Abstract
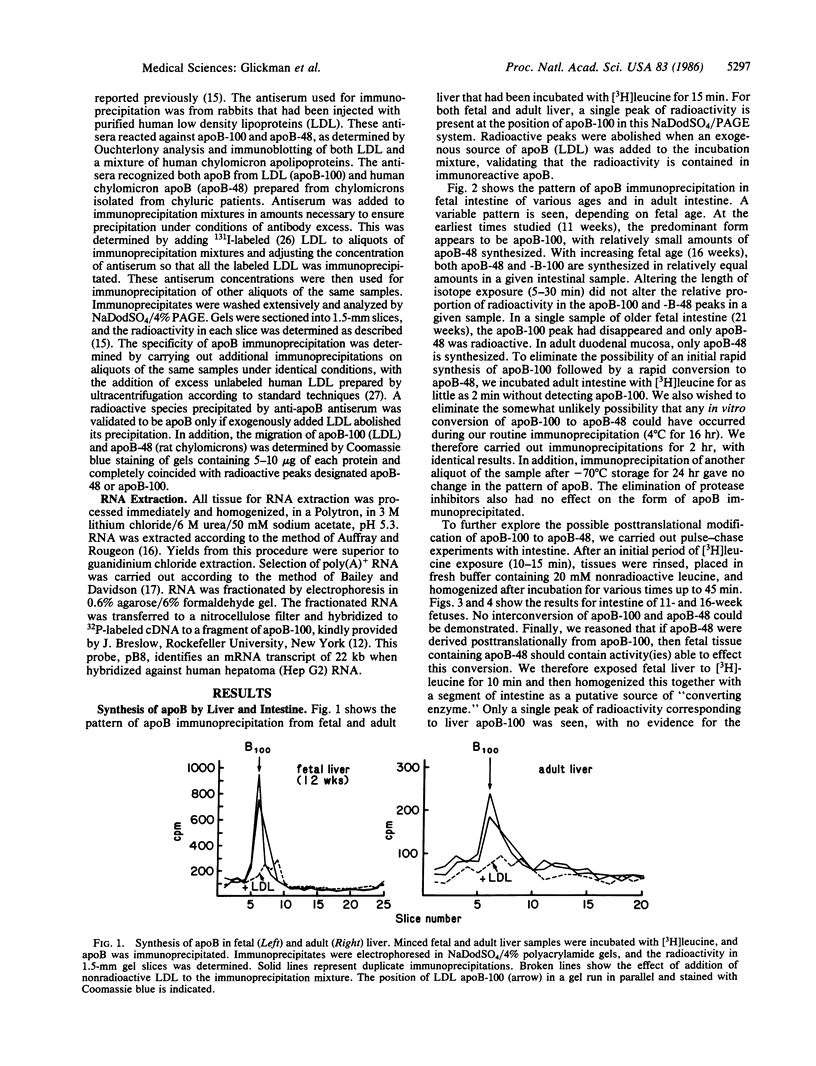
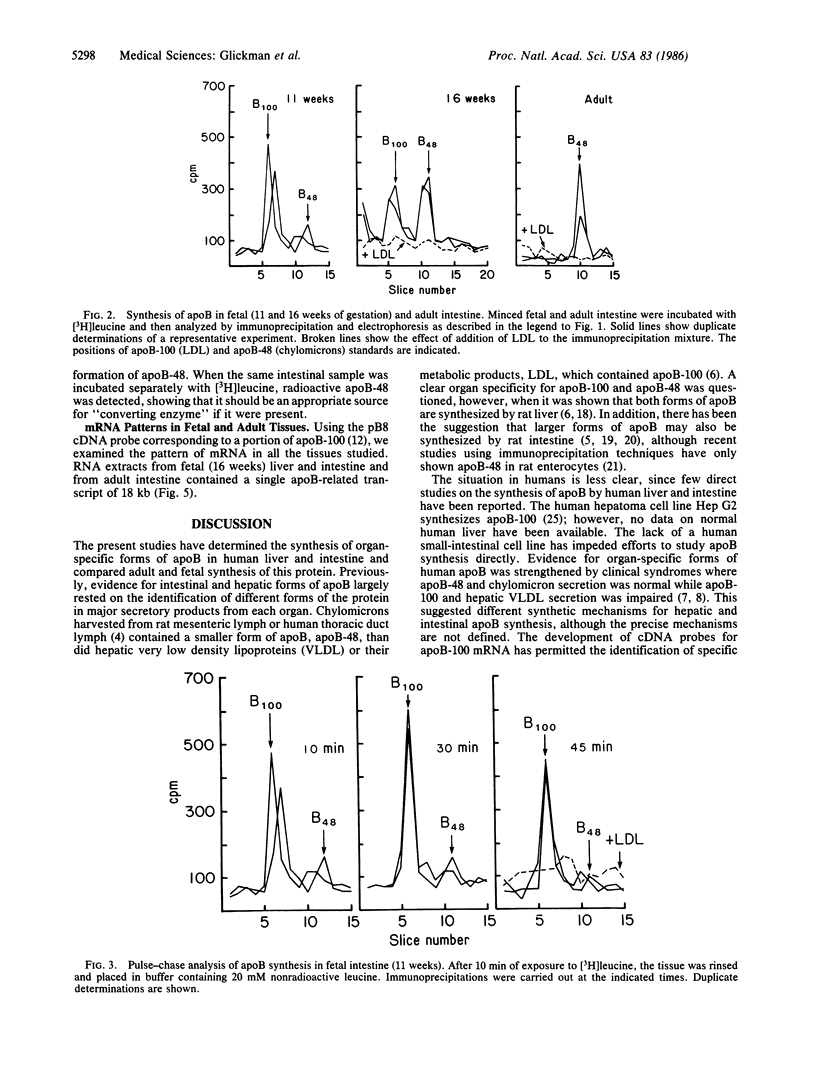
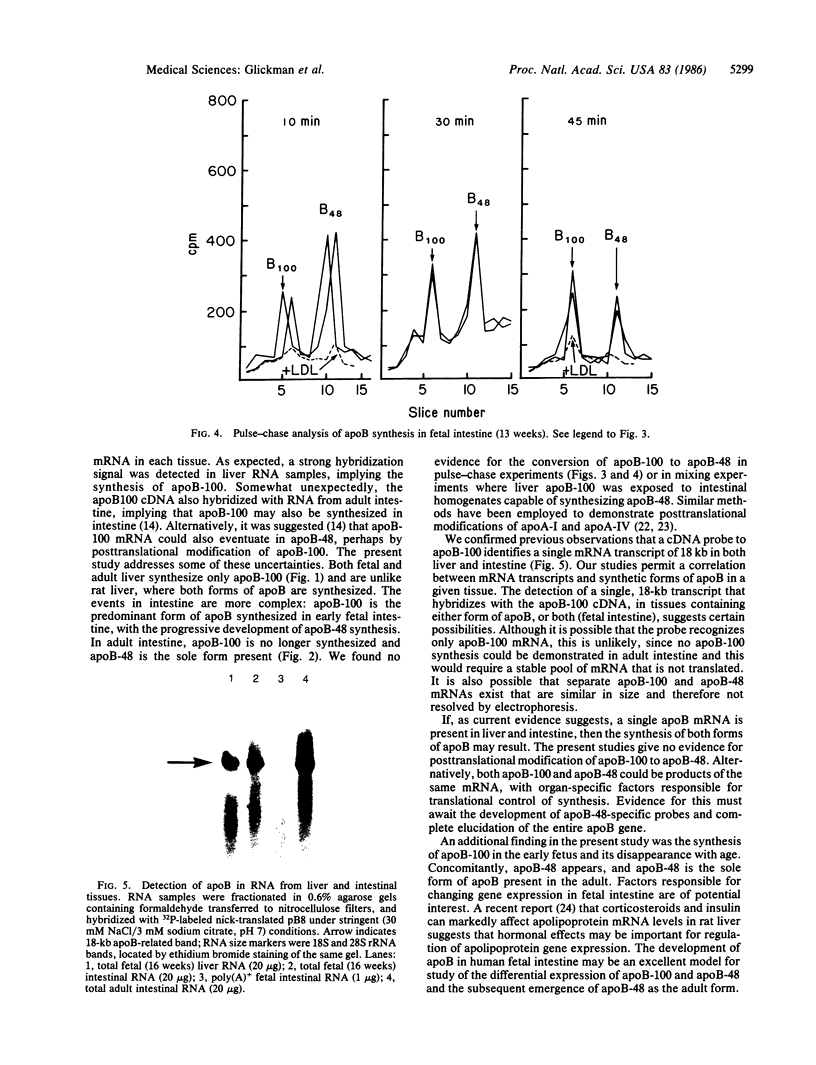
The synthesis of apolipoprotein B (apoB) was examined in human fetal and adult intestine and liver. Intestine and liver were minced and then incubated with [3H]leucine, homogenized, and subjected to immunoprecipitation with antiserum that recognized both apoB-100 and apoB-48 (forms of apoB found in low density lipoproteins and in chylomicrons, respectively). Immunoprecipitates of fetal and adult liver contained radioactivity in a single apoB-100 peak when examined by NaDodSO4/polyacrylamide gel electrophoresis. Intestine from fetuses at 11 weeks of gestation incorporated radioactivity mainly into apoB-100, with little incorporation into apoB-48. Sixteen-week fetal intestine showed both apoB-100 and apoB-48, whereas adult intestine incorporated radioactivity only into apoB-48. Pulse-chase experiments with 11- and 16-week fetal intestine showed no evidence for the conversion of apoB-100 to apoB-48. Incubation of intestinal homogenates with fetal liver apoB-100 did not result in the conversion of apoB-100 to smaller forms of apoB. A cDNA probe to hepatic apoB-100 identified a single, 18-kilobase transcript in poly(A)+ RNA from fetal and adult liver and fetal intestine of all ages. These studies define the developmental pattern of apoB synthesis in human fetal and adult liver and intestine. No evidence could be found for the conversion of apoB-100 to apoB-48. The finding of a single mRNA transcript despite the form of apoB synthesized in each tissue is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bisgaier C. L., Glickman R. M. Intestinal synthesis, secretion, and transport of lipoproteins. Annu Rev Physiol. 1983;45:625–636. doi: 10.1146/annurev.ph.45.030183.003205. [DOI] [PubMed] [Google Scholar]
