Abstract
Rheumatoid arthritis (RA) is a chronic inflammatory disease affecting synovial joints. Therapies blocking tumor necrosis factor-alpha (TNFα) are now routinely used in the management of RA. However, a significant number of patients with RA do not respond or develop resistance to anti-TNF therapies, and the participation of other cytokines in RA pathogenesis has been reported as well. Lymphotoxin alpha (LTα) is the closest homolog to TNFα and has been implicated in inflammation and autoimmunity since its original description in 1968. In spite of that, little is known about the role of LTα in RA or the potential of blocking this cytokine as an alternative therapeutic approach. In this review, we aim to summarize the general features of LTα and what is currently known about its participation in RA.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease affecting synovial joints. A hallmark of RA is the pseudotumoral expansion of fibroblast-like synoviocytes (FLSs), which invade and destroy the joint. Tumor necrosis factor-alpha (TNFα) plays a major role in promoting RA, and blocking this cytokine is effective for treating patients with RA [1]. However, a significant number of patients do not respond or become resistant to anti-TNF therapies; approximately 50% of the patients still receive anti-TNFs 5 years after the start of treatment [2]. The participation of other cytokines in RA has also been reported and could explain the absence of response to anti-TNFs. Often, patients treated with anti-TNFs show secondary effects such as recurrent infections [3]. Therefore, it is important to define additional therapeutic strategies in order to better control synovial inflammation and joint destruction observed in RA. Although lymphotoxin alpha (LTα) has been associated with autoimmune and inflammatory diseases and is the closest homolog to TNFα, few data pointing to a role for LTα in RA are available [4-10]. In this review, we aim to summarize the general features of LTα and what at present is known about its role in RA.
Lymphotoxin alpha in general
LTα, formerly known as TNFβ, was originally described in 1968 as a cytotoxic factor produced by T lymphocytes after antigenic or mitogenic stimulation [11]. Later on, in 1984, human LTα was purified from a B-lymphoblastoid cell line [12,13] and its structure was determined by classic protein- sequencing methods, making LTα the first member of the TNF superfamily to be characterized [14]. TNFα was subsequently purified, and sequence comparison and receptor competition experiments revealed that these two proteins were homologous [15,16]. Indeed, LTα is the closest homolog to TNFα.
LTα and TNFα are 30% homologous in their primary amino acid sequence, but of greater significance is the observation that the regions of major sequence homology indicated a similarity in their tertiary and quaternary structures [15]. LTα is structurally similar to TNFα: LTα is a soluble homotrimer composed of 17-kDa monomers and binds to and signals specifically through TNF receptors 1 and 2 (TNFR1 and TNFR2) to exert its biological activities.
Although LTα and TNFα have many similarities, there are some distinct molecular and biological differences [17,18]. Like TNFα, LTα binds with high affinity to TNFR1 and TNFR2 [19]. However, the N-terminus of LTα, unlike that of TNFα, resembles a traditional signal peptide, making its conversion to a soluble form extremely efficient. Thus, LTα is never found at the cell surface, a unique feature among the TNF superfamily members. LTα is anchored to the cell membrane only in association with membrane-bound LTβ, as LTαβ hetero-trimers [20]. LTαβ is structurally distinct from LTα and comprises two membrane-anchored heterotrimers, the predominant LTα1β2 form and a minor LTα2β1 form, both of which interact with the LTβ receptor (LTβR) [18,21,22]. Besides binding to TNFR1 and TNFR2, LTα binds to HVEM (herpesvirus entry mediator), a receptor discovered as an entry route for herpes simplex virus, but this binding is relatively weak [23].
LTα is expressed by CD4+ T helper type 1 (Th 1) cells, CD8+ cells, natural killer (NK) cells, B cells, and macro-phages [18]. LTα has specific roles in the development and function of the immune system, mainly in lymphoid organ development, organization and maintenance of lymphoid microenvironments, host defense, and inflammation [18]. However, most of the evidence pointing to these roles came from genetically deficient mice and the relevance of LTα in humans is less clear. Moreover, these mice models make it difficult to determine the relative role of LTα in these systems. This is because the LTα gene is closely linked to the TNFα and LTβ genes and targeting the LTα gene can lead to collateral damage to the neighboring genes [24]. Additionally, LTα could some-how control the expression of TNFα and the absence of LTα could interfere with the production of this cytokine. In any case, although LTα was once considered to be redundant to TNFα, the fact that the same cell types express both LTα and TNFα and that knockout mice for either cytokines can manifest different phenotypes suggest that the two cytokines have overlapping and different functions.
