Abstract
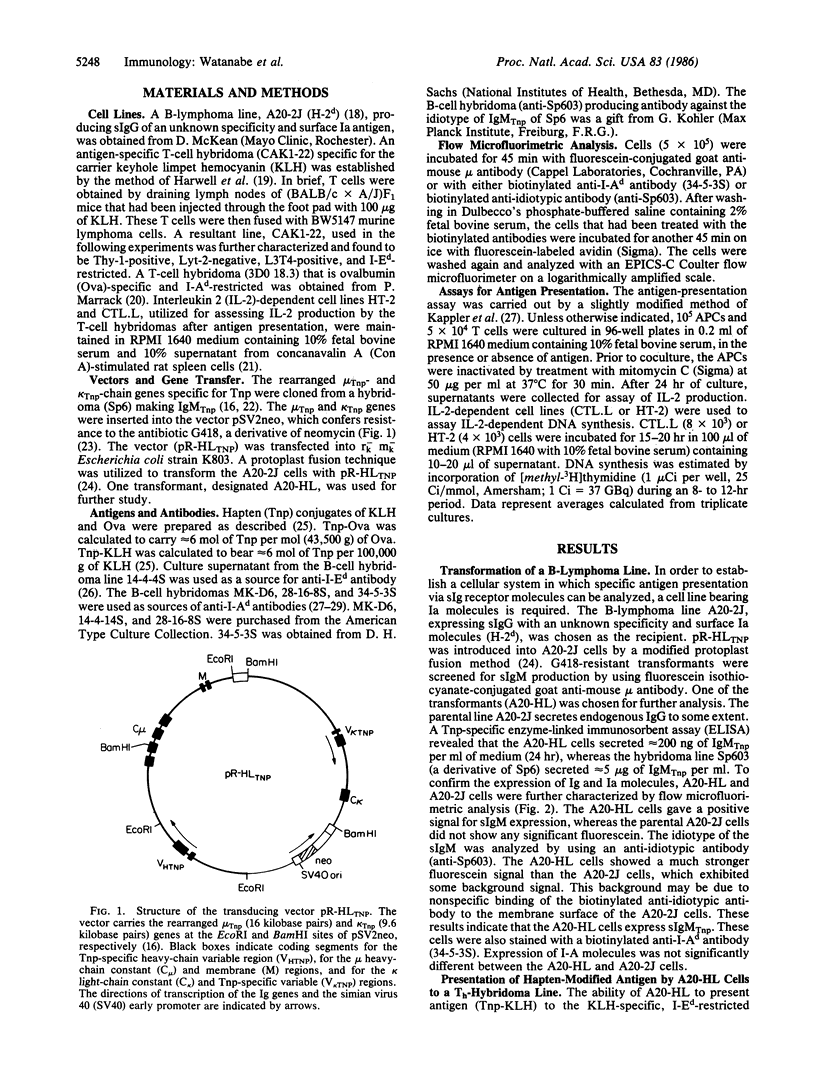
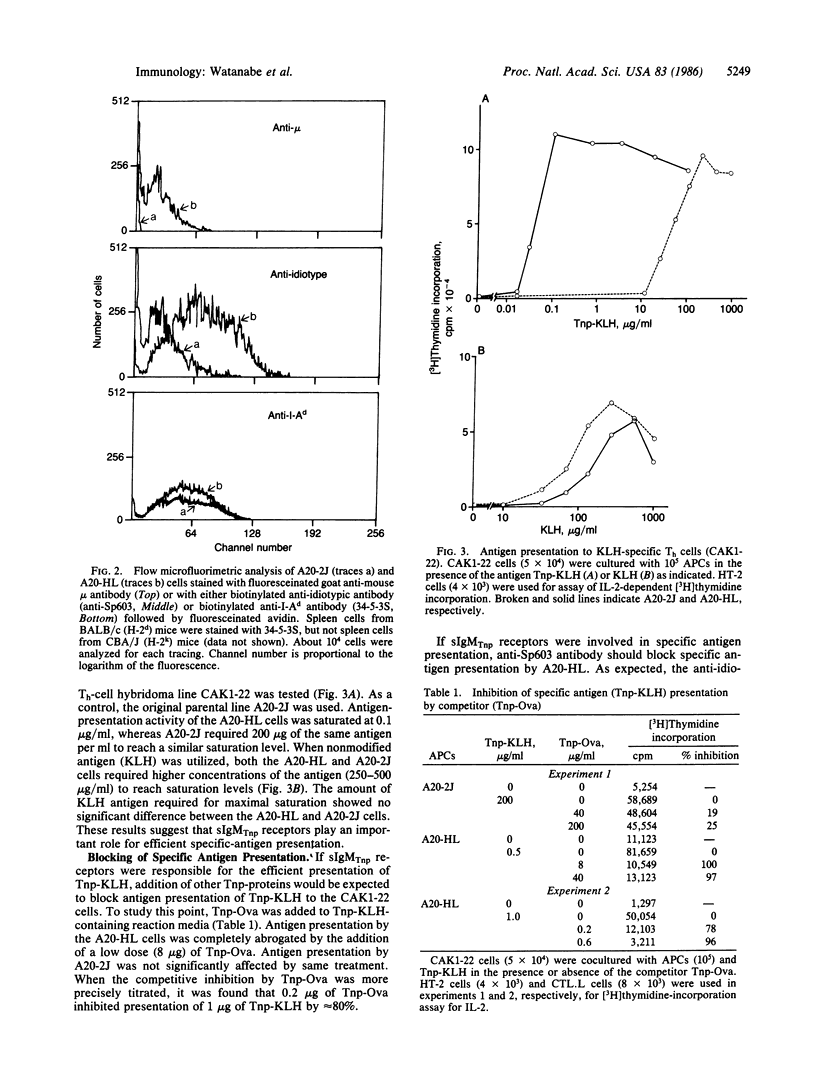
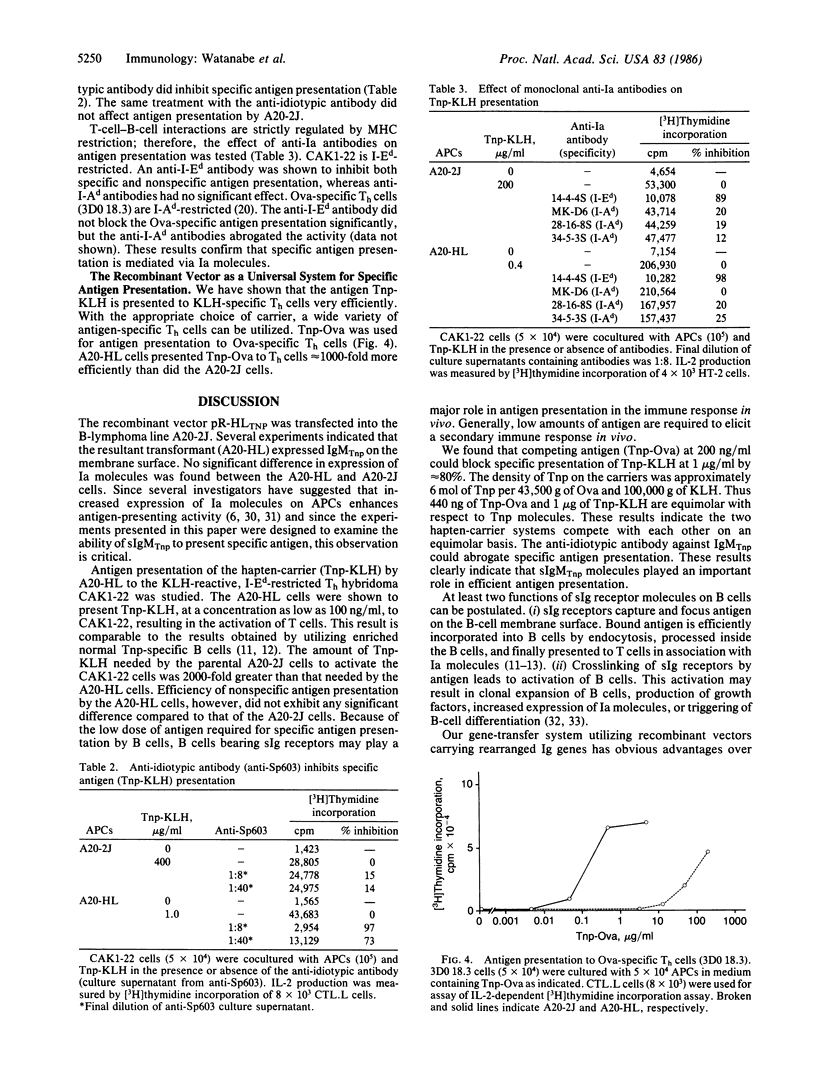
The rearranged genes encoding immunoglobulin heavy (mu) and light (kappa) chains specific for the hapten 2,4,6-trinitrophenyl (Tnp) were introduced into a B-lymphoma line that bears surface IgG with an unknown specificity and expresses surface Ia molecules. A transformant expressing surface IgM specific for Tnp was obtained. The transformant was found to present Tnp-proteins to antigen (protein)-specific T cells far more efficiently than the parenteral B-lymphoma line. This artificial system, utilizing recombinant DNA technology and gene transfer, provides several approaches for the study of T-cell-B-cell interactions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbas A. K., Haber S., Rock K. L. Antigen presentation by hapten-specific B lymphocytes. II. Specificity and properties of antigen-presenting B lymphocytes, and function of immunoglobulin receptors. J Immunol. 1985 Sep;135(3):1661–1667. [PubMed] [Google Scholar]
- Ashwell J. D., DeFranco A. L., Paul W. E., Schwartz R. H. Antigen presentation by resting B cells. Radiosensitivity of the antigen-presentation function and two distinct pathways of T cell activation. J Exp Med. 1984 Mar 1;159(3):881–905. doi: 10.1084/jem.159.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkhoucha F., Naquet P., Pierres A., Marchetto S., Pierres M. Efficiency of antigen presentation to T cell clones by (B cell X B cell lymphoma) hybridomas correlates quantitatively with cell surface ia antigen expression. Eur J Immunol. 1984 Sep;14(9):807–814. doi: 10.1002/eji.1830140908. [DOI] [PubMed] [Google Scholar]
- Beller D. I. Functional significance of the regulation of macrophage Ia expression. Eur J Immunol. 1984 Feb;14(2):138–143. doi: 10.1002/eji.1830140207. [DOI] [PubMed] [Google Scholar]
