Abstract
Introduction
Cathepsin K is a recently discovered cysteine protease which cleaves the triple helical domains of type I to II collagen. It has been shown to be up-regulated in synovial tissue from osteoarthritic and rheumatoid patients, and is a component in normal and nonarthritic cartilage, where it increases with aging. Studies on heart valve development have recently shown that receptor activator of nuclear factor-κB ligand (RANKL) acts during valve remodeling to promote cathepsin K expression. Since extracellular matrix remodeling is a critical component of disc structure and biomechanical function, we hypothesized that cathepsin K and RANKL may be present in the human intervertebral disc.
Methods
Studies were performed following approval of the authors' Human Subjects Institutional Review Board. Six annulus specimens from healthier Thompson grade I to II discs, and 12 specimens from more degenerate grade III to IV discs were utilized in microarray analysis of RANKL and cathepsin K gene expression. Immunohistochemistry was also performed on 15 additional disc specimens to assess the presence of RANKL and cathepsin K.
Results
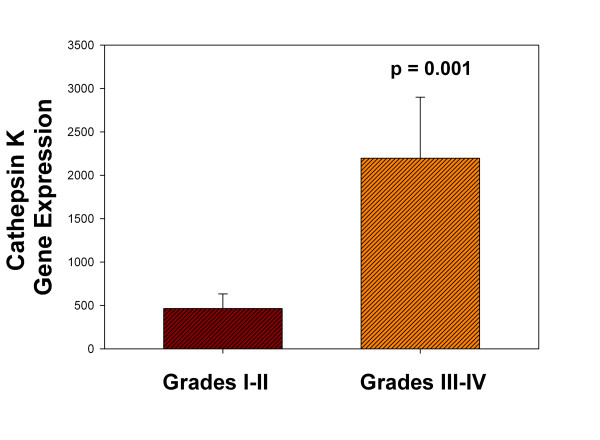
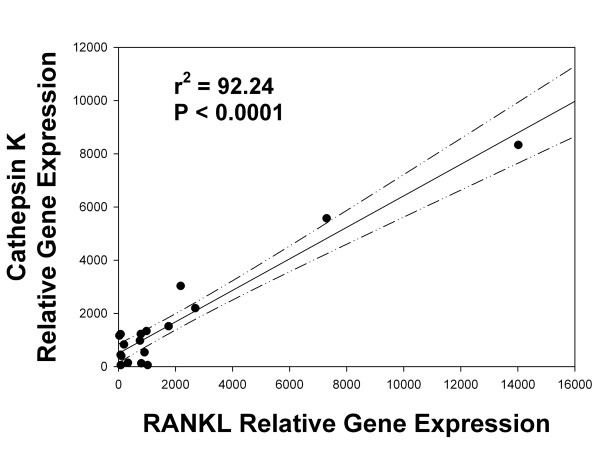
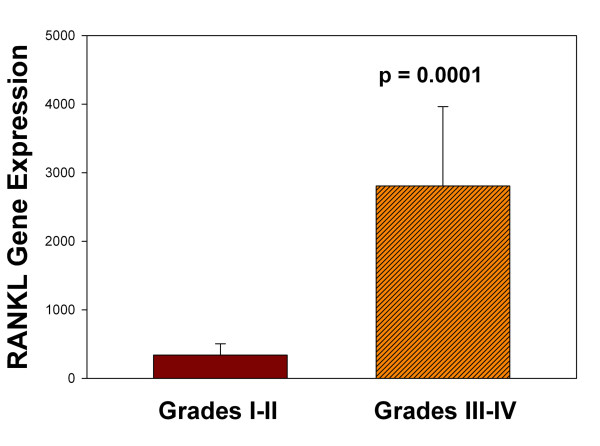
Cathepsin K gene expression was significantly greater in more degenerated grade III to IV discs compared to healthier grade I to II discs (P = 0.001). RANKL was also identified with immunohistochemistry and molecular analyses. RANKL gene expression was also significantly greater in more degenerated discs compared to healthier ones (P = 0.0001). A significant linear positive correlation was identified between expression of cathepsin K and RANKL (r2 = 92.2; P < 0.0001).
Conclusions
Extracellular matrix remodeling is a key element of disc biology. Our use of an appropriate antibody and gene expression studies showed that cathepsin K is indeed present in the human intervertebral disc. Immunolocalization and molecular analyses also confirmed that RANKL is present in the human disc. Expression of RANKL was found to be significantly greater in more degenerated compared to healthier discs (P = 0.0001). Cathepsin K gene expression levels showed a positive, significant correlation with RANKL expression. Based on these data, we propose that cathepsin K plays a significant role in disc matrix remodeling and in matrix degradation in the proinflammatory cytokine-rich microenvironment of the degenerating disc.
