Abstract
Considerable progress has been made in the past few years in the development of therapeutic interventions that can reduce mortality in sepsis. However, encouraging physicians to put the results of new studies into practice is not always simple. A roundtable was thus convened to provide guidance for clinicians on the integration and implementation of new interventions into the intensive care unit (ICU). Five topics were selected that have been shown in randomized, controlled trials to reduce mortality: limiting the tidal volume in acute lung injury or acute respiratory distress syndrome, early goal-directed therapy, use of drotrecogin alfa (activated), use of moderate doses of steroids, and tight control of blood sugar. One of the principal investigators for each study was invited to participate in the roundtable. The discussions and questions that followed the presentation of data by each panel member enabled a consensus recommendation to be derived regarding when each intervention should be used. Each new intervention has a place in the management of patients with sepsis. Furthermore, and importantly, the therapies are not mutually exclusive; many patients will need a combination of several approaches – an 'ICU package'. The present article provides guidelines from experts in the field on optimal patient selection and timing for each intervention, and provides advice on how to integrate new therapies into ICU practice, including protocol development, so that mortality rates from this disease process can be reduced.
Keywords: intensive care unit, intervention, mortality, sepsis
Introduction
Sepsis is the tenth most common cause of death in the US [1]. A recent US study reported that severe sepsis accounts for in excess of 215,000 deaths annually from a total population of approximately 750,000 patients – a mortality rate of approximately 29% (with published studies quoting a range of 28–50%) [2].
This persistent, high mortality rate is clearly unacceptable, given that it ranks sepsis above some of the higher profile causes of in-hospital death, including stroke (12–19% risk of death in the first 30 days) and acute myocardial infarction (AMI) (8% risk of death in the first 30 days) [3]. Moreover, the actual number of deaths associated with the condition may be even higher than current estimates suggest. Many sepsis patients have at least one comorbidity and deaths are often attributed to these conditions rather than to sepsis [4,5,6]. Unfamiliarity with the signs and symptoms of sepsis may further hinder accurate diagnosis.
There are many possible reasons for this high mortality. Sepsis is certainly a complex disease state; the pathophysiology is only now beginning to be unraveled, and it is complicated by heterogeneous presentation (possible signs of sepsis are presented in Table 1). While none of these signs alone is specific for sepsis, the otherwise unexplained presence of these signs should signal the possibility of a septic response.
Table 1.
Possible signs of sepsis (adapted from [7])
| Parameters | Signs |
|---|---|
| General | Fever, chills |
| Inflammatory | Altered white blood cell count, increased serum concentrations of C-reactive protein or procalcitonin |
| Coagulopathy | Increased D-dimers, low protein C, increased prothrombin time/activated partial thromboplastin time |
| Hemodynamic | Tachycardia, increased cardiac output, low systemic vascular resistance, low oxygen extraction ratio |
| Metabolic | Increased insulin requirements |
| Tissue perfusion | Altered skin perfusion, reduced urine output |
| Organ dysfunction | Increased urea and creatinine, low platelet count or other coagulation abnormalities, hyperbilirubinemia |
Many cases of sepsis are recognized late, and patients are often inappropriately treated before entering the intensive care unit (ICU) by physicians unfamiliar with the signs and symptoms of the condition. Furthermore, treatment may be initiated by any of a number of physicians (anesthetists, hematologists, intensivists, infectious disease specialists, pulmonologists, and emergency physicians). There are presently various defined supportive strategies for treating patients with sepsis, but improvements are needed to reduce the unacceptably high mortality rate.
Moreover, as with other areas of medicine, the application and integration of new but proven strategies for reducing morbidity and mortality into clinical practice has been slow.
Encouraging new data have recently been presented on new approaches to the management of patients with sepsis. Many of these approaches attempt to modulate or interrupt the sepsis cascade and to address the cause of multiorgan dysfunction. Although many of these approaches are in early phases of development (e.g. antibodies to tumor necrosis factor [TNF] alpha, bactericidal permeability increasing protein, high-flow hemofiltration to remove circulating inflammatory mediators, platelet-activating factor acetyl hydrolase, and antielastases), other approaches are more advanced and are already beginning to impact on outcomes in the ICU.
