area of nano-based sensing is growing rapidly and shows promise to create quick, high-throughput, sensitive and specific detection methods for cancer diagnosis and drug screening.
7. Multifunctional theranostic systems
7.1. Nanoparticles for molecular imaging and photothermal therapy
Molecular imaging provides a tool for visualization, characterization and quantification of biological processes at the cellular and subcellular levels within intact living organisms. Nuclear medicine molecular imaging (PET, SPECT) has high sensitivity, but it has some limitations in terms of resolution, short half-life of tracers, and high instrumentation cost and complexity. Optical imaging is highly sensitive, inexpensive, can yield high resolution, and can be used endoscopically for minimally-invasive approaches. However, clinical applications are restricted by the small depth of penetration, which creates a disadvantage for imaging of deep tumors compared to other imaging modalities such as CT and MRI. NIR wavelengths (800–1000 nm) can be used to improve tissue penetration in optical imaging by minimizing photon absorption by tissue components, thus allowing for in vivo opti cal imaging applications [147, 148]. The development of novel fluorescent agents has fueled interest in optical imaging applications for cancer detection. Targeted fluorescent contrast agents can help delineate the boundary between tumor and healthy tissue and be used as an adjuvant to direct surgical resection.
Nanoparticles have been used for CT, MRI, nuclear, and optical imaging applications [149, 150], and the design of multifunctional nanoparticles allows for simultaneous delivery of therapeutic and imaging agents in vivo [84]. In some cases, the intrinsic properties of the nanoparticle allow to be used as an imaging agent and/or an agent for hyperthermia, radiation, and photodynamic therapy applications. This provides opportunities for image-guided therapy and truly integrated theranostic systems. For instance, iron-oxide nanoparticles can be used as a guided hyperthermia agent based on the magnetic properties of the iron-oxide core along with MRI detection. In 2007, Yang et al prepared hydrophobic magnetic nanocrystals and DOX simultaneously incorporated into poly(lactic-co-glycolic acid) (PLGA)-PEG-COOH. The magnetopolymeric nanohybrids were then conjugated to HER-2 antibody for targeting purposes, and the group was able to use these multifunctional carriers for MRI detection as well as for inhibition of tumor growth [151]. Recently, Park et al published a review on multifunctional nanoparticles for cancer imaging and therapy [152]. Multifunctional approaches may be crucial to the development of customizable early detection systems, tailored cancer therapies, real-time monitoring of treatment progression, and clinically translatable advances in cancer diagnosis, intervention and prognosis.
Gold nanostructures (including nanospheres, nanorods, nanoshells, and nanocages) have strong absorption in the visible and NIR range, and have been applied to optical imaging, CT imaging, photothermal therapy, biosensing, drug delivery, and combined imaging and therapy [153–157]. Gold nanoparticles (figure 3) are tunable hyperthermia agents that can generate heat upon excitation at peaks corresponding to their surface plasmon resonance (SPR), which can in turn be controlled by modifying the nanoparticle aspect ratio [158]. The ability to control size for tailored applications (small particles for drug delivery, or large particles for imaging) can thus be coupled with the ability to customize the excitation wavelength for hyperthermia generation. Recently, Park et al [159] fabricated DOX-loaded PLGA-Au H-S NPs that can simultaneously deliver chemotherapy and heat to tumor sites [86]. In their study, there was a synergistic effect of the combined treatment that resulted in higher therapeutic efficacy and shorter treatment times. Animal studies have also shown that hyperthermia has a synergistic effect with other cancer therapeutic modalities [160], and phase I human clinical trials are ongoing to test the clinical potential of gold nanoparticles [161].
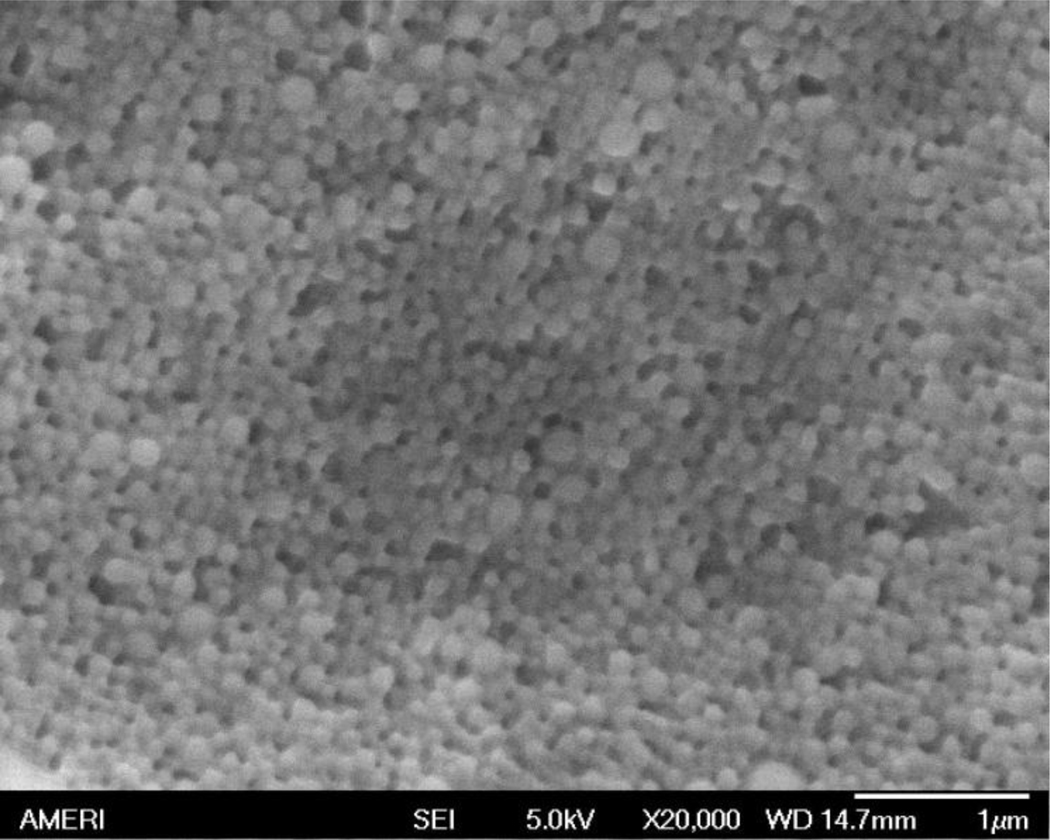
Figure 3.
SEM image of gold nanoparticles
NIR dyes such as cyanine dyes, rhodamine derivatives, phtalocyanine and napthalocyanine can also be used as imaging and photothermal agents. NIR dyes have been developed with a variety of narrow fluorescent excitation peaks and small spectral overlap, such as the members of the IRDye® family (IR800 CW, IR680LT, etc; see www.licor.com for details). These characteristics allow in vivo imaging combining different dyes to provide enhanced contrast to different tissues as needed (figure 4). Some NIR dyes can be used in multifunctional applications, as is the case of indocyanine green (ICG), which has be utilized in clinical measurement of cardiac output, evaluation of liver and kidney function, photodynamic therapy, photothermal therapy and imaging [162, 163]. Although it has a smaller heat generation efficiency than gold nanoparticles [164, 165], ICG still produces rapid temperature increases upon laser excitation and can be used as a localized hyperthermia agent. A challenge of ICG-mediated hyperthermia is the ability to deliver the dye to target tissues in sufficient quantities and without degradation, given its poor stability in aqueous solution. An option to overcome this problem is to use other cyanine dyes with similar properties but enhanced stability, such as IR820. Our group has performed comparative studies of IR820 and ICG in imaging and hyperthermia applications [166]. In vitro, IR820 can be used in live cell imaging and for cytotoxic hyperthermia, and gives comparable results to those obtained using ICG. In small animal imaging, IR820 provided longer-lasting windows for detection. Twenty-four hours after i.v. dye administration, IR820 resulted in a significantly more intense fluorescence signal and significantly higher organ dye content than ICG (p<0.05).