In regard to the development of secondary lymphoid organs, it was shown that mice deficient in LTα are completely devoid of peripheral lymphoid tissues, such as Peyer patches (PP) [25]. It has been demonstrated that LTα mediates PP formation through TNFR1 because TNFR-/- mice either lack or have abnormal PP whereas TNFα-/- mice have normal PP [26].
Several studies suggested a role for LTα in host defense against certain infections. Mice deficient in LTα are highly susceptible to Staphylococcus aureus infections [27]. Other studies showed the LTα requirement for granuloma formation and resistance to Mycobacterium, Leishmania, and Plasmodium infections in mice [28-31]. However, whether these functions are mediated by LTα, LTβ, or even TNFα is unclear. The contribution of LTα to host defense was further challenged by recently generated LTα-/- mice showing intact TNFα production, which allows the evaluation of LTα alone, as opposed to the earlier generated LTα-/- mice that showed altered expression of TNFα [32].
LTα has been implicated in inflammation since its initial description. LTα induces inflammation in vivo when expressed under the control of the rat insulin promoter (RIP) at the sites of transgene expression in the pancreas and kidney of RIPLT mice [33], and this occurs even in LTβ-/- mice [34], indicating that LTα alone induces inflammation. Additional data suggesting a proinflammatory role for LTα derive from studies on experimental allergic encephalomyelitis (EAE) and show that myelin basic protein-specific T-cell clones secrete LTα [35] and that LTα-/- mice are resistant to inflammation and clinical signs of EAE whereas LTβ-/- mice can still develop EAE [36]. The mechanisms through which LTα promotes inflammation and lymphoid organ development are still poorly understood. One possibility is the induction of adhesion molecules in endothelial cells. In vitro studies showed that recombinant human LTα induces expression of intercellular adhesion molecule (ICAM) and E-selectin in human endothelial cells [37]. RIPLT mice overexpressing LTα exhibited a high expression of ICAM-1 and vascular cell adhesion molecule-1 in the vasculature of the inflamed pancreas and kidney independently of T or B cell-derived cytokines [38]. LTα could also contribute to inflammation by the induction of chemokines. In this manner, LTα induces the expression of RANTES (regulated upon activation, normal T cell expressed and secreted) and monocyte chemoattractant protein-1 in a murine endothelial cell line [39]. Moreover, LTα contributes to lymphatic vessel functions in steady-state conditions and induces lymphangiogenesis in inflammation through mechanisms yet to be characterized [40].
LTα is required for the differentiation of NK cells and plays a role in the recruitment and antitumor activity of mature NK cells [41-43]. When inoculated subcutaneously with syngeneic B16F10 melanoma cells, LTα-/- mice develop enhanced tumor growth and metastasis in comparison with wild-type littermates. This was associated with a lower number of NK cells and with slower migration of these cells from the bone marrow to peripheral organs [44]. Established, preclinical graft-versus-host disease (GVHD) models showed that LTα contributes to the development of GVHD, the most frequent complication of allogeneic transplantation [45]. Naïve and alloreactive CD4+ T cells secrete soluble LTα after T-cell receptor stimulation. LTα participates in GVHD-mediated epithelial cell apoptosis, target organ damage, and mortality and this is mediated through TNFR1 signaling [45]. These effects were not redundant to TNFα, as GVHD patients treated with TNFRFc, which cross-reacts with and blocks LTα, have outcomes different from those of patients treated with anti-TNFα monoclonal antibody, as do patients with a chronic auto-immune disease such as RA [8].