- Davidson N. O., Glickman R. M. Apolipoprotein A-I synthesis in rat small intestine: regulation by dietary triglyceride and biliary lipid. J Lipid Res. 1985 Mar;26(3):368–379. [PubMed] [Google Scholar]
- Davidson N. O., Kollmer M. E., Glickman R. M. Apolipoprotein B synthesis in rat small intestine: regulation by dietary triglyceride and biliary lipid. J Lipid Res. 1986 Jan;27(1):30–39. [PubMed] [Google Scholar]
- Deeb S. S., Motulsky A. G., Albers J. J. A partial cDNA clone for human apolipoprotein B. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4983–4986. doi: 10.1073/pnas.82.15.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovson J., Huang Y. O., Baker N., Kannan R. Apolipoprotein B is structurally and metabolically heterogeneous in the rat. Proc Natl Acad Sci U S A. 1981 Jan;78(1):157–161. doi: 10.1073/pnas.78.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshourbagy N. A., Boguski M. S., Liao W. S., Jefferson L. S., Gordon J. I., Taylor J. M. Expression of rat apolipoprotein A-IV and A-I genes: mRNA induction during development and in response to glucocorticoids and insulin. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8242–8246. doi: 10.1073/pnas.82.23.8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. I., Bisgaier C. L., Sims H. F., Sachdev O. P., Glickman R. M., Strauss A. W. Biosynthesis of human preapolipoprotein A-IV. J Biol Chem. 1984 Jan 10;259(1):468–474. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert P. N., Hyams J. S., Bernier D. N., Berman M. M., Saritelli A. L., Lynch K. M., Nichols A. V., Forte T. M. Apolipoprotein B-100 deficiency. Intestinal steatosis despite apolipoprotein B-48 synthesis. J Clin Invest. 