Abstract
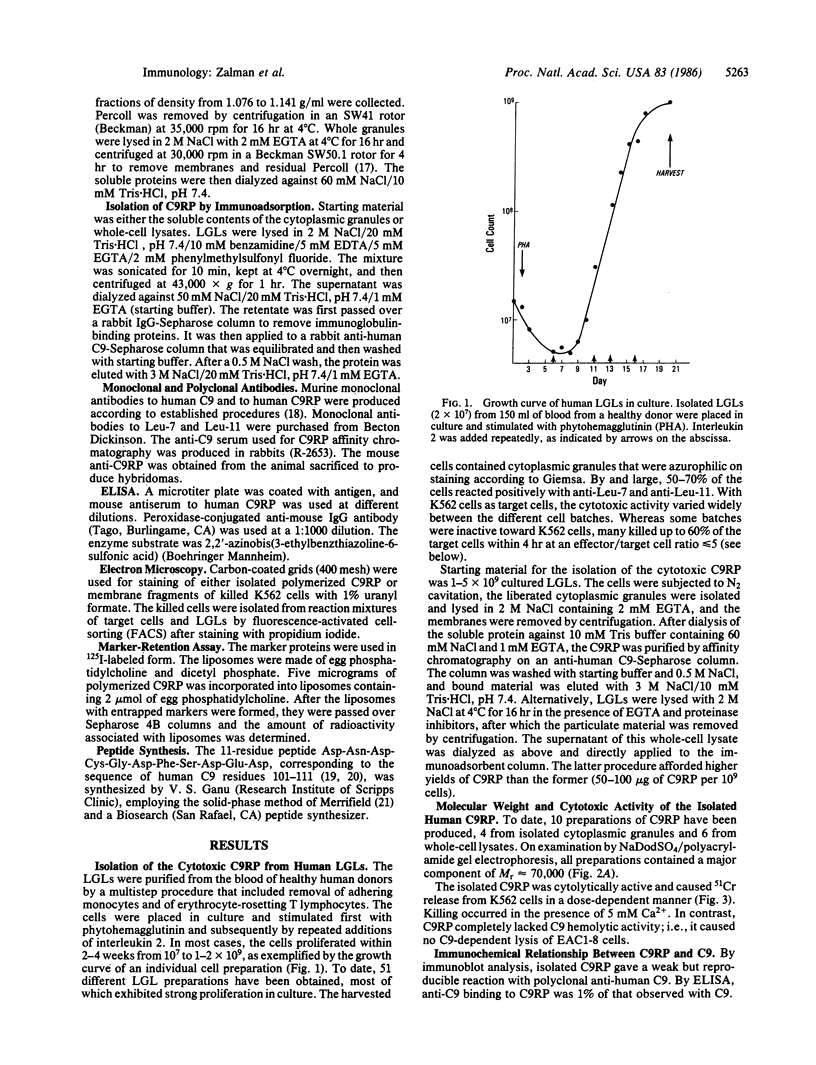
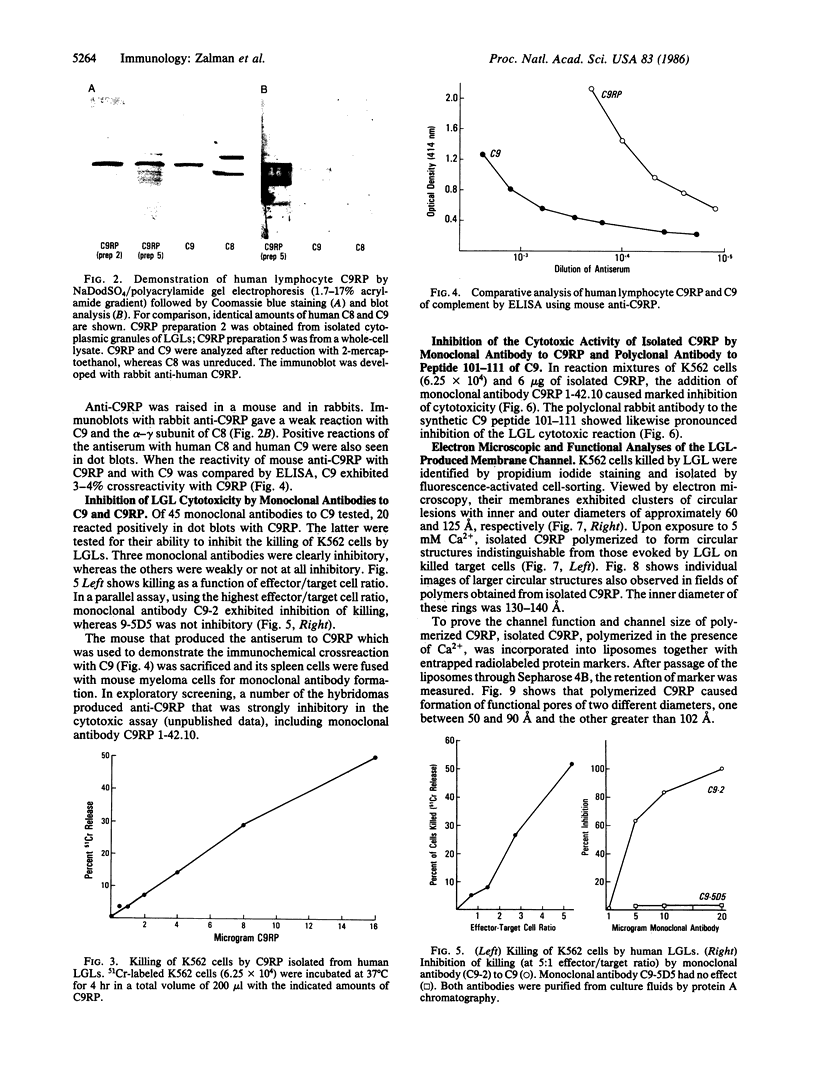
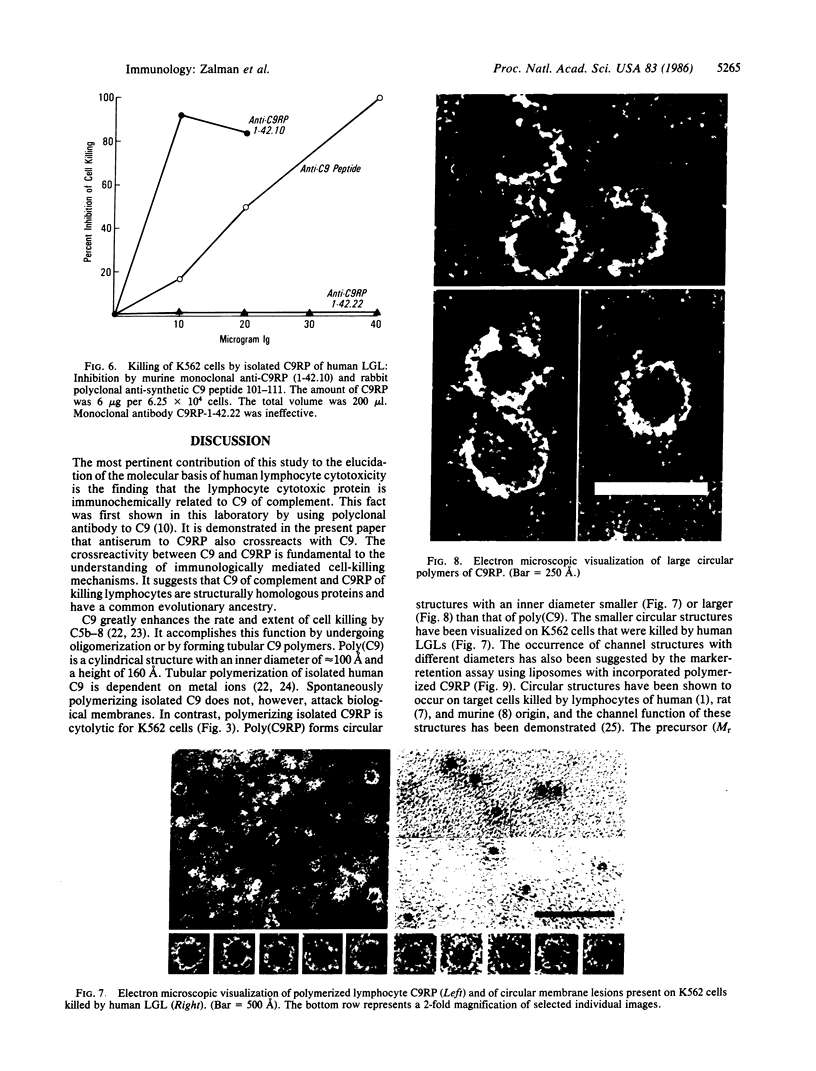
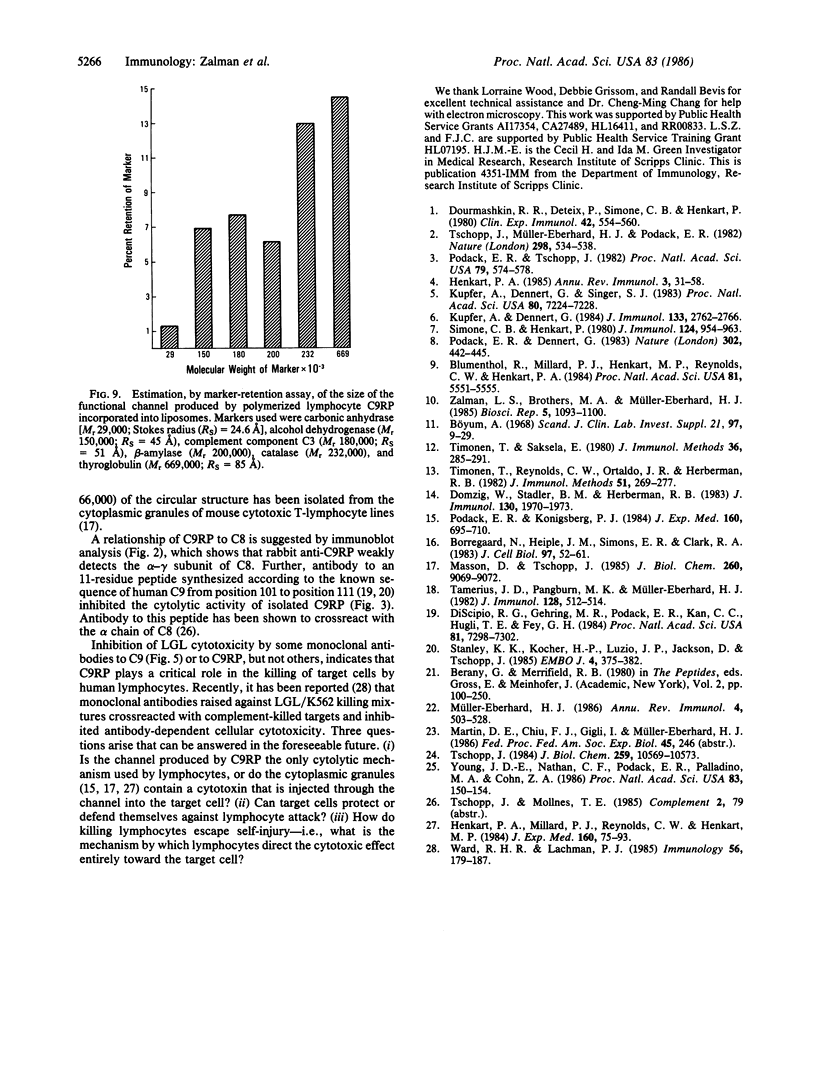
A Mr 70,000 protein was isolated from cytotoxic human large granular lymphocytes and shown to have cytotoxic activity. The protein was demonstrated to be immunochemically related to the ninth component (C9) of complement and was therefore designated C9-related protein (C9RP). This finding suggests that C9RP and C9 share homology in primary structure and have a common evolutionary ancestry. C9RP was isolated, by affinity chromatography employing anti-human C9-Sepharose, from either purified cytoplasmic granules or whole-cell lysates of cultured human large granular lymphocytes. The cells were isolated from healthy blood donors and maintained in interleukin-2-dependent cultures. The immunochemical crossreactivity of C9 with C9RP was 3-4%, using a murine anti-C9RP antiserum. Certain murine monoclonal antibodies to C9RP and to C9 inhibited killing of K562 cells by human large granular lymphocytes. Killed target cells, identified by propidium iodide staining and isolated by fluorescence-activated cell-sorting, exhibited clusters of circular membrane lesions that resembled poly(C9) in appearance. Polymerization of isolated C9RP in the presence of Ca2+ resulted in the formation of two different circular structures, one having an inner diameter of approximately equal to 60 A, and the other, of 125 A. Polymerized C9RP could be incorporated into liposomes and, as such, gave rise to channels of two different sizes. The smaller channel had a functional diameter of 50-90 A, and the bigger channel, a diameter greater than 102 A.