- Chesnut R. W., Colon S. M., Grey H. M. Requirements for the processing of antigens by antigen-presenting B cells. I. Functional comparison of B cell tumors and macrophages. J Immunol. 1982 Dec;129(6):2382–2388. [PubMed] [Google Scholar]
- Chesnut R. W., Grey H. M. Studies on the capacity of B cells to serve as antigen-presenting cells. J Immunol. 1981 Mar;126(3):1075–1079. [PubMed] [Google Scholar]
- Corley R. B., LoCascio N. J., Ovnic M., Haughton G. Two separate functions of class II (Ia) molecules: T-cell stimulation and B-cell excitation. Proc Natl Acad Sci U S A. 1985 Jan;82(2):516–520. doi: 10.1073/pnas.82.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb P., Feldmann M. The role of macrophages in the generation of T-helper cells. II. The genetic control of the macrophage-T-cell interaction for helper cell induction with soluble antigens. J Exp Med. 1975 Aug 1;142(2):460–472. doi: 10.1084/jem.142.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathman C. G., Frelinger J. G. T-lymphocyte clones. Annu Rev Immunol. 1983;1:633–655. doi: 10.1146/annurev.iy.01.040183.003221. [DOI] [PubMed] [Google Scholar]
- Harwell L., Skidmore B., Marrack P., Kappler J. Concanavalin A-inducible, interleukin-2-producing T cell hybridoma. J Exp Med. 1980 Oct 1;152(4):893–904. doi: 10.1084/jem.152.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins K., Kappler J., Marrack P. The major histocompatibility complex-restricted antigen receptor on T cells. Annu Rev Immunol. 1984;2:51–66. doi: 10.1146/annurev.iy.02.040184.000411. [DOI] [PubMed] [Google Scholar]