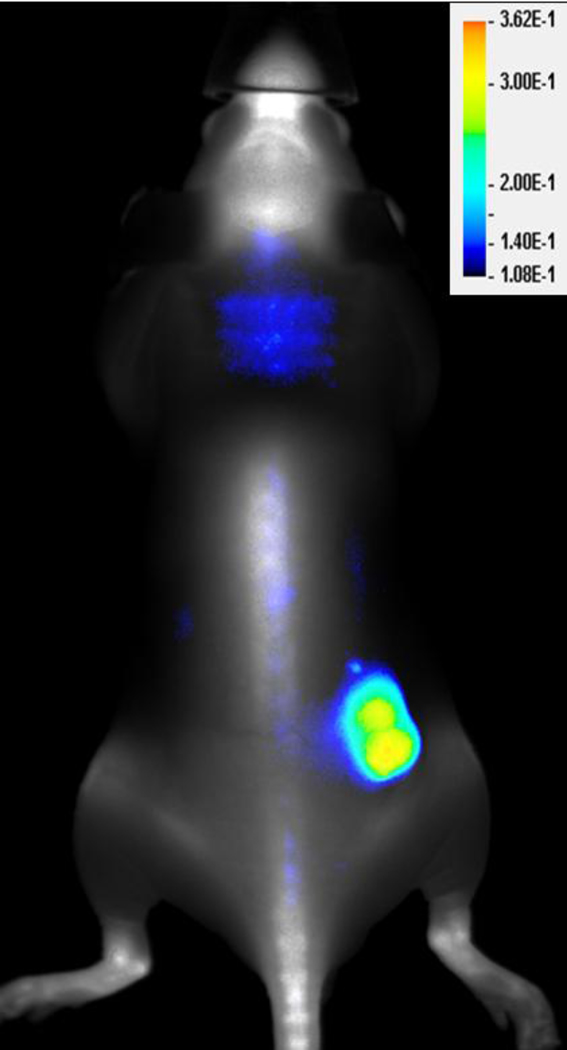
Figure 4.
An athymic nude mouse bearing a subcutaneous A431 tumor received an intraperitoneal injection of IRDye 680 BoneTag (4 nmole) 2 weeks prior to receiving the tumor specific optical probe, IRDye 800CW EGF (1 nmole). The mouse was imaged 72 hours later on the Pearl Imaging System (LI-COR Biosciences). Fluorescent signal for IRDye 680 BoneTag is represented in grayscale and IRDye 800CW EGF in pseudo color. Provided to: Anthony J McGoron, PhD, Florida International University, by LI-COR Biosciences.
Another approach to enhance delivery of NIR dyes to target tissues is to use nanocarriers. Nanoformulations of ICG, such as ICG-loaded PLGA nanoparticles, have shown increased plasma circulation times in vivo, along with significantly higher organ uptake compared to the free dye in mice [93]. When entrapped in nanoparticles, ICG can still produce hyperthermic cell killing [167], and our group has developed a multifunctional system for image-guided chemotherapy and hyperthermia in which ICG and DOX were simultaneously loaded into PLGA nanoparticles (figure 5) [168]. We subsequently studied the effect of the multifunctional system in cancer cell lines MES-SA, DX-5, and SKOV-3, and we showed that the delivery vehicle was able to bypass MDR in resistant cells, and that the combined chemotherapy/hyperthermia approach resulted in enhanced cell killing compared to hyperthermia or chemotherapy alone [167].
Figure 5.
SEM image of PLGA NPs simultaneously loaded with indocyanine green and doxorubicin.
7.2. Ultrasonic image-guided therapy
Ultrasound contrast agents in nanobubble form can preferentially extravasate into tumor tissue through the EPR effect, and be activated through tumor-directed ultrasound for imaging and controlled delivery purposes. Nanobubbles can enhance cell permeability through cavitation and, as a result, increase the cytotoxicity of delivered agents [169]. Gao et al reported the formulation of DOX-containing nanoemulsions created from perfluorocarbon nanodroplets stabilized by biodegradable block-copolymer micelles [170]. This group showed that the release of encapsulated drugs can be achieved via a cavitation effect that is limited to the tumor region, and can enhance tumor-specific drug uptake, along with the ability for real-time imaging using ultrasonography [170]. In a subsequent study from the same group, Rapoport et al created a second-generation formulation using perfluoro-15-crown-5-ether (PFCE) nanodroplets loaded with paclitaxel. These nanoagents showed both ultrasound and fluorine 19F MR contrast properties, allowing for multimodal monitoring of delivery and biodistribution through ultrasonography and MRI in mice [171]. Some challenges still remain for the application of this approach to cancer therapy and imaging, especially arising from the inhomogeneity of nanodroplet distribution within the tumor as a result of non-uniform vascularization. This issue, which can also affect other forms of nanotherapy, seems to be involved in the development of drug resistance in some areas of the tumor [172].
Multimodal approaches combining ultrasound-mediated release or imaging with other diagnostic and therapeutic techniques have also been explored. Watanabe et al reported successful transfection of mice skeletal muscles using ultrasound triggered nanobubbles for gene delivery along with PET for transfection rate monitoring [173]. Xu et al developed PLGA-nanobubble contrast agents encapsulating Texas Red dye that could be used for dual-mode optical and ultrasound imaging [174]. High intensity frequency ultrasound can also be used in thermal ablation and hyperthermia interventions, and several reports have demonstrated a synergistic effect of these approaches with chemotherapy [175, 176].
7.3. Carbon nanotubes
Carbon nanotubes (CNTs) are composed of carbon atoms arranged in hexagonal networks that are approximately 1 nm in diameter and 1–100 µm in length [177]. CNTs can be single-walled or multi-walled, have large electrical and thermal conductivities, and can be used in multifunctional applications, including photoacoustic imaging [178], biosensing and cancer cell detection [179], drug delivery [180], and photothermal therapy [181].
In drug delivery applications, CNTs are able to enter cells and even cell nuclei thanks to their small size, and they can be functionalized with different moieties in their inner and outer surfaces for targeting and conjugation [177]. Liu et al reported that single-walled CNTs conjugated to the chemotherapy drug paclitaxel showed 10 times higher tumor uptake than for the free drug in a murine 4T1 breast cancer model [180].
CNTs have also been used as localized "nanobombs" to destroy cancer cells, as described by Panchapakesan et al [181], who create localized explosions of CNTs by exposing them to a 800-nm laser at intensities of 50–200 mW/cm2 in a PBS solution. The nanobomb effect occurs by laser heating of water molecules adsorbed at the surface of the CNTs, which can reach temperatures exceeding 100°C and cause a localized explosion that destroys the nanotubes as well as their host BT474 cancer cells. Surrounding cells not exposed to nanotubes remained viable. Still, the main concern in clinical translation of CNT-based agents is the toxicity profile of these nanomaterials, with some groups reporting marked lung toxicity and asbestos-like pathogenesis in mice [182–184].