1985 Aug;76(2):403–412. doi: 10.1172/JCI111986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. S., Bock S. C., Feinstein S. I., Breslow J. L. Human apolipoprotein B cDNA clone isolation and demonstration that liver apolipoprotein B mRNA is 22 kilobases in length. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6825–6829. doi: 10.1073/pnas.82.20.6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J. P. Apolipoprotein B: structural and metabolic heterogeneity. Annu Rev Physiol. 1983;45:637–650. doi: 10.1146/annurev.ph.45.030183.003225. [DOI] [PubMed] [Google Scholar]
- Kane J. P., Hardman D. A., Paulus H. E. Heterogeneity of apolipoprotein B: isolation of a new species from human chylomicrons. Proc Natl Acad Sci U S A. 1980 May;77(5):2465–2469. doi: 10.1073/pnas.77.5.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott T. J., Rall S. C., Jr, Innerarity T. L., Jacobson S. F., Urdea M. S., Levy-Wilson B., Powell L. M., Pease R. J., Eddy R., Nakai H. Human apolipoprotein B: structure of carboxyl-terminal domains, sites of gene expression, and chromosomal localization. Science. 1985 Oct 4;230(4721):37–43. doi: 10.1126/science.2994225. [DOI] [PubMed] [Google Scholar]
- Lee D. M., Koren E., Singh S., Mok T. Presence of B-100 in rat mesenteric chyle. Biochem Biophys Res Commun. 1984 Sep 28;123(3):1149–1156. doi: 10.1016/s0006-291x(84)80253-3. [DOI] [PubMed] [Google Scholar]
- Lusis A. J., West R., Mehrabian M., Reuben M. A., LeBoeuf R. C., Kaptein J. S., Johnson D. F., Schumaker V. N., Yuhasz M. P., Schotz M. C. Cloning and expression of apolipoprotein B, the major protein of low and very low density lipoproteins. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4597–4601. doi: 10.1073/pnas.82.14.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy M. J., Kane J. P., Hardman D. A., Hamilton R. L., Dalal K. B. Normotriglyceridemic abetalipoproteinemia. absence of the B-100 apolipoprotein. J Clin Invest. 