- Hawley R. G., Shulman M. J., Murialdo H., Gibson D. M., Hozumi N. Mutant immunoglobulin genes have repetitive DNA elements inserted into their intervening sequences. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7425–7429. doi: 10.1073/pnas.79.23.7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakiuchi T., Chesnut R. W., Grey H. M. B cells as antigen-presenting cells: the requirement for B cell activation. J Immunol. 1983 Jul;131(1):109–114. [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. H., Unanue E. R. Critical role of determinant presentation in the induction of specific responses in immunocompetent lymphocytes. J Exp Med. 1973 Apr 1;137(4):967–990. doi: 10.1084/jem.137.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985 Apr 11;314(6011):537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- McKean D. J., Infante A. J., Nilson A., Kimoto M., Fathman C. G., Walker E., Warner N. Major histocompatibility complex-restricted antigen presentation to antigen-reactive T cells by B lymphocyte tumor cells. J Exp Med. 1981 Nov 1;154(5):1419–1431. doi: 10.1084/jem.154.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe J. G., Cambier J. C. B cell activation. III. B cell plasma membrane depolarization and hyper-Ia antigen expression induced by receptor immunoglobulin cross-linking are coupled. J Exp Med. 1983 Nov 1;158(5):1589–1599. doi: 10.1084/jem.158.5.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi A., Hawley R. G., Hawley T., Shulman M. J., Traunecker A., Köhler G., Hozumi N. Functional immunoglobulin M production after transfection of cloned immunoglobulin heavy and light chain genes into lymphoid cells. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6351–6355. doi: 10.1073/pnas.80.20.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi A., Hawley R. G., Shulman M. J., Hozumi N. Transfer of a cloned immunoglobulin light-chain gene to mutant hybridoma cells restores specific antibody production. Nature. 1983 Mar 24;302(5906):340–342. doi: 10.1038/302340a0. [DOI] [PubMed] [Google Scholar]