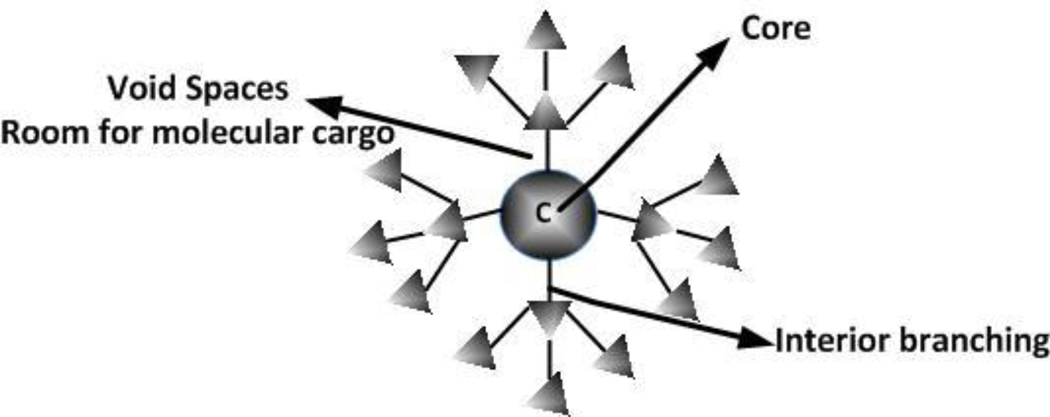
7.4. Dendrimers
Dendrimers (figure 6) are synthetic structures comprised of a core molecule giving rise to highly-branched tree-like extensions that provide a large surface area for functionalization with diverse targeting and task-specific moieties. Dendrimers have monodispersed, tunable nanoparticle sizes. These polymers were first reported in the 1980's by Tomalia and co-workers, who synthesized three-dimensional polyamindoamine (PAMAM) dendrimers containing tertiary amines and amide linkages [185]. Recently, Menjoge et al published a review on dendrimer8 based drugs and imaging conjugates [186]. Dendrimers have been used in imaging applications, boron neutron capture therapy, photodynamic therapy, and as drug delivery systems [187]. Dendrimer-based MRI imaging agents (i.e. Gadomer series) are currently in clinical trials by Bayer Schering Pharma AG [188].
Figure 6.
Schematic representation of a dendrimer.
The versatility of dendrimers makes them an ideal choice for simultaneous delivery of drugs that have very distinct physicochemical properties such as the degree of hydrophilicity. Tekade et al. co-encapsulated methotrexate (a hydrophobic chemotherapeutic agent) and all-trans retinoic acid (a hydrophilic compound with mild anticancer activity) in a generation 5 poly(propyleneimine) dendrimer [189]. Also, conjugation of dendrimer branches with PEG or polyethylene oxide (PEO) can be used to prolong blood circulation times and create stealth delivery platforms. An example is the work of Lee et al [190], who prepared a polyester-based dendrimer–PEO–doxorubicin conjugate that was able to treat DOX-insensitive C-26 tumors subcutaneously implanted in BALB/c mice, with an efficacy comparable to the commercially available liposomal form of DOX, Doxil®. Dendrimers have several advantages for in vivo systemic delivery of cancer drugs, such as increased stability in biological liquids, tumor-specific targeting, and nanoscale size that allows them to cross cell membrane and enter target cells effectively.
A single dendrimer can act as a platform for imaging agents, targeting and recognition molecules that identify cancer cells, multiple therapeutic agents with cytotoxic effects, and molecules that can detect cell death to monitor the effectiveness of treatment [191]. Quintana and colleagues [192] synthesized an ethylenediamine core PAMAM generation 5 dendrimer that was covalently attached to folic acid, fluorescein, and methotrexate. This complex has the potential to be used for targeting, imaging and intracellular drug delivery, and in this particular study it showed 100-fold higher cytotoxicity than free methotrexate. Combinational chemotherapy and siRNA therapy using dendrimers has also been reported [193], and a fifth generation polyamido-amine (PAMAM) dendrimer conjugated to fluorescein isothiocyanate (for imaging) and recombinant Fibroblast Growth Factor-1 (for tumor targeting) was recently engineered to track cell targeting and cellular internalization [194].
Dendrimers can also be used as therapeutic agents instead of carriers, as is the case of the anionic functionalized poly(L-lysine) dendrimer formulation Vivagel®, currently being evaluated in clinical trials for safety and efficacy as a microbicide [195].
7.5. Quantum Dots
Quantum dots (QDs, figure 7) are semiconductor nanocrystals that range from 2 to 10 nm in diameter and are made of elements from group II–VI or III–V. They have broad excitation spectra with narrow and tunable emission spectra, 10–50 times larger molar extinction coefficients than organic dyes, and they are exceptionally photo-chemically stable, which makes them useful in monitoring long-term interactions of multiple-labeled biological molecules within cells. QDs have the potential to be used as sensitive fluorescent probes for screening cancer markers in fluids, as specific labels for classifying tissue biopsies, and as high resolution contrast agents for medical imaging that can detect small tumors. An interesting property of QDs is that depending on their size and chemical composition, their fluorescent emission can be tuned to any wavelength between blue and infrared. As a result, several quantum dots can be visualized concurrently, offering the possibility to use various QDs, each conjugated to a different antibody to target different tumor markers. This can be used in real-time cancer imaging, particularly in the tracking of metastatic tumors [196].
Figure 7.
Schematic representation of a quantum dot
Typically QDs are synthesized in nonpolar organic solvents, which results in a capping of the quantum dots with a monolayer of the nonpolar solvent. In many occasions they need to be soluble in aqueous buffers, so their surface is modified by amphiphilic molecules. Different strategies have been developed to address aqueous solubility, including ligand exchange with simple thiol-containing molecules [197], dendrons [198], peptides [199], and encapsulation by a layer of amphiphilic copolymers. This strategy not only helps to facilitate solubilization, but also provides a linker for bioconjugation of peptides, antibodies, oligonucleotides, or small molecule drugs, hereby multi-functionalizing the QDs for tumor targeting, tumor imaging and drug delivery.
Multifunctional nanoparticles consisting of polymeric micelles encapsulating iron oxide nanoparticles and fluorescent QDs were recently reported as MRI-fluorescent ultrasensitive markers [200]. Gao and coworkers developed a new class of multifunctional probes for simultaneous targeting and imaging of tumors in live animals [201]. They used antibody conjugated QDs to target a prostate-specific membrane antigen, PSMA. Recent work by Derfus et al [202] indicates that CdSe QDs are highly toxic to cultured cells under UV illumination for extended periods of time. For human clinical applications, a major concern is the potential toxicity of QD probes, which has recently become a topic of considerable discussion and debate. One of the main issues is certainly related to long-term safety of nanomaterials, both developed for in vitro and in vivo applications. Recent research advances allow real-time testing of the cytotoxicity of nanoscale materials using whole-cell based electrical impedance measurements [203]. Toxicology tests have to be developed specifically for nanomaterials within a well-defined framework of risk assessment and management.