At a roundtable discussion in London in June 2002, Professor Jean-Louis Vincent brought together five experts to discuss more effective implementation of five exciting new interventions in the ICU setting to decrease the unacceptable burden of mortality in patients with severe sepsis.
Each of the roundtable panelists is a highly respected physician in the world of sepsis and critical care medicine. The interventions discussed encompassed low tidal volume in patients with acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) (Edward Abraham), early goal-directed therapy (EGDT) (Emanuel Rivers), drotrecogin alfa (activated) (Gordon Bernard), moderate-dose corticosteroids (Djillali Annane), and tight control of blood sugar (GreetVan den Berghe).
Objectives
The purpose of the roundtable discussion was to provide guidance for clinicians on the integration of new interventions into the ICU to reduce the mortality in sepsis, on appropriate patient selection for these interventions, and on appropriate timing of these interventions.
The present review reports the discussions and recommendations of the panel.
Unacceptable mortality
The overall 30-day mortality in the ICU is typically ~20% [8]. The 30-day mortality in the population with severe sepsis, defined as sepsis with organ dysfunction, is 30–50%. It is clear from this figure that severe sepsis contributes disproportionately to the overall 30-day mortality in the ICU and compares unfavorably with some of the higher profile acute killers in hospital (e.g. stroke and AMI) [3]. Despite the general improvements in medicine overall, this mortality rate has remained essentially unchanged for the past 25 years. This has contributed to a feeling of pessimism among intensivists and other medical professionals regarding treatment prospects for severe sepsis, and a reluctance to rapidly incorporate new interventions into clinical practice [9].
Mortality reductions: slow and steady wins the race
Although the sepsis mortality rates are unacceptable, they camouflage some significant developments that are and have been occurring for hospital patients, for the general ICU population and, particularly, for those with severe sepsis. Direct comparison of mortality rates among patients with identical Acute Pysiology and Chronic Health Evaluation (APACHE) scores in the placebo arm of anti-TNF or anti-endotoxin studies published 10–15 years ago [10,11,12] with more recent studies [13,14], demonstrates that the mortality rate is much lower in more recent studies. Interestingly, this decrease was apparent even before the five interventions discussed in the present article were published, reflecting improvements in the general supportive care of sepsis patients.
Indeed, the panel contends that mortality from septic shock has already been reduced. Some patients who in the recent past would have died from severe sepsis or septic shock do not reach the ICU now because they are well managed on the wards, in the emergency department, and even during preoperative and postoperative care. For example, those sepsis patients that receive prompt antibiotic therapy have a 10–15% lower mortality rate than those who receive antibiotic therapy later in their care [15].
Progress is also being made in diagnosing sepsis: more patients are being tested to identify the source of infection and the pathogens involved, supportive care measures have been improved (e.g. hemodynamic support), and other measures have been put in place to reduce the incidence of nosocomial infections (e.g. reducing the need for pulmonary artery catheters by using echo techniques to assess cardiac function). There has also been a realization of the importance of specially trained intensive care physicians in the ICU. It has been internationally recognized that changing the ICU from an 'open format', whereby patients are cared for by their admitting physician, to a 'closed format', whereby patients are managed by appointed intensivists, reduces mortality rates [16].
Although the mortality rate is beginning to decline, it still remains unacceptably high. Furthermore, the number of patients with severe sepsis and septic shock is increasing; people are living longer, and there has been a rise in the number of immunocompromised patients due to aggressive cancer therapy and the increased prevalence of HIV.
Learning from other specialties
In-hospital AMI-associated mortality rates averaged approximately 25–30% in the 1960s [3]. This clearly unacceptable mortality rate was addressed by the development of a number of new pharmacological and mechanical interventions together with improvements in supportive care.
In the landmark Second International Study of Infarct Survival trial, published in 1988, 17,187 suspected AMI patients were treated with either streptokinase or aspirin, with both drugs, or with neither. The mortality rate in the combination group of this trial was 8%, compared with 13.2% in those patients given neither streptokinase nor aspirin [17].
Cardiologists have effectively implemented multiple pharmacologic and supportive care interventions to reduce mortality in AMI from 25–30% to 8% and lower. Not satisfied with this already remarkable figure, they are trying to reduce it further.