Introduction
Cathepsin K, discovered and isolated from a rabbit osteoclast library in 1994 [1], is an interesting cysteine protease which cleaves the triple helical domains of types I to II collagen [1]. Research has shown that its collagenolytic action requires that chondroitin or keratin sulfate be bound to the protease [2]. Cathepsin K is present in osteoclasts, human ovary, heart and skeletal muscle, lung, placenta, testis, small intestine and colon [3]. Studies have identified upregulation of cathepsin K in fibroblast-like cells in synovial tissue of osteoarthritic and rheumatoid patients [4-7]. A link to heightened expression in the presence of IL-1ß or TNFα was also shown by in vitro culture of fibroblasts derived from rheumatoid patients in the work of Hou et al. [5]. In addition, an association with aging and increased cathepsin K levels has been suggested by Dejica et al. since they found increased cathepsin K content in nonarthritic cartilage from older compared to younger subjects [8]. Work by Ruettger et al. showed that cathepsin K is regulated via activation of the classical protein kinase C and p38 MAP kinase in articular chondrocytes [9].
Two interesting reports have shown spontaneous development of synovitis and cartilage degeneration in transgenic mice, which overexpress cathepsin K (transgenic UTU17 mice) [10,11]. In analysis of this model, transgenic animals showed osteoarthritis and increased levels of cathepsin K with aging. During growth and aging, cathepsin K was found to be the most abundant cysteine proteinase in the mouse knee joint, where cathepsin K was present near sites of matrix degeneration and destruction in articular cartilage.
Cathepsin K is a recognized component of osteoclasts, where it plays a central role in bone resorption [7]. RANKL (receptor activator of nuclear factor κB ligand, also known as TRANCE, TNFSF11, OPGL, and ODF) is a member of the tumor necrosis factor family of signaling molecules, which functions in promoting osteoclast differentiation. RANKL, well known for its role in production of extracellular matrix remodeling enzymes, is one of the factors capable of inducing cathepsin K production. This aspect of the biology of cathepsin K has recently been studied by Combs and Yutzey in an analysis of the regulation of heart valve development which found that RANKL and cathepsin K are expressed by endocardial cushion endothelial cells; RANKL acted during valve remodeling to promote cathepsin K expression [12].
Although there is not a large body of literature addressing cathepsin K and its role in disc degeneration, there are several previous interesting studies. The work of Ariga et al. pointed to an association with endplate separation and disorganization of the annulus in spinal degenerative disorders [13]. Neidhart et al. found strong expression of cathepsin K in a number of regions of the spine in patients with ankylosing spondylitis [14]. Mwale et al. have studied the effect of a collagen type II collagen fragment (the 245-270 peptide) on disc cells [15]. This work showed that the addition of this peptide fragment at levels of 1 μg/ml to cultured annulus cells resulted in a significant increase in cathepsin K expression by one day of culture; expression levels decreased over the following four days of culture and reached control levels. Nucleus cells exposed to this fragment also showed stimulated cathepsin K expression at one day of exposure.
In the present study, we hypothesized that cathepsin K might be an important component of matrix remodeling overlooked to date in the intervertebral disc. We tested this hypothesis by assessing gene expression of cathepsin K and RANKL in human disc tissue, and also applied immunohistochemistry analyses to disc tissues.
Materials and methods
Clinical study population
Experimental study of human disc specimens was approved prospectively by the authors' Human Subjects Institutional Review Board at Carolinas Medical Center. The need for informed consent was waived by the ethical board since disc tissue was removed as part of routine surgical practice. Scoring of disc degeneration utilized the Thompson scoring system; this system scores disc degeneration over the spectrum from a healthy disc (Thompson grade I) to discs with advanced degeneration (grade V, the most advanced stage of degeneration) [16]. Patient specimens were derived from surgical disc procedures performed on individuals with herniated discs and degenerative disc disease. Surgical specimens were transported to the laboratory in sterile tissue culture medium. Care was taken to remove all granulation tissue and to sample only disc tissue. Non-surgical control donor disc specimens were obtained via the National Cancer Institute Cooperative Human Tissue Network (CHTN); they were shipped overnight to the laboratory in sterile tissue culture medium and processed as described below. Specimen procurement from the CHTN was included in our approved protocol by our human subjects Institutional Review board.
Expression of cathepsin K and RANKL in vivo
Analysis of human disc tissue was carried out as previously described using laser capture microdissection methods [17]. Total RNA was extracted from cells using the TRIzol reagent (Gibco, Carlsbad, CA, USA), reverse transcribed to double-stranded cDNA, subjected to two rounds of transcription, and hybridized to the DNA microarray in the Affymetrix Fluidics Station 400. Affymetrix human U133 X3P arrays were used. The GCOS Affymetrix GeneChip Operating System (version 1.2, Affymetrix, Santa Clara, CA, USA) was used for determining gene expression levels of RANKL and cathepsin K (Affymetrix gene identifications AF053712.1 for RANKL, and NM_000396.1 for cathepsin K).