Lymphotoxin alpha in rheumatoid arthritis
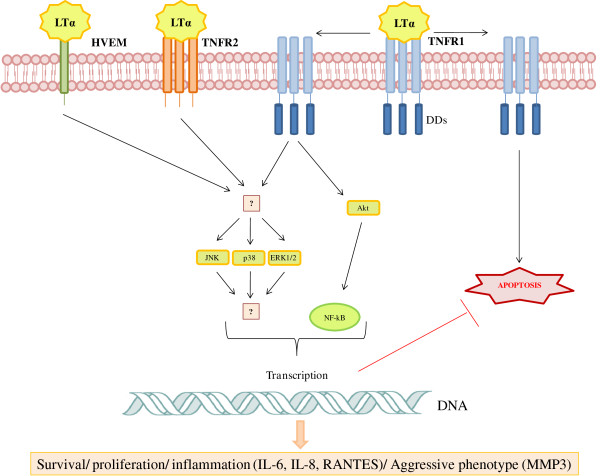
The first reports suggesting a role for LTα in RA came from an analysis in patients with RA by enzyme-linked immunosorbent assay (ELISA), reverse transcription-polymerase chain reaction, and immunohistochemistry. It has been reported that LTα levels are elevated in the serum and the synovial tissue of patients with RA in comparison with the healthy controls or patients with osteoarthritis [6,46]. A relevant piece of evidence linking LTα to RA was provided by a case report describing an RA patient with no beneficial clinical effect after therapy with infliximab, a monoclonal antibody that specifically blocks TNFα. Interestingly, subsequent treatment of this patient with etanercept, a TNFR2-Fc fusion protein that also blocks LTα, resulted in clinical remission of the disease [8]. The different ligand specificities of etanercept and infliximab could account for the different outcomes of this patient after both treatments. Increased LTα expression has been shown in the synovial tissue of this patient [8]. These data, together with the biological similarities between LTα and TNFα, suggest that resis-tance to TNFα blockage may occur when TNFα is not the dominant inflammatory cytokine and that LTα may play a role in the disease. An important advancement in the understanding of the participation of LTα in RA came from a study using the collagen-induced arthritis (CIA) mouse model, the most commonly used animal model for arthritis [47]. In this model, the blocking of LTα with a monoclonal antibody significantly improved the disease [47]. Th e main mechanism responsible for this improvement in the CIA model appears not to be the blocking of soluble LTα but the depletion of LTα expressing Th1 and Th17 cells [47]. Still, the anti-LTα antibody applied in this study also binds to soluble LTα and inhibits its binding to a TNFR2.Ig in a competition ELISA [47]. An example of a dual functionality of an antagonist in RA is the well-established monoclonal antibody infliximab, which binds specifically to TNFα. Besides blocking secreted TNFα, infliximab can activate the complement cascade and deplete membrane-bound TNFα-expressing cells through a cytotoxic mechanism [18]. Recently, our group provided more evidence for a role of LTα in RA when we demonstrated that LTα can trigger activation (that is, proliferation and induction of an inflammatory and aggressive phenotype) of FLSs [48]. The mechanisms through which LTα activates FLSs are depicted in Figure 1, in a proposed model for the action of LTα in RA FLSs. To better evaluate the role of LTα in RA, our group analyzed LTα levels in whole sera, plasma, and synovial fluid of patients with RA, patients with osteoarthritis, and healthy controls. We were unable detect LTα reliably with the commercially available ELISA kits in these samples. However, this does not mean LTα is not expressed locally in joints of patients with RA. While it would be interesting to detect circulating LTα in synovial fluid, it would be equally or even more important to obtain in situ evidence of LTα expression in arthritic tissue, where it might exert effects such as those we reported on synovial fibroblasts.
Figure 1.
Proposed model for the action of lymphotoxin alpha (LTα) in rheumatoid arthritis (RA) fibroblast-like synoviocytes (FLSs). RA FLSs express all LTα receptors (TNFR1, TNFR2, and HVEM). TNFR1 contains a cytoplasmic death domain (DD). Although the specific contribution of each receptor for LTα signaling remains to be clarified, RA FLSs are activated upon LTα binding through the phosphorylation of the mitogen-activated protein kinases p38 and ERK1/2 and of the phosphatidylinositol 3-kinase (PI3K) Akt. Transcription factors such as nuclear factor-kappa-B (NF-κB), in turn, are activated. These events lead to cell responses involved in the pathogenesis of RA, such as proliferation, survival, and secretion of proinflammatory cytokines, chemokines, and matrix metalloproteinases (MMPs). Based on [48]. ERK, extracellular signal-regulated kinase; HVEM, herpesvirus entry mediator; IL, interleukin; JNK, c-jun N-terminal kinase; RANTES, regulated upon activation, normal T cell expressed and secreted; TNFR, tumor necrosis factor receptor.
Conclusions
TNFα is known to play a crucial role in RA, but several other proinflammatory cytokines have been identified to contribute to the disease as well [49]. LTα can easily be placed in the context of the RA synovium as it is secreted by CD4+ Th1 cells, CD8+ T cells, NK cells, and macrophages, cell types that are increased in the arthritic joint. The fact that LTα activates RA FLSs and thus may contribute to synovial hyperplasia suggests that LTα can also play a disease-promoting role in RA [48]. It will be important to further characterize the relevance of LTα in RA by detecting it in vivo in patients with RA.