Physicians treating patients with sepsis are clearly faced with a very different situation to those treating patients with AMI, and so direct comparisons are not possible. However, several factors have contributed to the success of AMI therapy and possibly to the lack of such success in sepsis (Table 2).
Table 2.
A comparison of acute myocardial infarction (AMI) and sepsis
| AMI | Sepsis | |
|---|---|---|
| Market issues | Significant publicity surrounding and general awareness of the condition; large trials | Lack of understanding among physicians and the general public |
| Diagnosis | A relatively straightforward and relatively common diagnosis (electrocardiogram, enzymes, troponin), and one that can be made by generalists, not just cardiology specialists | Complicated by a long list of signs and symptoms and few objective tools for validation |
| Comorbidities | Generally single organ disease (notable exception when complicated by cardiogenic shock) | Often chronic or acute comorbidities |
| Physician education | Generalists have been taught to recognize the signs and symptoms of AMI; initial treatment is usually provided by emergency physicians, who are trained to treat these patients | Sepsis patients often come 'second hand' from a specialist who may not be appropriately trained to diagnose, manage, and refer patients with sepsis |
New interventions
Sepsis is undoubtedly complicated. However, many of the lessons that have been learned through effective application of therapies in other disease states can be applied to severe sepsis. Furthermore, the encouraging data that are beginning to appear in the literature indicate that sepsis may not be as intractable to treat as once thought.
The following sections provide salient information on five interventions that have shown a significant positive impact on mortality rates in sepsis, severe sepsis, septic shock, or sepsis-related diseases in recent clinical trials. The interventions were presented at the roundtable by one of the principal investigators of the key trial of the intervention. Each section concludes with recommendations for the integration of the particular intervention into clinical practice.
Low tidal volume in ALI/ARDS
Background
The traditional approach in patients with ALI/ARDS is to ventilate using tidal volumes between 10 and 15 ml/kg body weight, almost twice the average tidal volume at rest (7–8 ml/kg body weight), and to maintain a low positive end-expiratory pressure (PEEP). The purpose of this approach is to achieve normal values for the pH and partial pressure of arterial carbon dioxide. However, this method leads to high inspiratory airway pressures and to excessive stretch of the aerated lung.
In 1997, Tremblay et al. examined the effect of ventilation strategy on lung inflammatory mediators in the presence and absence of a pre-existing inflammatory stimulus in Sprague–Dawley rats [18]. In both stimulated and non-stimulated groups, the presence of inflammatory mediators (TNF-α, IL-1β, IL-6, IL-10, macrophage inflammatory protein 2, and IFN-γ) was highest in those rats ventilated with a large tidal volume and zero PEEP. Furthermore, in a study by Ranieri et al. in 1999 [19], the concentration of inflammatory mediators 36 hours after randomization of the groups was significantly lower in the lung-protective strategy group (tidal volume, 7.6 ± 1.1 ml/kg) than in the control group (tidal volume, 11.1 ± 1.3 ml/kg) (P < 0.05).
Following on from the positive results in the Tremblay et al. trial [18], a small study (53 patients) was carried out by Amato et al. in Brazil [20]. The mortality rate was 38% in patients given 'protective' ventilation (PEEP above the lower inflection point on the static pressure–volume curve, tidal volume <6 ml/kg ideal body weight, driving pressures <20 cmH2O above the PEEP value, permissive hypercapnia, and preferential use of pressure-limited ventilatory modes) compared with 71% in patients on conventional ventilation (P < 0.001). This impressive reduction in mortality was tempered by the higher than normal mortality level in the control group, prompting the National Institutes of Health-funded Acute Respiratory Distress Syndrome Network to set up a similar, larger (861 patients), prospective, multicenter, randomized trial in the US [21].
Protocol
For a summary of the protocol used in this study, see Appendix 1.