Gene array data related to human disc tissue reported here have been uploaded to the Gene Expression Omnibus (GEO) website [GEO:GSE23130] and may be accessed at sample numbers GSM569830 - GSM569848.
Immunolocalization of RANKL
Disc specimens were fixed in 10% neutral buffered formalin for no longer than 24 hours and changed to 70% ethanol. Undecalcified specimens were embedded in paraffin and sections cut at 4 μm, collected on PLUS slides (Allegiance, McGaw Park, IL, USA) and dried at 60°C. Sections were deparaffinized in xylene (Allegiance) and rehydrated through graded alcohols (AAPER, Shelbyville, KY, USA) to distilled water. Antigen retrieval was performed using Dako Target Retrieval Solution, pH 6.0 (Dako, Carpenteria, CA, USA) for 20 minutes at 95°C followed by cooling for 20 minutes. The remainder of the procedure was performed using the Dako Autostainer Plus (Dako) automated stainer. Endogenous peroxidase was blocked using 3% H202 (Humco, Texarcana, TX, USA). Slides were incubated for one hour with anti-RANKL (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a 1:50 dilution. Secondary antibody was 4+Biotinylated Universal Goat Link (Biocare Medical, Concord, CA, USA) for 10 minutes followed by 4+ streptavidin HRP Label (Biocare) for 10 minutes and Vector NovaRed (Vector Laboratories, Burlingame, CA, USA) for 5 minutes. Slides were removed from the stainer, rinsed in water, counterstained with light green, dehydrated, cleared and mounted with resinous mounting media. Mouse IgG (Dako) was used as a negative control. Human tonsil was utilized as a positive control.
Immunolocalization of cathepsin K
Following fixation and embedding as described above, paraffin sections were cut at 4 μm, collected on PLUS slides (Allegiance) and dried at 60°C. Sections were deparaffinized in xylene (Allegiance) and rehydrated through graded alcohols (AAPER) to distilled water. The remainder of the procedure was performed using the Dako Autostainer Plus (Dako) automated stainer. Endogenous peroxidase was blocked using 3% H202 (Humco). Slides were incubated for one hour with anti-cathepsin K (Abcam, Cambridge, MA, USA) at a 1:100 dilution. Mouse IgG (Dako) was used as a negative control. A secondary antibody was 4+Biotinylated Universal Goat Link (Biocare Medical,) for 10 minutes followed by 4+ streptavidin HRP Label (Biocare) for 10 minutes and DAB (Biocare)) for 5 minutes. Slides were removed from the stainer, rinsed in water, counterstained with light green, dehydrated, cleared and mounted with resinous mounting media. Rat physeal tissue was used as a positive control.
Statistical analyses
Standard statistical analyses were performed utilizing InStat (GraphPad Software, Inc., San Diego, CA, USA). Unpaired t-tests and linear regression analyses were performed; means ± s.e.m. were calculated. P = 0.05 was considered to be the level of significant.
Results
Molecular confirmation of in vivo expression of Cathepsin k and RANKL expression in the human annulus
Six annulus specimens from healthier Thompson grade I to II discs, and 13 specimens from more degenerate grade III to IV discs were utilized in microarray analyses of RANKL and cathepsin K gene expression. Demographic features for this study population are listed in Table 1. As shown in Figure 1, cathepsin K expression was significantly greater in more degenerate Thompson grade III to IV discs than in healthier grade I to II discs (P = 0.001). Statistical evaluation also found a positive, significant correlation between the expression levels of cathepsin K and those of RANKL (Figure 2; r2 = 92.2; P < 0.0001). RANKL gene expression was also found to be significantly greater in more degenerate Thompson grade III to IV discs compared to healthier grade I to II discs (Figure 3, P = 0.0001). Statistical analysis showed that there was no relationship between age and either cathepsin K or RANKL gene expression levels.
Table 1.