1981 May;67(5):1441–1450. doi: 10.1172/JCI110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash J. M., Rothblat G. H., Sparks C. E. Lipoprotein apolipoprotein synthesis by human hepatoma cells in culture. Biochim Biophys Acta. 1981 Nov 23;666(2):294–298. doi: 10.1016/0005-2760(81)90120-x. [DOI] [PubMed] [Google Scholar]
- Sparks C. E., Marsh J. B. Metabolic heterogeneity of apolipoprotein B in the rat. J Lipid Res. 1981 Mar;22(3):519–527. [PubMed] [Google Scholar]
- Takashima Y., Kodama T., Iida H., Kawamura M., Aburatani H., Itakura H., Akanuma Y., Takaku F., Kawade M. Normotriglyceridemic abetalipoproteinemia in infancy: an isolated apolipoprotein B-100 deficiency. Pediatrics. 1985 Mar;75(3):541–546. [PubMed] [Google Scholar]
- Wei C. F., Chen S. H., Yang C. Y., Marcel Y. L., Milne R. W., Li W. H., Sparrow J. T., Gotto A. M., Jr, Chan L. Molecular cloning and expression of partial cDNAs and deduced amino acid sequence of a carboxyl-terminal fragment of human apolipoprotein B-100. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7265–7269. doi: 10.1073/pnas.82.21.7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. L., Windmueller H. G. Variant forms of plasma apolipoprotein B. Hepatic and intestinal biosynthesis and heterogeneous metabolism in the rat. J Biol Chem. 1981 Apr 25;256(8):3615–3618. [PubMed] [Google Scholar]
- Zannis V. I., Karathanasis S. K., Keutmann H. T., Goldberger G., Breslow J. L. Intracellular and extracellular processing of human apolipoprotein A-I: secreted apolipoprotein A-I isoprotein 2 is a propeptide. Proc Natl Acad Sci U S A. 1983 May;80(9):2574–2578. doi: 10.1073/pnas.80.9.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]