Key data
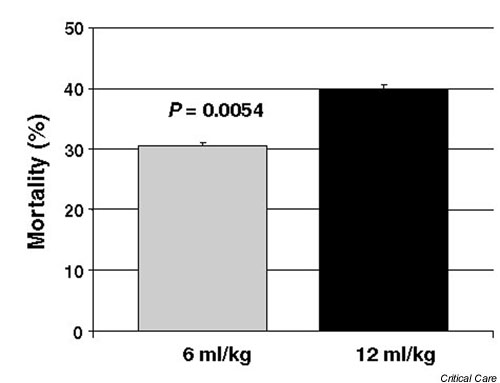
The trial was stopped after the fourth interim analysis because the use of lower tidal volumes was found to be associated with a significantly reduced mortality (P = 0.005 for the difference in mortality between groups).
The primary endpoints were mortality prior to hospital discharge with unassisted breathing and ventilator-free days (days alive, off mechanical ventilation, between enrollment and day 28). Both of these endpoints were achieved (Figs 1,2,3).
Figure 1.
Mortality prior to hospital discharge in patients receiving a tidal volume of 6 and 12 ml/kg ideal body weight.
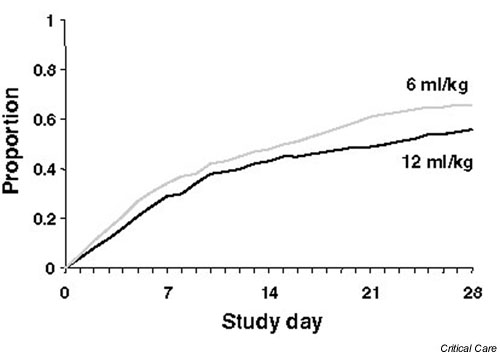
Figure 2.
Proportion of patients alive and off the ventilator having been ventilated with a tidal volume of 6 and 12 ml/kg ideal body weight.
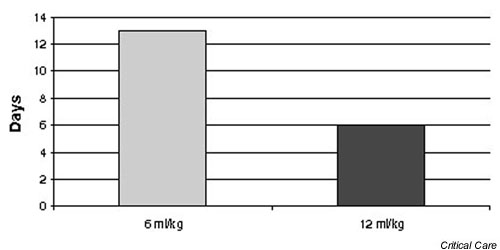
Figure 3.
Median number of ventilator-free days in patients receiving a tidal volume of 6 and 12 ml/kg ideal body weight.
In addition, patients receiving a tidal volume of 6 ml/kg ideal body weight had increased organ failure free days and lower IL-6 levels.
Implications
ALI is seen in 25–42% of patients with sepsis [22]. Although the approach has only been tested in patients with ALI/ARDS, a tidal volume of 6 ml/kg ideal body weight is at the lower end of the range of physiologic ventilation. Hence, this approach should be suitable for most patients in the ICU setting.
Furthermore, as many patients with severe sepsis or septic shock progress to frank ALI/ARDS, the panel believes that low tidal volume therapy is a valid option in these patients, and an option that may indeed prevent the development of ALI/ARDS.
Although patient selection in the clinical trial specified both blood gas and lung infiltrate criteria, at least 90% of patients in the general ICU setting meet the criteria for blood gas but not those for infiltrates. At the Vanderbilt University School of Medicine, patients receive low tidal volumes regardless of whether they have bilateral infiltrates.
Further questions
Does low tidal volume ventilation increase the occurrence of acidosis?
Acidosis is more likely to develop in patients with severe lung problems rather than in those exhibiting milder disease when tidal volumes are kept low. However, acidosis is seldom a clinical problem and rarely requires administration of bicarbonates.
Is low tidal volume therapy distressing for the patient?
One of the issues with low tidal volume therapy is that the patients are often more uncomfortable, at least initially, when they are being ventilated with a tidal volume of 6 ml/kg ideal body weight. The patients tend to exhibit tachypnea and may become more agitated. Sedation is generally required, but the ventilator setting can be maintained. Of more concern is that ICU staff may consider a respiration rate of 40/min to be a sign of something more serious and may attempt to terminate the intervention. Education of staff is clearly essential.
Would provision of extrinsic PEEP cause hemodynamic problems?