- Ochi A., Hozumi N. Enhanced transcription in a pre-B-like cell line transformed with cloned antibody genes after antigen-specific stimulation. J Immunol. 1986 Apr 1;136(7):2705–2708. [PubMed] [Google Scholar]
- Ozato K., Mayer N. M., Sachs D. H. Monoclonal antibodies to mouse major histocompatibility complex antigens. Transplantation. 1982 Sep;34(3):113–120. doi: 10.1097/00007890-198209000-00001. [DOI] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Ozato K., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981 Jan;126(1):317–321. [PubMed] [Google Scholar]
- Rock K. L., Benacerraf B., Abbas A. K. Antigen presentation by hapten-specific B lymphocytes. I. Role of surface immunoglobulin receptors. J Exp Med. 1984 Oct 1;160(4):1102–1113. doi: 10.1084/jem.160.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Benacerraf B. MHC-restricted T cell activation: analysis with T cell hybridomas. Immunol Rev. 1983;76:29–57. doi: 10.1111/j.1600-065x.1983.tb01096.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. S., Shevach E. M. Function of macrophages in antigen recognition by guinea pig T lymphocytes. I. Requirement for histocompatible macrophages and lymphocytes. J Exp Med. 1973 Nov 1;138(5):1194–1212. doi: 10.1084/jem.138.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Sunshine G. H., Katz D. R., Feldmann M. Dendritic cells induce T cell proliferation to synthetic antigens under Ir gene control. J Exp Med. 1980 Dec 1;152(6):1817–1822. doi: 10.1084/jem.152.6.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swierkosz J. E., Rock K., Marrack P., Kappler J. W. The role of H-2 linked genes in helper T-cell function. II. Isolation on antigen-pulsed macrophages of two separate populations of F1 helper T cells each specific for antigen and one set of parental H-2 products. J Exp Med. 1978 Feb 1;147(2):554–570. doi: 10.1084/jem.147.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]