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenthal R., Millard P. J., Henkart M. P., Reynolds C. W., Henkart P. A. Liposomes as targets for granule cytolysin from cytotoxic large granular lymphocyte tumors. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5551–5555. doi: 10.1073/pnas.81.17.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiScipio R. G., Gehring M. R., Podack E. R., Kan C. C., Hugli T. E., Fey G. H. Nucleotide sequence of cDNA and derived amino acid sequence of human complement component C9. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7298–7302. doi: 10.1073/pnas.81.23.7298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domzig W., Stadler B. M., Herberman R. B. Interleukin 2 dependence of human natural killer (NK) cell activity. J Immunol. 1983 Apr;130(4):1970–1973. [PubMed] [Google Scholar]
- Dourmashkin R. R., Deteix P., Simone C. B., Henkart P. Electron microscopic demonstration of lesions in target cell membranes associated with antibody-dependent cellular cytotoxicity. Clin Exp Immunol. 1980 Dec;42(3):554–560. [PMC free article] [PubMed] [Google Scholar]
- Henkart P. A. Mechanism of lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 1985;3:31–58. doi: 10.1146/annurev.iy.03.040185.000335. [DOI] [PubMed] [Google Scholar]
- Henkart P. A., Millard P. J., Reynolds C. W., Henkart M. P. Cytolytic activity of purified cytoplasmic granules from cytotoxic rat large granular lymphocyte tumors. J Exp Med. 1984 Jul 1;160(1):75–93. doi: 10.1084/jem.160.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Dennert G. Reorientation of the microtubule-organizing center and the Golgi apparatus in cloned cytotoxic lymphocytes triggered by binding to lysable target cells. J Immunol. 1984 Nov;133(5):2762–2766. [PubMed] [Google Scholar]