The strategy assessed in this trial not only includes ventilation with a low tidal volume, but also the provision of extrinsic PEEP. There may be some concern that an increased respiratory rate may result in intrinsic PEEP and hemodynamic problems (e.g. decreased cardiac filling, decreased cardiac output, and diminished blood pressure). The panel believes that auto PEEP was not an issue in the Acute Respiratory Distress Syndrome Network study. In addition, in the groups with low tidal volume, at least 10% more oxygen was required to maintain the fraction of inspired oxygen (FiO2), suggesting that there was very little auto PEEP occurring.
Recommendations of the panel
When mechanical ventilation is indicated for treatment of patients with ALI/ARDS, the tidal volume should be limited to ~6 ml/kg ideal body weight.
Early goal-directed therapy (EGDT)
Background
Goal-directed therapy represents an attempt to adjust the cardiac preload, afterload, and contractility to balance systemic oxygen delivery with oxygen demand. In patients with severe sepsis and septic shock, such an approach would seem eminently reasonable as part of general supportive measures to restore and maintain adequate cellular perfusion and to prevent organ dysfunction. In the setting of the ICU, however, supranormal and normal approaches have met with little or no success [23,24]. It is possible that, by the time these therapies are applied in the ICU, any such intervention may have been too late. Hence, the focus has shifted towards hemodynamic optimization in the early presentation of disease, such as in the emergency department.
A prospective, randomized, predominantly blinded study was initiated by the Early Goal-Directed Therapy Collaborative Group to examine the results of hemodynamic interventions in the emergency department [25]. In this study, patients were randomly assigned to either 6 hours of EGDT or to standard therapy prior to admission to the ICU.
Protocol
Baseline characteristics (including the adequacy and duration of antibiotic therapy) in the EGDT and standard therapy groups were not significantly different. The vital signs, resuscitation endpoints, organ dysfunction scores, and coagulation-related variables were similar in these groups at baseline [25]. However, there were some important differences between the treatment groups (see Table 3).
Table 3.
Therapeutic interventions: standard therapy versus EGDT
| Intervention | 0–6 hours | 7–72 hours | 0–72 hours |
|---|---|---|---|
| Fluid therapy (l) | -2.49a | +1.98b | +0.085 |
| Receiving vasopressors (%) | +2.9 | +13.8d | +14.5c |
| Receiving inotropes (%) | -12.9a | -6.1 | -6.2 |
| Receiving red blood cell transfusion (%) | -45.6a | -21.7a | -23.9a |
| Mechanical ventilation instituted (%) | +0.8 | +14.6a | +15.0c |
| Pulmonary artery catheter use (%) | +3.4 | +10.6e | +13.9b |
A negative or positive value indicates how the control group therapy compares with the treatment group. a P < 0.001, b P = 0.01, c P = 0.02, d P = 0.03, e P = 0.04. EGDT, early goal-directed therapy.
Patients who were randomized to standard therapy received a central venous pressure (CVP) arterial line as well as Foley catheterization. The endpoints were adjusted for a CVP of 8–12 mmHg, a mean arterial pressure (MAP) >65 mmHg, and a urine output of at least 0.5 ml/kg/hour.
Patients randomized to EGDT received the same therapy but, in addition, were monitored for the endpoint of central venous oxygen saturation (ScvO2) >70%. EGDT patients were given more intravenous fluids (including blood transfusions) and more inotropic support (mostly dobutamine).
For more information on the protocol used in this study, see Appendix 2.
Key data
Key data are presented in Table 4. The in-hospital mortality was 30.5% in the group assigned to EGDT and was 46.5% in the group assigned to standard therapy (P = 0.009), indicating that EGDT provides significant benefits in improving outcomes in patients with severe sepsis and septic shock.
Table 4.
Outcome measures: percentage change or improvement, baseline to 72 hours
| Outcome measure | Control | Treatment | P value |
|---|---|---|---|
| ScvO2 * | 32.7 | 44.9 | 0.001 |
| Lactate* | 43.5 | 61.0 | 0.02 |
| Base deficit* | 42.7 | 77.5 | 0.001 |
| pH* | 5.4 | 12.3 | 0.001 |
| APACHE II* | 24.5 | 40.2 | 0.002 |
| Mortality in hospital | 46.5 | 30.5 | 0.009 |
| Mortality at 28 days | 49.2 | 33.3 | 0.01 |
| Mortality at 60 days | 56.9 | 44.3 | 0.03 |
| Length of hospital stay† | - | 20.7 | 0.04 |
APACHE II, Acute Physiology and Chronic Health Evaluation; ScvO2, central venous oxygen saturation. * Baseline to 72 hours. †Surviving to hospital discharge.