8. The future of nanoscale cancer theranostics
Nanosize delivery platforms have distinct advantages in cancer therapy, starting with their inherent ability to accumulate at tumor sites due to the EPR effect. More importantly, their versatility provides opportunities for multifunctionalization and creation of "smart particles", so that a single platform can be used to detect tumors, treat them, monitor treatment response, and guide therapeutic regimes. Nanoformulations can be functionalized to minimize clearance by the immune system and prolong circulation times, and they can be targeted to specific cells by the addition of surface ligands that hone in to specific receptors. This allows for enhanced accumulation at tumor sites, where the particles can then provide sustained customizable release of therapeutic agents such as chemotherapy drugs, or be used for other therapy modalities such as hyperthermia. Recent advances in biosensing have allowed the development of nano-based assays that can be used in high-throughput screening and detection of cancer cells and biomarkers, opening new avenues for point-of-care diagnostics. Combinations of diagnostic and therapeutic applications based on nanomaterials allow for holistic patient management approaches. Nanotheranostics have great potential for clinically translatable advances that can positively impact the overall process of cancer diagnosis and management, and result in enhanced quality of life for cancer patients.
Despite these advantages, the design and fabrication of nanoparticles for cancer therapy and diagnosis still present many challenges including biocompatibility, pharmacokinetics, in vivo targeting efficacy, and cost-effectiveness. The optimization of these variables depends on nanoparticle design parameters such as size, shape, surface charge, composition, preparation protocols, decorating moieties, and drug loading and release rate. The most crucial aspect in future development of nano-based medicine will likely be the ability for multifunctionalization and successful engineering and fabrication of multimodal nanotheranostic designs. The ultimate goal will be to maximize the amount of diagnostic information and therapeutic efficacy, minimize the time frame for early diagnosis, and reduce the degree and frequency of invasive interventions. In order to achieve these advances, an important issue that requires further exploration is the long-term safety of nanomaterials, as highlighted by the reports on toxicity of carbon nanotubes and quantum dots that were discussed in this review. A solid framework of protocols for testing nanomaterial safety in vitro and in vivo needs to be developed in order to allow a full assessment of the risk factors derived from the use of nanomaterials, to understand their impact on human health and the environment, and to develop specific regulatory guidelines for manufacturing and safe human use.
Acknowledgments
A.F.F. received support from NIH/NIGMS R25 GM061347.
References
- 1.Ahmedin JD, Siegel R, Xu J, Ward E. A Cancer Journal for Clinics. 2010 [Google Scholar]
- 2.Wang X, Wang Y, Chen ZG, Shin DM. Cancer Res Treat. 2009;41:1–11. doi: 10.4143/crt.2009.41.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehlrich P. Collected Studies on Immunity. New York, NY: John Wiley and Sons, Ltd.; 1906. pp. 404–443. [Google Scholar]
- 4.Kreuter J. J Anat. 1996;189(Pt 3):503–505. [PMC free article] [PubMed] [Google Scholar]
- 5.Breimer DD. Adv Drug Deliv Rev. 1998;33:265–268. doi: 10.1016/s0169-409x(98)00034-9. [DOI] [PubMed] [Google Scholar]
- 6.Brandl M. Biotechnol Annu Rev. 2001;7:59–85. doi: 10.1016/s1387-2656(01)07033-8. [DOI] [PubMed] [Google Scholar]
- 7.Estella-Hermoso de Mendoza A, Campanero MA, Mollinedo F, Blanco-Prieto MJ. J Biomed Nanotechnol. 2009;5:323–343. doi: 10.1166/jbn.2009.1042. [DOI] [PubMed] [Google Scholar]
- 8.Matsumura Y, Hamaguchi T, Ura T, et al. Br J Cancer. 2004;91:1775–1781. doi: 10.1038/sj.bjc.6602204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JH, Jung SW, Kim IS, et al. Int J Pharm. 2003;251:23–32. doi: 10.1016/s0378-5173(02)00582-3. [DOI] [PubMed] [Google Scholar]
- 10.Liu ZH, Jiao YP, Wang YF, et al. Adv Drug Deliv Rev. 2008;60:1650–1662. doi: 10.1016/j.addr.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Xu ZP, Zeng QH, Lu GQ, Yu AB. Chem Eng Sci. 2006;61:1027–1040. [Google Scholar]
- 12.Dakhil S, Ensminger W, Cho K, et al. Cancer. 1982;50:631–635. doi: 10.1002/1097-0142(19820815)50:4<631::aid-cncr2820500403>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 13.Nelken N, Schneider PA. Surg Clin N Am. 2004;84:1203–1236. doi: 10.1016/j.suc.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Gregoriadis G, Leathwood PD, Ryman BE. FEBS Lett. 1971;14:95–99. doi: 10.1016/0014-5793(71)80109-6. [DOI] [PubMed] [Google Scholar]
- 15.Gregoriadis G, Ryman BE. Eur J Biochem. 1972;24:485–491. doi: 10.1111/j.1432-1033.1972.tb19710.x. [DOI] [PubMed] [Google Scholar]
- 16.Banerjee R. J Biomater Appl. 2001;16:3–21. doi: 10.1106/RA7U-1V9C-RV7C-8QXL. [DOI] [PubMed] [Google Scholar]
- 17.Axelsson B. Adv Drug Deliv Rev. 1989;3:391–404. [Google Scholar]
- 18.Bakker-Woudenberg I, Lokerse A, ten Kate M, et al. Eur J Clin Microbiol. 1993;12:S61–S67. doi: 10.1007/BF02389881. [DOI] [PubMed] [Google Scholar]
- 19.Lestini BJ, Sagnella SM, Xu Z, et al. J Control Release. 2002;78:235–247. doi: 10.1016/s0168-3659(01)00505-3. [DOI] [PubMed] [Google Scholar]
- 20.Tarahovsky YS. Biochemistry (Mosc) 2010;75:811–824. doi: 10.1134/s0006297910070023. [DOI] [PubMed] [Google Scholar]
- 21.Strother R, Matei D. Ther Clin Risk Manag. 2009;5:639–650. doi: 10.2147/tcrm.s5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fassas A, Anagnostopoulos A. Leukemia & Lymphoma. 2005;46:795–802. doi: 10.1080/10428190500052438. [DOI] [PubMed] [Google Scholar]
- 23.Sapra P, Tyagi P, Allen TM. Curr Drug Deliv. 2005;2:369–381. doi: 10.2174/156720105774370159. [DOI] [PubMed] [Google Scholar]
- 24.Sarris AH, Hagemeister F, Romaguera J, et al. Ann Oncol. 2000;11:69–72. doi: 10.1023/a:1008348010437. [DOI] [PubMed] [Google Scholar]