- Kupfer A., Dennert G., Singer S. J. Polarization of the Golgi apparatus and the microtubule-organizing center within cloned natural killer cells bound to their targets. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7224–7228. doi: 10.1073/pnas.80.23.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson D., Tschopp J. Isolation of a lytic, pore-forming protein (perforin) from cytolytic T-lymphocytes. J Biol Chem. 1985 Aug 5;260(16):9069–9072. [PubMed] [Google Scholar]
- Müller-Eberhard H. J. The membrane attack complex of complement. Annu Rev Immunol. 1986;4:503–528. doi: 10.1146/annurev.iy.04.040186.002443. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Dennert G. Assembly of two types of tubules with putative cytolytic function by cloned natural killer cells. 1983 Mar 31-Apr 6Nature. 302(5907):442–445. doi: 10.1038/302442a0. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Konigsberg P. J. Cytolytic T cell granules. Isolation, structural, biochemical, and functional characterization. J Exp Med. 1984 Sep 1;160(3):695–710. doi: 10.1084/jem.160.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Tschopp J. Polymerization of the ninth component of complement (C9): formation of poly(C9) with a tubular ultrastructure resembling the membrane attack complex of complement. Proc Natl Acad Sci U S A. 1982 Jan;79(2):574–578. doi: 10.1073/pnas.79.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone C. B., Henkart P. Permeability changes induced in erthrocyte ghost targets by antibody-dependent cytotoxic effector cells: evidence for membrane pores. J Immunol. 1980 Feb;124(2):954–963. [PubMed] [Google Scholar]
- Stanley K. K., Kocher H. P., Luzio J. P., Jackson P., Tschopp J. The sequence and topology of human complement component C9. EMBO J. 1985 Feb;4(2):375–382. doi: 10.1002/j.1460-2075.1985.tb03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamerius J. D., Pangburn M. K., Müller-Eberhard H. J. Selective inhibition of functional sites of cell-bound C3b by hybridoma-derived antibodies. J Immunol. 1982 Jan;128(1):512–514. [PubMed] [Google Scholar]