Abbreviations
CIA: collagen-induced arthritis; EAE: experimental allergic encephalomyelitis; ELISA: enzyme-linked immunosorbent assay; FLS: fibroblast-like synoviocyte; GVHD: graft-versus-host disease; ICAM: intercellular adhesion molecule; LTα: lymphotoxin alpha; NK: natural killer; PP: Peyer patches; RA: rheumatoid arthritis; RIP: rat insulin promoter; RIPLT: rat insulin promoter lymphotoxin; Th: T helper; TNF: tumor necrosis factor; TNFR: tumor necrosis factor receptor.
Competing interests
Wyeth as part of Pfizer participate in the funding of a project on the effect of anti-TNF (soluble receptor and monoclonal antibodies) on LTa in rheumatoid arthritis
Contributor Information
Flavia Calmon-Hamaty, Email: flavia.calmon-hamaty@igmm.cnrs.fr.
Bernard Combe, Email: b-combe@chu-montpellier.fr.
Michael Hahne, Email: hahne@igmm.cnrs.fr.
Jacques Morel, Email: j-morel@chu-montpellier.fr.
Acknowledgements
We thank Wyeth (now part of Pfizer Inc, New York, NY, USA) and GERIR (Groupe d'Etudes et de Recherches Immuno-Rhumatologiques, Montpellier, France) for their financial support. This study was supported by contract interface number 05.524-DRV/MC/SS.
References
- Arend WP, Dayer JM. Cytokines and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum. 1990;33(Suppl 3):305–315. doi: 10.1002/art.1780330302. [DOI] [PubMed] [Google Scholar]
- Soliman MM, Ashcroft DM, Watson KD, Lunt M, Symmons DP, Hyrich KL. on behalf of the British Society for Rheumatology Biologics Register. Impact of concomitant use of DMARDs on the persistence with anti-TNF therapies in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70:583–589. doi: 10.1136/ard.2010.139774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen NJ, Stein CM. New drugs for rheumatoid arthritis. N Engl J Med. 2004;350(Suppl 21):2167–2179. doi: 10.1056/NEJMra032906. [DOI] [PubMed] [Google Scholar]
- Saxne T, Palladino MA Jr, Heinegård D, Talal N, Wollheim FA. Detection of tumor necrosis factor alpha but not tumor necrosis factor beta in rheumatoid arthritis synovial fluid and serum. Arthritis Rheum. 1988;31(Suppl 8):1041–1045. doi: 10.1002/art.1780310816. [DOI] [PubMed] [Google Scholar]
- Brennan FM, Chantry D, Jackson AM, Maini RN, Feldmann M. Cytokine production in culture by cells isolated from the synovial membrane. J Autoimmun. 1989;2(Suppl):177–186. doi: 10.1016/0896-8411(89)90129-7. [DOI] [PubMed] [Google Scholar]
- Robak T, Gladalska A, Stepień H. The tumour necrosis factor family of receptors/ligands in the serum of patients with rheumatoid arthritis. Eur Cytokine Netw. 1998;9(Suppl 2):145–154. [PubMed] [Google Scholar]
- Takemura S, Braun A, Crowson C, Kurtin PJ, Cofield RH, O'Fallon WM, Goronzy JJ, Weyand CM. Lymphoid neogenesis in rheumatoid synovitis. J Immunol. 2001;167(Suppl 2):1072–1080. doi: 10.4049/jimmunol.167.2.1072. [DOI] [PubMed] [Google Scholar]
- Buch MH, Conaghan PG, Quinn MA, Bingham SJ, Veale D, Emery P. True infliximab resistance in rheumatoid arthritis: a role for lymphotoxin alpha? Ann Rheum Dis. 2004;63(Suppl 10):1344–1346. doi: 10.1136/ard.2003.014878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudbrandsdottir S, Bliddal H, Petri A, Terslev L, Danneskiold-Samsoe B, Bjørnhart B, Bendtzen K, Müller K. Plasma TNF binding capacity profiles during treatment with etanercept in rheumatoid arthritis. Scand J Rheumatol. 2004;33(Suppl 6):385–388. doi: 10.1080/03009740410000921. [DOI] [PubMed] [Google Scholar]