- 25.Chhikara BS, Parang K. Expert Opin Drug Deliv. 2010;7:1399–1414. doi: 10.1517/17425247.2010.527330. [DOI] [PubMed] [Google Scholar]
- 26.Cattel L, Ceruti M, Dosio F. Tumori. 2003;89:237–249. doi: 10.1177/030089160308900302. [DOI] [PubMed] [Google Scholar]
- 27.Salzberg M, Thurlimann B, Bonnefois H, et al. Oncology. 2005;68:293–298. doi: 10.1159/000086967. [DOI] [PubMed] [Google Scholar]
- 28.Cheung TW, Remick SC, Azarnia N, et al. Clin Cancer Res. 1999;5:3432–3437. [PubMed] [Google Scholar]
- 29.Koukourakis MI, Koukouraki S, Giatromanolaki A, et al. J Clin Oncol. 1999;17:3512–3521. doi: 10.1200/JCO.1999.17.11.3512. [DOI] [PubMed] [Google Scholar]
- 30.Caponigro F, Comella P, Budillon A, et al. Ann Oncol. 2000;11:339–342. doi: 10.1023/a:1008319618638. [DOI] [PubMed] [Google Scholar]
- 31.Roerdink F, Dijkstra J, Hartman G, et al. BBA Gen Subjects. 1981;677:79–89. doi: 10.1016/0304-4165(81)90148-3. [DOI] [PubMed] [Google Scholar]
- 32.Blume G, Cevc G, Crommelin MDJA, et al. BBA Rev Biomembranes. 1993;1149:180–184. doi: 10.1016/0005-2736(93)90039-3. [DOI] [PubMed] [Google Scholar]
- 33.Mori A, Klibanov AL, Torchilin VP, Huang L. FEBS Letters. 1991;284:263–266. doi: 10.1016/0014-5793(91)80699-4. [DOI] [PubMed] [Google Scholar]
- 34.Allen TM. Adv Drug Deliv Rev. 1994;13:285–309. [Google Scholar]
- 35.Burger JJ, Tomlinson E, Mulder EMA, McVie JG. Int J Pharm. 1985;23:333–344. [Google Scholar]
- 36.Anderson JM, Shive MS. Adv Drug Deliv Rev. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 37.Birrenbach GSP. J Pharm Sci. 1976;65:1763. doi: 10.1002/jps.2600651217. [DOI] [PubMed] [Google Scholar]
- 38.Avgoustakis K. Curr Drug Deliv. 2004;1:321–333. doi: 10.2174/1567201043334605. [DOI] [PubMed] [Google Scholar]
- 39.Fahmy TM, Fong PM, Goyal A, Saltzman WM. Materials Today. 2005;8:18–26. [Google Scholar]
- 40.Chung YI, Kim JC, Kim YH, et al. J Control Release. 2010;143:374–382. doi: 10.1016/j.jconrel.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 41.Nicolas J, Couvreur P. Wiley Interdisciplinary Reviews-Nanomedicine and Nanobiotechnology. 2009;1:111–127. doi: 10.1002/wnan.15. [DOI] [PubMed] [Google Scholar]
- 42.Kumari A, Yadav SK, Yadav SC. Colloid Surface B. 2009;75:1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Nagpal K, Singh SK, Mishra DN. Chem Pharm Bull (Tokyo) 2010;58:1423–1430. doi: 10.1248/cpb.58.1423. [DOI] [PubMed] [Google Scholar]
- 44.Jayakumar R, Prabaharan M, Nair SV, Tamura H. Biotechnol Adv. 2010;28:142–150. doi: 10.1016/j.biotechadv.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Dash M, Chiellini F, Ottenbrite R, Chiellini E. Progress in Polymer Science. 2011;36:981–1014. [Google Scholar]
- 46.Corsi K, Chellat F, Yahia L, Fernandes JC. Biomaterials. 2003;24:1255–1264. doi: 10.1016/s0142-9612(02)00507-0. [DOI] [PubMed] [Google Scholar]
- 47.Erbacher P, Zou S, Bettinger T, et al. Pharm Res. 1998;15:1332–1339. doi: 10.1023/a:1011981000671. [DOI] [PubMed] [Google Scholar]
- 48.Kumar M, Behera AK, Lockey RF, et al. Hum Gene Ther. 2002;13:1415–1425. doi: 10.1089/10430340260185058. [DOI] [PubMed] [Google Scholar]
- 49.Kievit FM, Veiseh O, Bhattarai N, et al. Adv Funct Mater. 2009;19:2244–2251. doi: 10.1002/adfm.200801844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tripathi SK, Goyal R, Kumar P, Gupta KC. Nanomedicine. 2011 Jul 12; [Epub ahead of print] [Google Scholar]
- 51.Aktas Y, Andrieux K, Alonso MJ, et al. Int J Pharm. 2005;298:378–383. doi: 10.1016/j.ijpharm.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 52.Karatas H, Aktas Y, Gursoy-Ozdemir Y, et al. J Neurosci. 2009;29:13761–13769. doi: 10.1523/JNEUROSCI.4246-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Son YJ, Jang JS, Cho YW, et al. J Control Release. 2003;91:135–145. doi: 10.1016/s0168-3659(03)00231-1. [DOI] [PubMed] [Google Scholar]
- 54.Mitra S, Gaur U, Ghosh PC, Maitra AN. J Control Release. 2001;74:317–323. doi: 10.1016/s0168-3659(01)00342-x. [DOI] [PubMed] [Google Scholar]
- 55.Janes KA, Fresneau MP, Marazuela A, et al. J Control Release. 2001;73:255–267. doi: 10.1016/s0168-3659(01)00294-2. [DOI] [PubMed] [Google Scholar]
- 56.Lee E, Lee J, Lee IH, et al. J Med Chem. 2008;51:6442–6449. doi: 10.1021/jm800767c. [DOI] [PubMed] [Google Scholar]
- 57.Min KH, Park K, Kim YS, et al. J Control Release. 2008;127:208–218. doi: 10.1016/j.jconrel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 58.Hwang HY, Kim IS, Kwon IC, Kim YH. J Control Release. 2008;128:23–31. doi: 10.1016/j.jconrel.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 59.Ohya Y, Takei T, Kobayashi H, Ouchi T. J Microencapsul. 1993;10:1–9. doi: 10.3109/02652049309015307. [DOI] [PubMed] [Google Scholar]
- 60.Wang X, Li Y, Du Y. J Nanosci Nanotechnol. 2009;9:6866–6875. doi: 10.1166/jnn.2009.1889. [DOI] [PubMed] [Google Scholar]
- 61.Cheong SJ, Lee CM, Kim SL, et al. Int J Pharm. 2009;372:169–176. doi: 10.1016/j.ijpharm.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 62.Nam T, Park S, Lee SY, et al. Bioconjug Chem. 2010;21:578–582. doi: 10.1021/bc900408z. [DOI] [PubMed] [Google Scholar]
- 63.Baldrick P. Regul Toxicol Pharm. 2010;56:290–299. doi: 10.1016/j.yrtph.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 64.Acharya S, Sahoo SK. Adv Drug Deliv Rev. 2010 doi: 10.1016/j.addr.2010.10.008. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 65.Yoo HS, Lee KH, Oh JE, Park TG. J Control Release. 2000;68:419–431. doi: 10.1016/s0168-3659(00)00280-7. [DOI] [PubMed] [Google Scholar]
- 66.Park J, Fong PM, Lu J, et al. Nanomedicine. 2009;5:410–418. doi: 10.1016/j.nano.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhardwaj V, Ankola DD, Gupta SC, et al. Pharm Res. 2009;26:2495–2503. doi: 10.1007/s11095-009-9965-4. [DOI] [PubMed] [Google Scholar]
- 68.Gradishar WJ,TS, Davidson N, Shaw H, Desai N, Bhar P, Hawkins M, O'Shaughnessy J. J Clin Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 69.Reynolds C, Barrera D, Jotte R, et al. J Thorac Oncol. 2009;4:1537–1543. doi: 10.1097/JTO.0b013e3181c0a2f4. [DOI] [PubMed] [Google Scholar]