Demographic features for specimens studied for immunocytochemical localization of cathepsin K and RANKL
| Subject number | Site | Thompson score | Age/gender | Other information (cause of death) |
|---|---|---|---|---|
| Annulus specimens | ||||
| 1 | L4 to 5 | 1.5 | 45/F | CHTN (unknown) |
| 1 | L3 to 4 | II | 45/F | CHTN (unknown) |
| 2 | L5 to S1 | II | 21/M | Surgical specimen |
| 3 | L4 to 5 | 2.5 | 40/M | CHTN (MI) |
| 4 | L1 to 2 | III | 33/F | CHTN (PE) |
| 5 | L4 to 5 | III | 33/F | Surgical specimen |
| 6 | L3 to 4 | III | 46/F | Surgical specimen |
| 7 | L5 to S1 | III | 53/M | Surgical specimen |
| 8 | L4 to 5 | III | 29/F | Surgical specimen |
| 9 | L2 to 3 | III | 54/M | Surgical specimen |
| 4 | L3 to 4 | IV | 33/F | CHTN (PE) |
| 10 | L3 to 4 | IV | 59/F | Surgical specimen |
| 10 | L1 to 2 | IV | 59/F | Surgical specimen |
| 10 | L2 to 3 | IV | 59/F | Surgical specimen |
| 11 | L3 to 4 | IV | 78/M | Surgical specimen |
| 12 | L2 to 3 | IV | 56/F | Surgical specimen |
| 13 | L4 to 5 | IV | 39/F | Surgical specimen |
| 14 | L5 to S1 | V | 44/F | Surgical specimen |
| 15 | L5 to S1 | V | 39/F | Surgical specimen |
| Nucleus specimens | ||||
| 16 | L4 to 5 | 3.5 | 68/F | CHTN (stroke) |
| 17 | L3 to 4 | V | 69/M | CHTN (MI) |
| 18 | L5 to S1 | III | 54/F | CHTN (unknown) |
| 19 | L2 to 3; L3 to 4 |
II | 45/F | CHTN (unknown) |
| 20 | L3 to 4; T12 to L1 |
IV; 3.5 | 33/F | CHTN (PE) |
CHTN, Cooperative Human Tissue Network; F, female; age is presented in years; L, Lumbar; M, male; MI, myocardial infarction; PE, pulmonary embolism; S, sacral; T, thoracic.
Figure 1.
Cathepsin K expression and stages of disc degeneration. Cathepsin K gene expression was significantly greater in annulus tissue from more degenerated discs than in healthier discs (P = 0.001). Data are means ± s.e.m.
Figure 2.
Relationship between RANKL expression and cathepsin K expression. A significant, positive correlation was present between gene expression levels of RANKL and cathepsin K (r2 = 92.2; P < 0.0001). (Dashed line shows the 95% confidence interval for the correlation).
Figure 3.
RANKL expression and stages of disc degeneration. RANKL gene expression was significantly greater in annulus tissue from more degenerated discs than in healthier discs (P = 0.0001). Data are means ± s.e.m.
In vivo immunolocalization of cathepsin K and RANKL in the human disc
Immunohistochemical studies were performed on specimens derived from subjects whose demographic features are described in Table 1. Cells in the outer annulus showed strong immunolocalization of cathepsin K (Figure 4A). Inner annulus and nucleus regions showed that some, but not all, cells were positive for cathepsin K immunolocalization (Figure 4C, D). In the inner annulus, cells in clusters also showed that not all cells were positive for localization.
Figure 4.
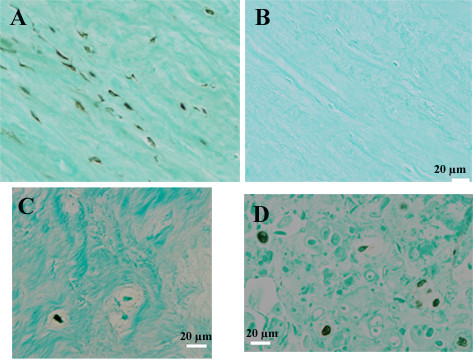
Representative images showing immunohistochemical localization of cathepsin K in the human disc. Immunohistochemical localization (black localization product) shows the presence of cathepsin K in the outer annulus (Figure 4A). Figure 4B shows an adjacent negative control section from the outer annulus. Within the inner annulus (Figure 4C) and nucleus (Figure 4D), both positive and negative cells were present. (Scale bar for Figure 4A is the same as that shown for Figure 4B).
RANKL immunohistochemical analyses required antigen retrieval during the localization procedure. Figure 5 shows cells positive for localization in the outer annulus (Figure 5A), and in cells present in clusters in the inner annulus (Figure 5B). Occasional positive cells were identified in the nucleus (data not shown).
Figure 5.
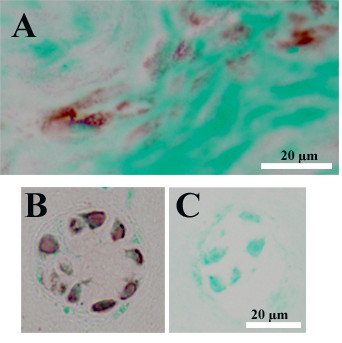
Representative images showing immunohistochemical localization of RANKL in the human disc. Immunohistochemical localization of RANKL (red localization product) using antigen retrieval in the outer annulus (Figure 5A) and inner annulus (Figure 5B). Figure 5C shows an adjacent negative control.
Discussion
Improved understanding of the regulation of extracellular matrix turnover during normal homeostasis and during advancing disc degeneration is an important topic in disc research [18]. As noted by Millward-Sadler et al., matrix turnover has implications for the pathogenesis of human disc degeneration [19]. Historically, the matrix metalloproteinases, including MMP-1, -2, -3, -7, -8, -9, -13, -19 and -28 [20-31], have been considered the key players in disc matrix destruction during disc degeneration.