- Laivoranta-Nyman S, Möttönen T, Hannonen P, Korpela M, Kautiainen H, Leirisalo-Repo M, Julkunen H, Luukkainen R, Hakala M, Vuori K, Laine AP, Toivanen A, Ilonen J. FIN-RACo Trial Group. Association of tumour necrosis factor a, b and c microsatellite polymorphisms with clinical disease activity and induction of remission in early rheumatoid arthritis. Clin Exp Rheumatol. 2006;24(Suppl 6):636–642. [PubMed] [Google Scholar]
- Williams TW, Granger GA. Lymphocyte in vitro cytotoxicity: lymphotoxins of several mammalian species. Nature. 1968;219(Suppl 5158):1076–1077. doi: 10.1038/2191076a0. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Moffat B, Harkins RN. Human lymphotoxin. Production by a lymphoblastoid cell line, purification, and initial characterization. J Biol Chem. 1984;259(Suppl 1):686–691. [PubMed] [Google Scholar]
- Aggarwal BB, Henzel WJ, Moffat B, Kohr WJ, Harkins RN. Primary structure of human lymphotoxin derived from 1788 lymphoblastoid cell line. J Biol Chem. 1985;260(Suppl 4):2334–2344. [PubMed] [Google Scholar]
- Aggarwal BB, Kohr WJ, Hass PE, Moffat B, Spencer SA, Henzel WJ, Bringman TS, Nedwin GE, Goeddel DV, Harkins RN. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985;260(Suppl 4):2345–2354. [PubMed] [Google Scholar]
- Pennica D, Nedwin GE, Hayflick JS, Seeburg PH, Derynck R, Palladino MA, Kohr WJ, Aggarwal BB, Goeddel DV. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984;312(Suppl 5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Eessalu TE, Hass PE. Characterization of receptors for human tumour necrosis factor and their regulation by gamma-interferon. Nature. 1985;318(Suppl 6047):665–667. doi: 10.1038/318665a0. [DOI] [PubMed] [Google Scholar]
- Gommerman JL, Browning JL. Lymphotoxin/light, lymphoid microenvironments and autoimmune disease. Nat Rev Immunol. 2003;3(Suppl 8):642–655. doi: 10.1038/nri1151. [DOI] [PubMed] [Google Scholar]
- Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005;23:787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- Medvedev AE, Espevik T, Ranges G, Sundan A. Distinct roles of the two tumor necrosis factor (TNF) receptors in modulating TNF and lymphotoxin alpha effects. J Biol Chem. 1996;271(Suppl 16):9778–9784. doi: 10.1074/jbc.271.16.9778. [DOI] [PubMed] [Google Scholar]
- Browning JL, Dougas I, Ngam-ek A, Bourdon PR, Ehrenfels BN, Miatkowski K, Zafari M, Yampaglia AM, Lawton P, Meier W, Benjamin CP, Hession C. Characterization of surface lymphotoxin forms. Use of specific monoclonal antibodies and soluble receptors. J Immunol. 1995;154(Suppl 1):33–46. [PubMed] [Google Scholar]
- Crowe PD, VanArsdale TL, Walter BN, Ware CF, Hession C, Ehrenfels B, Browning JL, Din WS, Goodwin RG, Smith CA. A lymphotoxin-beta-specific receptor. Science. 1994;264(Suppl 5159):707–710. doi: 10.1126/science.8171323. [DOI] [PubMed] [Google Scholar]
- Williams-Abbott L, Walter BN, Cheung TC, Goh CR, Porter AG, Ware CF. The lymphotoxin-alpha (LTalpha) subunit is essential for the assembly, but not for the receptor specificity, of the membrane-anchored LTalpha1beta2 heterotrimeric ligand. J Biol Chem. 1997;272(Suppl 31):19451–19456. doi: 10.1074/jbc.272.31.19451. [DOI] [PubMed] [Google Scholar]
- Mauri DN, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu GL, Ruben S, Murphy M, Eisenberg RJ, Cohen GH, Spear PG, Ware CF. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998;8(Suppl 1):21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- Kuprash DV, Alimzhanov MB, Tumanov AV, Grivennikov SI, Shakhov AN, Drutskaya LN, Marino MW, Turetskaya RL, Anderson AO, Rajewsky K, Pfeffer K, Nedospasov SA. Redundancy in tumor necrosis factor (TNF) and lymphotoxin (LT) signaling in vivo: mice with inactivation of the entire TNF/LT locus versus single-knockout mice. Mol Cell Biol. 2002;22(Suppl 24):8626–8634. doi: 10.1128/MCB.22.24.8626-8634.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YX, Chaplin DD. Development and maturation of secondary lymphoid tissues. Annu Rev Immunol. 1999;17:399–433. doi: 10.1146/annurev.immunol.17.1.399. [DOI] [PubMed] [Google Scholar]