- 70.Nyman DW, Campbell KJ, Hersh E, et al. J Clin Oncol. 2005;23:7785–7793. doi: 10.1200/JCO.2004.00.6148. [DOI] [PubMed] [Google Scholar]
- 71.Tang Y, Lei T, Manchanda R, et al. Pharm Res. 2010;27:2242–2253. doi: 10.1007/s11095-010-0231-6. [DOI] [PubMed] [Google Scholar]
- 72.Song XR, Cai Z, Zheng Y, et al. Eur J Pharm Sci. 2009;37:300–305. doi: 10.1016/j.ejps.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 73.Agueros M, Areses P, Campanero MA, et al. Eur J Pharm Sci. 2009;37:231–240. doi: 10.1016/j.ejps.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 74.Balthasar S, Michaelis K, Dinauer N, et al. Biomaterials. 2005;26:2723–2732. doi: 10.1016/j.biomaterials.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 75.Cheng FY, Su CH, Wu PC, Yeh CS. Chem Commun (Camb) 2010;46:3167–3169. doi: 10.1039/b919172k. [DOI] [PubMed] [Google Scholar]
- 76.Cho HS, Dong Z, Pauletti GM, et al. ACS Nano. 2010;4:5398–5404. doi: 10.1021/nn101000e. [DOI] [PubMed] [Google Scholar]
- 77.Dai H, Jiang X, Tan GC, et al. Int J Nanomed. 2006;1:507–522. doi: 10.2147/nano.2006.1.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Danhier F, Vroman B, Lecouturier N, et al. J Control Release. 2009;140:166–173. doi: 10.1016/j.jconrel.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 79.Derakhshandeh K, Soheili M, Dadashzadeh S, Saghiri R. Int J Nanomed. 2010;5:463–471. doi: 10.2147/ijn.s11586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.ElBayoumi TA, Torchilin VP. Clin Cancer Res. 2009;15:1973–1980. doi: 10.1158/1078-0432.CCR-08-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huh MS, Lee SY, Park S, et al. J Control Release. 2010;144:134–143. doi: 10.1016/j.jconrel.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 82.Jain AK, Swarnakar NK, Godugu C, et al. Biomaterials. 2011;32:503–515. doi: 10.1016/j.biomaterials.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 83.Jiang X, Dai H, Leong KW, et al. J Gene Med. 2006;8:477–487. doi: 10.1002/jgm.868. [DOI] [PubMed] [Google Scholar]
- 84.Kim K, Kim JH, Park H, et al. J Control Release. 2010;146:219–227. doi: 10.1016/j.jconrel.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 85.Kocbek P, Obermajer N, Cegnar M, et al. J Control Release. 2007;120:18–26. doi: 10.1016/j.jconrel.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 86.Lee SM, Park H, Yoo KH. Adv Mater. 2010;22:4049–4053. doi: 10.1002/adma.201001040. [DOI] [PubMed] [Google Scholar]
- 87.Liu Y, Li K, Liu B, Feng SS. Biomaterials. 2010;31:9145–9155. doi: 10.1016/j.biomaterials.2010.08.053. [DOI] [PubMed] [Google Scholar]
- 88.Misra R, Sahoo SK. Eur J Pharm Sci. 2010;39:152–163. doi: 10.1016/j.ejps.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 89.Musumeci T, Ventura CA, Giannone I, et al. Int J Pharm. 2006;325:172–179. doi: 10.1016/j.ijpharm.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 90.Pille JY, Li H, Blot E, et al. Hum Gene Ther. 2006;17:1019–1026. doi: 10.1089/hum.2006.17.1019. [DOI] [PubMed] [Google Scholar]
- 91.Qu J, Liu G, Wang Y, Hong R. Advanced Powder Technology. 2010;21:461–467. [Google Scholar]
- 92.Sahana B, Santra K, Basu S, Mukherjee B. Int J Nanomedicine. 2010;5:621–630. doi: 10.2147/IJN.S9962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saxena V, Sadoqi M, Shao J. Int J Pharm. 2006;308:200–204. doi: 10.1016/j.ijpharm.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 94.Wang H, Zhao P, Su W, et al. Biomaterials. 2010;31:8741–8748. doi: 10.1016/j.biomaterials.2010.07.082. [DOI] [PubMed] [Google Scholar]
- 95.Wang J, Tao X, Zhang Y, et al. Biomaterials. 2010;31:4426–4433. doi: 10.1016/j.biomaterials.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 96.Wu P, He X, Wang K, et al. J Biomed Nanotechnol. 2009;5:557–564. doi: 10.1166/jbn.2009.1073. [DOI] [PubMed] [Google Scholar]
- 97.Yallapu MM, Gupta BK, Jaggi M, Chauhan SC. J Colloid Interface Sci. 2010;351:19–29. doi: 10.1016/j.jcis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 98.Maeda H, Wu J, Sawa T, et al. Journal of Controlled Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 99.Maeda H, Bharate GY, Daruwalla J. Eur J Pharm Biopharm. 2009;71:409–419. doi: 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 100.Hall JB, Dobrovolskaia MA, Patri AK, McNeil SE. Nanomedicine. 2007;2:789–803. doi: 10.2217/17435889.2.6.789. [DOI] [PubMed] [Google Scholar]
- 101.Seymour LW, Miyamoto Y, Maeda H, et al. Eur J Cancer. 1995;31:766–770. doi: 10.1016/0959-8049(94)00514-6. [DOI] [PubMed] [Google Scholar]
- 102.Tolcher AW, Sugarman S, Gelmon KA, et al. J Clin Oncol. 1999;17:478–484. doi: 10.1200/JCO.1999.17.2.478. [DOI] [PubMed] [Google Scholar]
- 103.Brennan FR, Shaw L, Wing MG, Robinson C. Mol Biotechnol. 2004;27:59–74. doi: 10.1385/MB:27:1:59. [DOI] [PubMed] [Google Scholar]
- 104.Chang HR. Cancer. 2010;116:2856–2867. doi: 10.1002/cncr.25120. [DOI] [PubMed] [Google Scholar]
- 105.Glass B, Ziepert M, Reiser M, et al. Ann Oncol. 2010;21:2255–2261. doi: 10.1093/annonc/mdq235. [DOI] [PubMed] [Google Scholar]
- 106.Yoon DH, Choi DR, Ahn HJ, et al. Eur J Haematol. 2010;85:149–157. doi: 10.1111/j.1600-0609.2010.01467.x. [DOI] [PubMed] [Google Scholar]
- 107.Frampton JE, Keating GM. Biodrugs. 2008;22:113–120. doi: 10.2165/00063030-200822020-00004. [DOI] [PubMed] [Google Scholar]
- 108.Kanwar JR, Mahidhara G, Kanwar RK. Drug Discov Today. 2011;16:188–202. doi: 10.1016/j.drudis.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 109.Lee JF, Hesselberth JR, Meyers LA, Ellington AD. Nucleic Acids Res. 2004;32:D95–D100. doi: 10.1093/nar/gkh094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ruckman JGL, Beeson J, et al. J Biol Chem. 1998;273:20556–20567. doi: 10.1074/jbc.273.32.20556. [DOI] [PubMed] [Google Scholar]