- Timonen T., Reynolds C. W., Ortaldo J. R., Herberman R. B. Isolation of human and rat natural killer cells. J Immunol Methods. 1982;51(3):269–277. doi: 10.1016/0022-1759(82)90393-3. [DOI] [PubMed] [Google Scholar]
- Timonen T., Saksela E. Isolation of human NK cells by density gradient centrifugation. J Immunol Methods. 1980;36(3-4):285–291. doi: 10.1016/0022-1759(80)90133-7. [DOI] [PubMed] [Google Scholar]
- Tschopp J. Circular polymerization of the membranolytic ninth component of complement. Dependence on metal ions. J Biol Chem. 1984 Aug 25;259(16):10569–10573. [PubMed] [Google Scholar]
- Tschopp J., Müller-Eberhard H. J., Podack E. R. Formation of transmembrane tubules by spontaneous polymerization of the hydrophilic complement protein C9. Nature. 1982 Aug 5;298(5874):534–538. doi: 10.1038/298534a0. [DOI] [PubMed] [Google Scholar]
- Ward R. H., Lachmann P. J. Monoclonal antibodies which react with lymphocyte-lysed target cells and which cross-react with complement-lysed ghosts. Immunology. 1985 Sep;56(1):179–188. [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Nathan C. F., Podack E. R., Palladino M. A., Cohn Z. A. Functional channel formation associated with cytotoxic T-cell granules. Proc Natl Acad Sci U S A. 1986 Jan;83(1):150–154. doi: 10.1073/pnas.83.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalman L. S., Brothers M. A., Müller-Eberhard H. J. A C9 related channel forming protein in the cytoplasmic granules of human large granular lymphocytes. Biosci Rep. 1985 Dec;5(12):1093–1100. doi: 10.1007/BF01119631. [DOI] [PubMed] [Google Scholar]