In the present work we confirm that cathepsin K is constitutively expressed in the human disc. Our analysis showed that there was significantly greater expression of cathepsin K in degenerated discs compared to healthier ones (Figure 1). This finding suggests a role for cathepsin K in disc degeneration, and is in agreement with the findings of Konttinen et al. that cathepsin K expression increased with the severity of osteoarthritis [6].
We now know that the degenerating disc has increased expression of a number of important genes related to the extracellular matrix [32] and proteoglycans [33], inflammatory cytokines, and matrix-degrading agents, neurotrophins, and cytokines (for reviews and studies see [26,33-41]). In vitro studies have been especially helpful in advancing our understanding of inflammatory cytokine production [42-45]. Hou et al. have shown that IL-1ß or TNFα in vitro stimulation of synovial fibroblasts derived from rheumatoid or osteoarthritic subjects produced increased cathepsin K expression. These two proinflammatory cytokines have well-recognized roles in intervertebral disc degeneration [46]. We hypothesize that in the cytokine rich milieu of the degenerating disc cathepsin K plays a significant role in disc matrix degeneration.
RANKL is recognized as a factor capable of inducing cathepsin K production, and is known to be regulated by IL-1ß and TNFα (see [7] for a recent review). In addition to its acknowledged role in osteoclast development and function [7], RANKL signaling activates a number of pathways important in a number of cell types including bone marrow stromal cells, fibroblasts, mammary endothelial cells, epithelial cells, osteoblasts, osteoclasts and T lymphocytes (see [47,48] for reviews). In 2009, Mackiewicz et al. showed the immunohistochemical presence of RANKL in human annulus tissue [49].
In the present work, we identified significantly increased RANKL gene expression in more degenerated discs compared to healthier discs (Figure 3). Our analyses of gene expression data from disc tissue also showed a significant, positive linear relationship between cathepsin K and RANKL expression which accounted for a high proportion of the variability in this relationship (92.2%, Figure 2). This finding is also consistent with regulation of cathepsin K expression during disc degeneration; we note, however, that future mechanistic studies should be undertaken to further explore this control mechanism. Other aspects of RANKL function in the disc merit future studies to determine whether it acts to inhibit proliferation and to induce apoptosis as was seen by McGonigle et al. in endothelial cells [50].
It should be noted that the present analyses utilized only annulus cells in the microarray expression studies; we currently are adding nucleus specimens so that future work can explore expression patterns in nucleus cells as well as the annulus. We look forward to data from other disc research labs on this topic. Important future studies should include in vivo analyses to determine the exact relationship between cathepsin K and RANKL expression, correlations of cathepsin K levels to collagen fragments within the disc (as pioneered in the important work of Neidart et al. [14] and Mwale et al. [15], and expanded in vitro studies with annulus and nucleus cells.
In closing, a note should also be made concerning the current clinical interest in development of cathepsin K inhibitors because of their ability to inhibit bone resorption [3,7,51,52]. As we continue to move towards the application of biologic therapies for human disc degeneration, it may be efficacious to consider inclusion of selective, specific approaches to inhibit cathepsin K. Yasuca et al. reported in 2005 that some cathepsin K inhibitors are now in clinical trials for osteoporosis therapy, and noted that cathepsin K is a preferable drug target for non-inflammatory osteoarthritis with positive pre-clinical data [3].
Conclusions
Collectively, our results demonstrate the constitutive expression of cathepsin K and RANKL within human intervertebral disc tissue. Gene expression studies showed that both cathepsin K and RANKL expression levels are significantly greater in annulus tissue from more degenerated discs compared to levels in present healthier discs (P = 0.001 and 0.0001, respectively). A positive significant correlation was identified between expression levels of cathepsin K and RANKL (r2 = 92.2; P < 0.0001). Based on these data, we suggest that cathepsin K may play a significant role in disc matrix remodeling and in matrix degradation in the proinflammatory cytokine-rich microenvironment of the degenerating disc.
Abbreviations
CHTN: Cooperative Human Tissue Network; GEO: Gene Expression Omnibus; IL-1ß: interleukin-1-beta; MMP: matrix metalloproteinase; p38 MAP kinase: p38 mitogen-activated protein kinase; RANKL: receptor activator of nuclear factor-κ-B ligand; TNFα: tumor necrosis factor-alpha.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
HEG, in collaboration with ENH, conceived and planned the study. GLH performed microarray analyses, while JAI and NZ performed histology and immunohistochemistry. HEG wrote the manuscript and performed statistical analyses with HJN. Co-authors agreed with the finalized submission. All authors read and approved the final manuscript.
Contributor Information
Helen E Gruber, Email: helen.gruber@carolinashealthcare.org.
Jane A Ingram, Email: jane.ingram@carolinashealthcare.org.
Gretchen L Hoelscher, Email: gretchen.hoelscher@carolinashealthcare.org.