- 111.Kourlas H, Schiller DS. Clin Ther. 2006;28:36–44. doi: 10.1016/j.clinthera.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 112.Farokhzad OC, Cheng JJ, Teply BA, et al. P Natl Acad Sci USA. 2006;103:6315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Toffoli G, Cernigoi C, Russo A, et al. Int J Cancer. 1997;74:193–198. doi: 10.1002/(sici)1097-0215(19970422)74:2<193::aid-ijc10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 114.Parker N, Turk MJ, Westrick E, et al. Anal Biochem. 2005;338:284–293. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 115.Park JW, Hong KL, Kirpotin DB, et al. Clin Cancer Res. 2002;8:1172–1181. [PubMed] [Google Scholar]
- 116.Lei T, Srinivasan S, Tang Y, et al. Nanomed Nanotechnol. 2011;7:324–332. doi: 10.1016/j.nano.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Agrawal V, Paul MK, Mukhopadhyay AK. J Liposome Res. 2005;15:141–155. doi: 10.1080/08982100500364081. [DOI] [PubMed] [Google Scholar]
- 118.Song XR, Zheng Y, He G, et al. J Pharm Sci. 2010;99:4874–4879. doi: 10.1002/jps.22200. [DOI] [PubMed] [Google Scholar]
- 119.Soma CE, Dubernet C, Bentolila D, et al. Biomaterials. 2000;21:1–7. doi: 10.1016/s0142-9612(99)00125-8. [DOI] [PubMed] [Google Scholar]
- 120.Fan L, Li F, Zhang HT, et al. Biomaterials. 2010;31:5634–5642. doi: 10.1016/j.biomaterials.2010.03.066. [DOI] [PubMed] [Google Scholar]
- 121.Tardi P, Johnstone S, Harasyrn N, et al. Leukemia Res. 2009;33:129–139. doi: 10.1016/j.leukres.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 122.Harasym TO, Tardi PG, Harasym NL, et al. Oncol Res. 2007;16:361–374. doi: 10.3727/000000006783980937. [DOI] [PubMed] [Google Scholar]
- 123.Guo J, Zhou J, Ying X, et al. J Pharm Pharmac Sci. 2010;13:136–151. doi: 10.18433/j3p88z. [DOI] [PubMed] [Google Scholar]
- 124.Jiang JA, Yang SJ, Wang JC, et al. Eur J Pharm Biopharm. 2010;76:170–178. doi: 10.1016/j.ejpb.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 125.Zhang L, Radovic-Moreno AF, Alexis F, et al. ChemMedChem. 2007;2:1268–1271. doi: 10.1002/cmdc.200700121. [DOI] [PubMed] [Google Scholar]
- 126.Fayette M, Soria JC, Armand JP. Eur J Cancer. 2005;41:1109–1116. doi: 10.1016/j.ejca.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 127.Emanuel S, Gruninger RH, Fuentes-Pesquera A, et al. Mol Pharm. 2004;66:635–647. doi: 10.1124/mol.104.000638. [DOI] [PubMed] [Google Scholar]
- 128.Ma J, Waxman DJ. Mol Cancer Ther. 2008;7:3670–3684. doi: 10.1158/1535-7163.MCT-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Couzin J. Science. 2002;296:2314–2315. doi: 10.1126/science.296.5577.2314b. [DOI] [PubMed] [Google Scholar]
- 130.Sengupta S, Eavarone D, Capila I, et al. Nature. 2005;436:568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- 131.Liu F, Shollenberger LM, Huang L. Faseb Journal. 2004;18:1779–1781. doi: 10.1096/fj.04-2187fje. [DOI] [PubMed] [Google Scholar]
- 132.Wang Y, Gao SJ, Ye WH, et al. Nature Materials. 2006;5:791–796. doi: 10.1038/nmat1737. [DOI] [PubMed] [Google Scholar]
- 133.Wang H, Zhao P, Su W, et al. Biomaterials. 2010;31:8741–8748. doi: 10.1016/j.biomaterials.2010.07.082. [DOI] [PubMed] [Google Scholar]
- 134.Devi GR. Cancer Gene Ther. 2006;13:819–829. doi: 10.1038/sj.cgt.7700931. [DOI] [PubMed] [Google Scholar]
- 135.Zhu C, Jung S, Luo S, et al. Biomaterials. 2006;31:2408–2416. doi: 10.1016/j.biomaterials.2009.11.077. [DOI] [PubMed] [Google Scholar]
- 136.Chen YC, Bathula SR, Li J, Huang L. J Biol Chem. 2010;285:22639–22650. doi: 10.1074/jbc.M110.125906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen YC, Wu JZJ, Huang L. Mol Ther. 2010;18:828–834. doi: 10.1038/mt.2009.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Saad M, Garbuzenko OB, Minko T. Nanomedicine. 2008;3:761–776. doi: 10.2217/17435889.3.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sun TM, Du JZ, Yao YD, et al. Acs Nano. 2011 doi: 10.1021/nn103349h. [DOI] [PubMed] [Google Scholar]
- 140.Minelli C, Lowe SB, Stevens MM. Small. 2010;6:2336–2357. doi: 10.1002/smll.201000523. [DOI] [PubMed] [Google Scholar]
- 141.Grubisha DS, Lipert RJ, Park HY, et al. Anal Chem. 2003;75:5936–5943. doi: 10.1021/ac034356f. [DOI] [PubMed] [Google Scholar]
- 142.Gerion D, Chen F, Kannan B, et al. Anal Chem. 2003;75:4766–4772. doi: 10.1021/ac034482j. [DOI] [PubMed] [Google Scholar]
- 143.Xia Z, Xing Y, So MK, et al. Anal Chem. 2008;80:8649–8655. doi: 10.1021/ac801562f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Asami Y, Oishi J, Kitazaki H, et al. Anal Biochem. 2011 doi: 10.1016/j.ab.2011.06.041. In press, [Epub July 6] [DOI] [PubMed] [Google Scholar]
- 145.Hill HD, Mirkin CA. Nat Protoc. 2006;1:324–336. doi: 10.1038/nprot.2006.51. [DOI] [PubMed] [Google Scholar]
- 146.Stoeva SI, Lee JS, Smith JE, et al. J Am Chem Soc. 2006;128:8378–8379. doi: 10.1021/ja0613106. [DOI] [PubMed] [Google Scholar]
- 147.Simpson CR, Kohl M, Essenpreis M, Cope M. Phys Med Biol. 1998;43:2465–2478. doi: 10.1088/0031-9155/43/9/003. [DOI] [PubMed] [Google Scholar]
- 148.Weissleder R, Tung CH, Mahmood U, Bogdanov A. Nature Biotechnol. 1999;17:375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 149.Lee HY, Li Z, Chen K, et al. J Nucl Med. 2008;49:1371–1379. doi: 10.2967/jnumed.108.051243. [DOI] [PubMed] [Google Scholar]
- 150.Kang KW. Open Nucl Med J. 2010;2:153–156. [Google Scholar]
- 151.Yang J, Lee CH, Ko HJ, et al. Angew Chem Int Ed Engl. 2007;46:8836–8839. doi: 10.1002/anie.200703554. [DOI] [PubMed] [Google Scholar]
- 152.Park K, Lee S, Kang E, et al. Adv Funct Mat. 2009;19:1553–1566. [Google Scholar]
- 153.Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Lasers Med Sci. 2008;23:217–228. doi: 10.1007/s10103-007-0470-x. [DOI] [PubMed] [Google Scholar]
- 154.Huff TB, Hansen MN, Zhao Y, et al. Langmuir. 2007;23:1596–1599. doi: 10.1021/la062642r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Oyelere AK, Chen PC, Huang XH, et al. Bioconjug Chem. 2007;18:1490–1497. doi: 10.1021/bc070132i. [DOI] [PubMed] [Google Scholar]
- 156.von Maltzahn G, Centrone A, Park JH, et al. Adv Mater. 2009;21:3175–3180. doi: 10.1002/adma.200803464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li CZ, Male KB, Hrapovic S, Luong JH. Chem Commun (Camb) 2005:3924–3926. doi: 10.1039/b504186d. [DOI] [PubMed] [Google Scholar]
- 158.Ann-Ann D, Ying-Yi C, Wang CC, et al. HER-2 Antibody Conjugated Gold Nano Rod for in Vivo Photothermal Therapy. 8th IEEE conference on Nanotechnology NANO '08; 2008. pp. 882–885. [Google Scholar]