- Neumann B, Luz A, Pfeffer K, Holzmann B. Defective Peyer's patch organogenesis in mice lacking the 55-kD receptor for tumor necrosis factor. J Exp Med. 1996;184(Suppl 1):259–264. doi: 10.1084/jem.184.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgren O, Eugster HP, Sedgwick JD, Körner H, Tarkowski A. TNF/lymphotoxin-alpha double-mutant mice resist septic arthritis but display increased mortality in response to Staphylococcus aureus. J Immunol. 1998;161(Suppl 11):5937–5942. [PubMed] [Google Scholar]
- Roach DR, Briscoe H, Saunders B, France MP, Riminton S, Britton WJ. Secreted lymphotoxin-alpha is essential for the control of an intracellular bacterial infection. J Exp Med. 2001;193(Suppl 2):239–246. doi: 10.1084/jem.193.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers S, Hölscher C, Scheu S, Tertilt C, Hehlgans T, Suwinski J, Endres R, Pfeffer K. The lymphotoxin beta receptor is critically involved in controlling infections with the intracellular pathogens Mycobacterium tuberculosis and Listeria monocytogenes. J Immunol. 2003;170(Suppl 10):5210–5218. doi: 10.4049/jimmunol.170.10.5210. [DOI] [PubMed] [Google Scholar]
- Engwerda CR, Mynott TL, Sawhney S, De Souza JB, Bickle QD, Kaye PM. Locally up-regulated lymphotoxin alpha, not systemic tumor necrosis factor alpha, is the principle mediator of murine cerebral malaria. J Exp Med. 2002;195(Suppl 10):1371–1377. doi: 10.1084/jem.20020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engwerda CR, Ato M, Stäger S, Alexander CE, Stanley AC, Kaye PM. Distinct roles for lymphotoxin-alpha and tumor necrosis factor in the control of Leishmania donovani infection. Am J Pathol. 2004;165(Suppl 6):2123–2133. doi: 10.1016/s0002-9440(10)63262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepinsh DJ, Grivennikov SI, Klarmann KD, Lagarkova MA, Drutskaya MS, Lockett SJ, Tessarollo L, McAuliffe M, Keller JR, Kuprash DV, Nedospasov SA. Novel lymphotoxin alpha (LTalpha) knockout mice with unperturbed tumor necrosis factor expression: reassessing LTalpha biological functions. Mol Cell Biol. 2006;26(Suppl 11):4214–4225. doi: 10.1128/MCB.01751-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picarella DE, Kratz A, Li CB, Ruddle NH, Flavell RA. Insulitis in transgenic mice expressing tumor necrosis factor beta (lymphotoxin) in the pancreas. Proc Natl Acad Sci USA. 1992;89(Suppl 21):10036–10040. doi: 10.1073/pnas.89.21.10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacca R, Cuff CA, Lesslauer W, Ruddle NH. Differential activities of secreted lymphotoxin-alpha3 and membrane lymphotoxin-alpha1beta2 in lymphotoxin-induced inflammation: critical role of TNF receptor 1 signaling. J Immunol. 1998;160(Suppl 1):485–491. [PubMed] [Google Scholar]
- Powell MB, Mitchell D, Lederman J, Buckmeier J, Zamvil SS, Graham M, Ruddle NH, Steinman L. Lymphotoxin and tumor necrosis factor-alpha production by myelin basic protein-specific T cell clones correlates with encephalitogenicity. Int Immunol. 1990;2(Suppl 6):539–544. doi: 10.1093/intimm/2.6.539. [DOI] [PubMed] [Google Scholar]