Natalia Zinchenko, Email: natalia.zinchenko@carolinashealthcare.org.
H James Norton, Email: james.norton@carolinashealthcare.org.
Edward N Hanley, Jr, Email: edward.hanley@carolinashealthcare.org.
Acknowledgements
We wish to thank the Brooks Back Pain Research Endowment for support.
References
- Tezuka K, Tezuka Y, Maejima A, Sato T, Nemoto K, Kamioka H, Hakeda Y, Kamegawa M. Molecular cloning of a possible cysteine proteinase predominantly expressed in osteoclasts. J Biol Chem. 1994;269:1106–1109. [PubMed] [Google Scholar]
- Li A, Hou WS, Escalante-Torres CR, Gelb BD, Brömme D. Collagenase activity of cathepsin K depends on complex formation with chondroitin sulfate. J Biol Chem. 2002;277:28669–28676. doi: 10.1074/jbc.M204004200. [DOI] [PubMed] [Google Scholar]
- Yasuda Y, Kaleta J, Brömme D. The role of cathepsins in osteoporosis and arthritis: rationale for the design of new therapeutics. Adv Drug Deliv Rev. 2005;57:973–993. doi: 10.1016/j.addr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Hummell KM, Petrow PK, Franz JK, Muller-Ladner U, Aicher WK, Gay RE, Bromme D, Gay S. Cysteine proteinase cathepsin K mRNA is expressed in synovium of patients with rheumatoid arthritis and is detected at sites of synovial bone descruction. J Rheumatol. 1998;25:1887–1894. [PubMed] [Google Scholar]
- Hou W-S, Li W, Keyszer G, Weber E, Levy R, Klein MJ, Gravallese EM, Goldring SR, Brömme D. Comparison of cathepsins K and S expression within the rheumatoid and osteoarthritic synovium. Arthritis Rheum. 2002;46:663–674. doi: 10.1002/art.10114. [DOI] [PubMed] [Google Scholar]
- Konttinen YT, Mandelin J, Li T-F, Salo J, Lassus J, Liljeström M, Hukkanen M, Tagai M, Virtanen I, Santavirta S. Acidic cysteine endoproteinase cathepsin K in the degeneration of the superficial articular hyaline cartilage in osteoarthritis. Arthritis Rheum. 2002;46:953–960. doi: 10.1002/art.10185. [DOI] [PubMed] [Google Scholar]
- Salminen-Mankonen HJ, Morko J, Vuorio E. Role of cathepsin K in normal joints and in the development of arthritis. Curr Drug Targets. 2007;8:315–323. doi: 10.2174/138945007779940188. [DOI] [PubMed] [Google Scholar]
- Dejica VM, Mort JS, Laverty S, Percival MD, Antoniou J, Zukor DJ, Poole AR. Cleavage of type II collagen by cathepsin K in human osteoarthritic cartilage. Amer J Pathol. 2008;173:161–169. doi: 10.2353/ajpath.2008.070494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruettger A, Schueler S, Mollenhauer JA, Weideranders B. Cathepsins B, K, and L are regulated by a defined collagen type II peptide via activation of classical protein kinase C and p38 MAP kinase in articular chondrocytes. J Biol Chem. 2008;283:1043–1051. doi: 10.1074/jbc.M704915200. [DOI] [PubMed] [Google Scholar]
- Morko J, Kiviranta R, Joronen K, Säämänen A-M, Vuorio E, Salminen-Mankonen H. Spontaneous development of synovitis and cartilage degeneration in transgenic mice overexpressin cathepsin K. Arthritis Rheum. 2005;52:3713–3717. doi: 10.1002/art.21423. [DOI] [PubMed] [Google Scholar]
- Morko JP, Söderström M, Säämänen AM, Salminen HJ, Vuorio EI. Up regulation of cathepsin K expression in articular chondrocytes in a transgenic mouse model for osteoarthritis. Ann Rheum Dis. 2004;63:649–655. doi: 10.1136/ard.2002.004671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombs MD, Yutzey KE. VEGF and RANKL regulation of NFATc1 in heart valve development. Circ Res. 2009;105:565–574. doi: 10.1161/CIRCRESAHA.109.196469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariga K, Yonenobu K, Nakase T, Kaneko M, Okuda S, Uchiyama Y, Yoshikawa H. Localization of cathepsins D, K, and L in degenerated human intervertebral discs. Spine. 2001;26:2666–2672. doi: 10.1097/00007632-200112150-00007. [DOI] [PubMed] [Google Scholar]
- Neidhart M, Baraliakos X, Seemayer C, Zelder D, Gay RE, Michel BA, Boehm H, Gay S, Braun J. Expression of cathepsin K and matrix metalloproteinase 1 indicate persistent osteodestructive activity in long-standing ankylosing spondylitis. Ann Rheum Dis. 2009;68:1334–1339. doi: 10.1136/ard.2008.092494. [DOI] [PubMed] [Google Scholar]
- Mwale F, Wang HT, Zukor DJ, Huk OL, Petit A, Antoniou J. Effect of a type II collagen fragment on the expression of genes of the extracellular matrix in cells of the intervertebral disc. Open Orthop J. 2008;2:1–9. doi: 10.2174/1874325000802010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IKY, Bishop PB. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15:411–415. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Mougeot J-L, Hoelscher GL, Ingram JA, Hanley EN Jr. Microarray analysis of laser capture microdissected annulus cells from the human intervertebral disc. Spine. 2007;32:1181–1187. doi: 10.1097/BRS.0b013e318053ec89. [DOI] [PubMed] [Google Scholar]