- 159.Park H, Yang J, Lee J, et al. Acs Nano. 2009;3:2919–2926. doi: 10.1021/nn900215k. [DOI] [PubMed] [Google Scholar]
- 160.Wust P, Gneveckow U, Johannsen M, et al. Int J Hyperther. 2006;22:673–685. doi: 10.1080/02656730601106037. [DOI] [PubMed] [Google Scholar]
- 161.Day ES, Morton JG, West JL. J Biomech Eng. 2009;131 doi: 10.1115/1.3156800. 074001. [DOI] [PubMed] [Google Scholar]
- 162.Dorshow RB, Bugaj JE, Burleigh BD. J Biomed Opt. 1998;3:340–345. doi: 10.1117/1.429854. [DOI] [PubMed] [Google Scholar]
- 163.Johansen PL. Eur. Heart J. 1990;11 Suppl I:6–12. [Google Scholar]
- 164.O'Neal DP, Hirsch LR, Halas NJ, et al. Cancer Lett. 2004;209:171–176. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 165.Hirsch LR, Stafford RJ, Bankson JA, et al. Proc Natl Acad Sci USA. 2003;100:13549–13554. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fernandez-Fernandez A, Manchanda R, Lei T, et al. Mol Imaging. 2011 In Press. [PubMed] [Google Scholar]
- 167.Tang Y, Lei T, Manchanda R, et al. Pharm Res. 2010;27:2242–2253. doi: 10.1007/s11095-010-0231-6. [DOI] [PubMed] [Google Scholar]
- 168.Manchanda R, Fernandez-Fernandez A, Nagesetti A, McGoron AJ. Colloid Surface B. 2010;75:260–267. doi: 10.1016/j.colsurfb.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 169.Wang Y, Li X, Zhou Y, et al. Int J Pharm. 2010;384:148–153. doi: 10.1016/j.ijpharm.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 170.Gao Z, Kennedy AM, Christensen DA, Rapoport NY. Ultrasonics. 2008;48:260–270. doi: 10.1016/j.ultras.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rapoport N, Nam KH, Gupta R, et al. J Control Release. 2011;153:4–15. doi: 10.1016/j.jconrel.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rapoport N, Kennedy AM, Shea JE, et al. Mol Pharm. 2010;7:22–31. doi: 10.1021/mp900128x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Watanabe Y, Horie S, Funaki Y, et al. J Nucl Med. 2010;51:951–958. doi: 10.2967/jnumed.109.074443. [DOI] [PubMed] [Google Scholar]
- 174.Xu JS, Huang J, Qin R, et al. Biomaterials. 2010;31:1716–1722. doi: 10.1016/j.biomaterials.2009.11.052. [DOI] [PubMed] [Google Scholar]
- 175.Paparel P, Curiel L, Chesnais S, et al. BJU Int. 2005;95:881–885. doi: 10.1111/j.1464-410X.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- 176.Prat F, Chapelon JY, el Fadil FA, et al. Br J Cancer. 1993;68:13–17. doi: 10.1038/bjc.1993.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sinha N, Yeow JT. IEEE Trans Nanobioscience. 2005;4:180–195. doi: 10.1109/tnb.2005.850478. [DOI] [PubMed] [Google Scholar]
- 178.De La Zerda A, Zavaleta C, Keren S, et al. Nat Nanotechnol. 2008;3:557–562. doi: 10.1038/nnano.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Panchapakesan B, Cesarone G, Teker K, Wickstrom E. Nano Biotechnol. 2006;1:353–360. [Google Scholar]
- 180.Liu Z, Chen K, Davis C, et al. Cancer Res. 2008;68:6652–6660. doi: 10.1158/0008-5472.CAN-08-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Panchapakesan B, Lu S, Sivakumar K, et al. Nano Biotechnol. 2005;1:133–140. [Google Scholar]
- 182.Poland CA, Duffin R, Kinloch I, et al. Nat Nanotechnol. 2008;3:423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 183.Shvedova AA, Kisin ER, Porter D, et al. Pharmacol Ther. 2009;121:192–204. doi: 10.1016/j.pharmthera.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 184.Park EJ, Roh J, Kim SN, et al. Arch Toxicol. 2011;85:1121–1131. doi: 10.1007/s00204-011-0655-8. [DOI] [PubMed] [Google Scholar]
- 185.Tomalia DA, Baker H, Dewald J, et al. Macromolecules. 1986;19:2466–2468. [Google Scholar]
- 186.Menjoge AR, Kannan RM, Tomalia DA. Drug Discov Today. 2010;15:171–185. doi: 10.1016/j.drudis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 187.Zhu SJ, Hong MH, Tang GT, et al. Biomaterials. 2009;31:1360–1371. doi: 10.1016/j.biomaterials.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 188.Gerretsen SC, Versluis B, Bekkers SCAM, Leiner T. Eur J Radiol. 2008;65:80–85. doi: 10.1016/j.ejrad.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 189.Tekade RK, Dutta T, Gajbhiye V, Jain NK. J Microencapsul. 2009;26:287–296. doi: 10.1080/02652040802312572. [DOI] [PubMed] [Google Scholar]
- 190.Lee CC, Gillies ER, Fox ME, et al. Proc Natl Acad Sci USA. 2006;103:16649–16654. doi: 10.1073/pnas.0607705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kobayashi H, Brechbiel MW. Mol Imaging. 2003;2:1–10. doi: 10.1162/15353500200303100. [DOI] [PubMed] [Google Scholar]
- 192.Quintana A, Raczka E, Piehler L, et al. Pharm Res. 2002;19:1310–1316. doi: 10.1023/a:1020398624602. [DOI] [PubMed] [Google Scholar]
- 193.Kaneshiro TL, Lu ZR. Biomaterials. 2009;30:5660–5666. doi: 10.1016/j.biomaterials.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 194.Thomas TP, Shukla R, Kotlyar A, et al. Bioorg Med Chem Lett. 2010;20:700–703. doi: 10.1016/j.bmcl.2009.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rupp R, Rosenthal SL, Stanberry LR. Int J Nanomedicine. 2007;2:561–566. [PMC free article] [PubMed] [Google Scholar]
- 196.Voura EB, Jaiswal JK, Mattoussi H, Simon SM. Nat Med. 2004;10:993–998. doi: 10.1038/nm1096. [DOI] [PubMed] [Google Scholar]
- 197.Chan WC, Nie S. Science. 1998;281:2016–2018. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- 198.Guo W, Li JJ, Wang YA, Peng X. J Am Chem Soc. 2003;125:3901–3909. doi: 10.1021/ja028469c. [DOI] [PubMed] [Google Scholar]
- 199.Pinaud F, King D, Moore HP, Weiss S. J Am Chem Soc. 2004;126:6115–6123. doi: 10.1021/ja031691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Park JH, von Maltzahn G, Ruoslahti E, et al. Angew Chem Int Ed Engl. 2008;47:7284–7288. doi: 10.1002/anie.200801810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gao X, Cui Y, Levenson RM, et al. Nat Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 202.Derfus AM, Chan WCW, Bhatia SN. Nano Lett. 2004;4:11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hondroulis E, Liu C, Li CZ. Nanotechnology. 2010;21:315103. doi: 10.1088/0957-4484/21/31/315103. [DOI] [PubMed] [Google Scholar]