- Suen WE, Bergman CM, Hjelmström P, Ruddle NH. A critical role for lymphotoxin in experimental allergic encephalomyelitis. J Exp Med. 1997;186(Suppl 8):1233–1240. doi: 10.1084/jem.186.8.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober JS, Lapierre LA, Stolpen AH, Brock TA, Springer TA, Fiers W, Bevilacqua MP, Mendrick DL, Gimbrone MA Jr. Activation of cultured human endothelial cells by recombinant lymphotoxin: comparison with tumor necrosis factor and interleukin 1 species. J Immunol. 1987;138(Suppl 10):3319–3324. [PubMed] [Google Scholar]
- Kratz A, Campos-Neto A, Hanson MS, Ruddle NH. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med. 1996;183(Suppl 4):1461–1472. doi: 10.1084/jem.183.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuff CA, Schwartz J, Bergman CM, Russell KS, Bender JR, Ruddle NH. Lymphotoxin alpha3 induces chemokines and adhesion molecules: insight into the role of LT alpha in inflammation and lymphoid organ development. J Immunol. 1998;161(Suppl 12):6853–6860. [PubMed] [Google Scholar]
- Mounzer RH, Svendsen OS, Baluk P, Bergman CM, Padera TP, Wiig H, Jain RK, McDonald DM, Ruddle NH. Lymphotoxin-alpha contributes to lymphangiogenesis. Blood. 2010;116(Suppl 12):2173–2182. doi: 10.1182/blood-2009-12-256065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka K, Chaplin DD, Wang Y, Wu Q, Pegg LE, Yokoyama WM, Fu YX. Requirement for membrane lymphotoxin in natural killer cell development. Proc Natl Acad Sci USA. 1999;96(Suppl 11):6336–6340. doi: 10.1073/pnas.96.11.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Sun Y, Wang J, Lin X, Wang Y, Pegg LE, Fütterer A, Pfeffer K, Fu YX. Signal via lymphotoxin-beta R on bone marrow stromal cells is required for an early checkpoint of NK cell development. J Immunol. 2001;166(Suppl 3):1684–1689. doi: 10.4049/jimmunol.166.3.1684. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Johnstone RW, Cretney E, Haynes NM, Sedgwick JD, Korner H, Poulton LD, Baxter AG. Multiple deficiencies underlie NK cell inactivity in lymphotoxin-alpha gene-targeted mice. J Immunol. 1999;163(Suppl 3):1350–1353. [PubMed] [Google Scholar]
- Ito D, Back TC, Shakhov AN, Wiltrout RH, Nedospasov SA. Mice with a targeted mutation in lymphotoxin-alpha exhibit enhanced tumor growth and metastasis: impaired NK cell development and recruitment. J Immunol. 1999;163(Suppl 5):2809–2815. [PubMed] [Google Scholar]
- Markey KA, Burman AC, Banovic T, Kuns RD, Raffelt NC, Rowe V, Olver SD, Don AL, Morris ES, Pettit AR, Wilson YA, Robb RJ, Randall LM, Korner H, Engwerda CR, Clouston AD, Macdonald KP, Hill GR. Soluble lymphotoxin is an important effector molecule in GVHD and GVL. Blood. 2010;115(Suppl 1):122–132. doi: 10.1182/blood-2009-01-199927. [DOI] [PubMed] [Google Scholar]
- O'Rourke KP, O'Donoghue G, Adams C, Mulcahy H, Molloy C, Silke C, Molloy M, Shanahan F, O'Gara F. High levels of Lymphotoxin-Beta (LT-Beta) gene expression in rheumatoid arthritis synovium: clinical and cytokine correlations. Rheumatol Int. 2008;28(Suppl 10):979–986. doi: 10.1007/s00296-008-0574-z. [DOI] [PubMed] [Google Scholar]
- Chiang EY, Kolumam GA, Yu X, Francesco M, Ivelja S, Peng I, Gribling P, Shu J, Lee WP, Refi no CJ, Balazs M, Paler-Martinez A, Nguyen A, Young J, Barck KH, Carano RA, Ferrando R, Diehl L, Chatterjea D, Grogan JL. Targeted depletion of lymphotoxin-alpha-expressing TH1 and TH17 cells inhibits autoimmune disease. Nat Med. 2009;15(Suppl 7):766–773. doi: 10.1038/nm.1984. [DOI] [PubMed] [Google Scholar]
- Calmon-Hamaty F, Combe B, Hahne M, Morel J. Lymphotoxin α stimulates proliferation and pro-inflammatory cytokine secretion of rheumatoid arthritis synovial fibroblasts. Cytokine. 2011;53(Suppl 2):207–214. doi: 10.1016/j.cyto.2010.10.010. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7(Suppl 6):429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]