- Auuerbach JD, Johnnessen W, Borthakur A, Wheaton AJ, Dolinskas CA, Balderston RA, Reddy R, Elliott DM. In vivo quantification of human lumbar disc degeneration using T(1rho)-weighted magnetic resonance imaging. Eur Spine J. 2006;15:S338–S344. doi: 10.1007/s00586-006-0083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward-Sadler SJ, Costello PW, Freemont AJ, Hoyland JA. Regulation of catabolic gene expression in normal and degenerate human intervertebral disc cells: implications for the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2009;11:R65. doi: 10.1186/ar2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber HE, Ingram JA, Hoelscher GL, Zinchenko N, Norton HJ, Hanley EN Jr. Matrix metalloproetinase 28, a novel matrix metalloproteinase, is constitutively expressed in human intervertebral disc tissue and is present in matrix of more degenerated discs. Arthritis Res Ther. 2009;11:R184. doi: 10.1186/ar2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber HE, Ingram JA, Hanley EN Jr. Immunolocalization of MMP-19 in the human intervertebral disc: implications for disc aging and degeneration. Biotech Histochem. 2005;80:157–162. doi: 10.1080/10520290500387607. [DOI] [PubMed] [Google Scholar]
- Crean JKG, Roberts S, Jaffray DC, Eisenstein SM, Duance VC. Matrix metalloproteinases in the human intervertebral disc: role in disc degeneration and scoliosis. Spine. 1997;22:2877–2884. doi: 10.1097/00007632-199712150-00010. [DOI] [PubMed] [Google Scholar]
- Kozaci LD, Guner A, Oktay G, Guner G. Alterations in biochemical components of extracellular matrix in intervertebral disc herniation: role of MMP-2 and TIMP-2 in type II collagen loss. Cell Biochem Funct. 2006;24:431–436. doi: 10.1002/cbf.1250. [DOI] [PubMed] [Google Scholar]
- Shen B, Melrose J, Ghosh P, Taylor TKF. Induction of matrix metalloproteinase-2 and -3 activity in ovine nucleus pulposus cells grown in three-dimensional agarose gel culture by interleukin-1β: a potential pathway of disc degeneration. Eur Spine J. 2003;12:66–75. doi: 10.1007/s00586-002-0454-2. [DOI] [PubMed] [Google Scholar]
- Nemoto O, Yamagishi M, Yamada H, Kikuchi T, Takaishi H. Matrix metalloproteinase-3 production by human degenerated intervertebral disc. J Spinal Disord. 1997;10:493–498. [PubMed] [Google Scholar]
- Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase - their role in disorders of the human intervertebral disc. Spine. 2000;25:3005–3013. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- Momohara S, Okamoto H, Komiya K, Ikari K, Tadkuchi M, Tomatsu T, Kamatani N. Matrix metalloproteinase 28/epilysin expression in cartilage from patients with rheumatoid arthritis and osteoarthritis: comment on the article by Kevorkian et al. Arthritis Rheum. 2004;50:4074–4080. doi: 10.1002/art.20799. [DOI] [PubMed] [Google Scholar]
- Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204:47–54. doi: 10.1002/path.1608. [DOI] [PubMed] [Google Scholar]
- Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N. 2002 SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with disc degradation and resorption. Eur Spine J. 2002;11:308–320. doi: 10.1007/s00586-002-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean JJ, Lee CR, Alini M, Iatridis JC. The effects of short-term load duration on anabolic and catabolic gene expression in the rat tail intervertebral disc. J Orthop Res. 2005;23:1120–1127. doi: 10.1016/j.orthres.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Richardson SM, Doyle P, Minogue BM, Gnanalinghan K, Hoyland JA. Increased expression of matrix metalloproteinase 10, nerve growth factor and substance P in the painful degenerate intervertebral disc. Arthritis Res Ther. 2009;11:R126. doi: 10.1186/ar2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Masuda K, Thonar EJMA, An HS, Cs-Szabo G. Age-related changes in the extracellular matrix of nucleus pulposus and anulus fibrosus of human intervertebral disc. Spine. 2008;34:10–16. doi: 10.1097/BRS.0b013e31818e5ddd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cs-Szabo G, Juan DRS, Turumella V, Masuda K, Thonar EJMA, An HS. Changes in mRNA and protein levels of proteoglycans of the anulus fibrosus and nucleus pulposus during intervertebral disc degeneration. Spine. 2002;27:2212–2219. doi: 10.1097/00007632-200210150-00006. [DOI] [PubMed] [Google Scholar]
- Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 2009;48:5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- Le Maitre CL, Pockert A, Buttle DJ, Freemont AJ, Hoyland JA. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soc Trans. 2007;35:652–655. doi: 10.1042/BST0350652. [DOI] [PubMed] [Google Scholar]
- Hadjipavlou AG, Tzermaidianos MN, Bogduk N, Zindrick MR. The pathophysiology of disc degeneration. A critical review. J Bone Joint Surg Br. 2008;90:1261–1270. doi: 10.1302/0301-620X.90B10.20910. [DOI] [PubMed] [Google Scholar]
- Purmessur D, Freemont AJ, Hoyland JA. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis Res Ther. 2008;10:R99. doi: 10.1186/ar2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockert AJ, Richardson SM, Le Maitre CL, Lyon M, Deakin JA, Buttle DJ, Freemont AJ, Hoyland JA. Modified expression of the ADAMTS enzymes and tissue inhibitor of metalloproteinases 3 during human intervertebral disc degeneration. Arthritis Rheum. 2009;60:482–491. doi: 10.1002/art.24291. [DOI] [PubMed] [Google Scholar]
- Roberts S, Evans EH, Kletsas D, Jaffray DC, Eisenstein SM. Senescence in human intervertebral discs. Eur Spine J. 2006;15:S312–S316. doi: 10.1007/s00586-006-0126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMaitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–R745. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathfield SK, Le Maitre CL, Hoyland JA. Caveolin-1 expression and stress-induced premature senescence in human intervertebral disc degeneration. Arthritis Res Ther. 2008;10:R87. doi: 10.1186/ar2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JD, Georgescu HI, McIntyre-Larkin L, Stefanovic-Racic M, Donaldson WF III, Evans CH. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine. 1996;21:271–277. doi: 10.1097/00007632-199602010-00003. [DOI] [PubMed] [Google Scholar]
- LeMaitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1ß and TNFa expression profile. Arthritis Res Ther. 2007;9:R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand N, Reichert F, Floman Y, Rotshenker S. Murine nucleus pulposus-derived cells secrete interleukins- 1-β, -6, and -10 and granulocyte-macrophage colony-stimulating factor in cell culture. Spine. 1997;22:2598–2601. doi: 10.1097/00007632-199711150-00002. [DOI] [PubMed] [Google Scholar]
- Huang KY, Chen W-Y, Lee C-L, Yan J-J, Chang M-S. IL-20 may contribute to the pathogenesis of human intervertebral disc herniation. Spine. 2008;33:2034–2040. doi: 10.1097/BRS.0b013e31817eb872. [DOI] [PubMed] [Google Scholar]
- Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatol. 2008;47:809–814. doi: 10.1093/rheumatology/ken056. [DOI] [PubMed] [Google Scholar]
- Theoleyre S, Wittrant Y, Tat SK, Fortun Y, Redini F, Heymann D. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004;15:457–475. doi: 10.1016/j.cytogfr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Leibbrandt A, Penninger JM. RANK/RANKL: regulators of immune responses and bone physiology. Ann N Y Acad Sci. 2008;1143:123–150. doi: 10.1196/annals.1443.016. [DOI] [PubMed] [Google Scholar]
- Mackiewicz A, Salo J, Konttinen YT, Holm AK, Indayl A, Pajarinen J, Holm S. Receptor activator of nuclear factor kappa B ligand in an experimental intervertebral disc degeneration. Clin Exp Rheumatol. 2009;27:299–306. [PubMed] [Google Scholar]
- McGonigle JS, Giachelli CM, Scatena M. Osteoprotegerin and RANKL differentially regulate angiogenesis and endothelial cell function. Angiogenesis. 2009;12:35–46. doi: 10.1007/s10456-008-9127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup GB, Lark MW, Veber DF, Bhattacharyya A, Blake S, Dare LC, Erhard KF, Hoffman SF, James IE, Marquis RW, Ru Y, Vasko-Moser JA, Tomaszet T, Gowen M. Potent and selective inhibition of human cathepsin K leads to inhibition of bone resorption in vivo in a nonhuman primate. J Bone Mineral Res. 2001;16:1739–1746. doi: 10.1359/jbmr.2001.16.10.1739. [DOI] [PubMed] [Google Scholar]
- Lecaille F, Choe Y, Brandt W, Li Z, Craik CS, Brömme D. Selective inhibition of the collagenolytic activity of human cathepsin K by altering its S2 subsite specificity. Biochemistry. 2002;41:8447–8454. doi: 10.1021/bi025638x. [DOI] [PubMed] [Google Scholar]