During the interval from 7 to 72 hours, patients assigned to EGDT exhibited a more significant improvement in mean ScvO2 (70.4 ± 10.7% versus 65.3 ± 11.4%), in lactate concentration (3.0 ± 4.4 mmol/l versus 3.9 ± 4.4 mmol/), in base deficit (2.0 ± 6.6 mmol/l versus 5.1 ± 6.7 mmol/l), and in pH (7.40 ± 0.12 versus 7.36 ± 0.12) than patients assigned to standard therapy (P ≤ 0.02 for all comparisons). During the same period, the mean APACHE II scores were significantly lower, indicating less severe organ dysfunction, in the patients assigned to EGDT than in those patients assigned to standard therapy (13.0 ± 6.3 versus 15.9 ± 6.4, P < 0.001).
Implications
The protocol was based predominantly on guidelines published in 1999 by the Society of Critical Care Medicine [26]. However, these guidelines have not been universally followed in clinical practice since their publication. An increasing number of critically ill patients are presenting to, and being treated in, emergency departments [27,28]. This is presenting significant resource challenges in the emergency department environment. The inability to institute EGDT may thus not be a conscious decision by the clinician not to follow the Society of Critical Care Medicine guidelines. Emergency medicine in general may have to develop and formulate the cost-benefit analysis to support or implement such care in this environment in order to improve outcomes.
There are sufficient evidence-based data to recommend that all patients with severe sepsis or septic shock should receive early and aggressive resuscitation based on this EGDT protocol (see Appendix 2). It is important that the interventions are individualized to each patient.
Further questions
Why was there no difference in mechanical ventilation or vasopressor use between the standard treatment and EGDT groups during the first 6 hours, but a large difference in fluid transfusions and, especially, in dobutamine administration?
With a goal-oriented protocol, patients are stratified based on hemodynamic derangements. Using measurements of ScvO2, it is possible to identify patients with profound global myocardial dysfunction who are hence at risk of impaired perfusion. These patients, almost 15% of those in the EGDT group, received dobutamine during the first 6 hours because myocardial suppression was diagnosed. Once myocardial dysfunction is corrected (and compliance improved), these patients become more suitable for volume loading, so this group received almost 3.5 liters more fluids in the first 6 hours than the control patients. Therefore, although vasopressor use was similar in the first 6 hours, patients in the EGDT group were more aggressively weaned off these agents during this period, resulting in fewer patients in this group entering the ICU on vasopressors than in the control group. The lack of aggressive volume loading in the control group led to greater use of vasopressors in patients over the subsequent 72 hours. In spite of more volume loading, the EGDT group received less mechanical ventilation over the subsequent 72 hours than in the standard treatment group.
Why was cardiovascular collapse a significant cause of death in the control group?
Cryptic shock (shock with normal vital signs) is a frequent occurrence in early severe sepsis and septic shock. Despite resuscitation to the goals for mean arterial blood pressure and CVP, almost 40% of control patients continued to exhibit global tissue hypoxia (decreased ScvO2 and increased lactate levels); in these patients, there was a twofold increase in hemodynamic deterioration, requiring more mechanical ventilation, pulmonary artery catheterization, and vasopressor use in the subsequent 72 hours.
How do severe sepsis and septic shock differ hemodynamically in the early stages compared with that classically described in the ICU?
Patients presenting with early sepsis and septic shock are characterized by hypovolemia (low CVP), normal to increased blood pressures, and decreased cardiac output (decreased central venous oxygen saturation and low cardiac index). This is in contrast to ICU patients who are euvolemic, have high ScvO2, and have elevated cardiac indices [29].
What are the most important ways in which EGDT can improve outcomes?
The key factors are early detection of high-risk patients in cryptic shock, early reversal of hemodynamic perturbations and global tissue hypoxia, prevention of acute cardiovascular collapse, and the possibility of preventing the inflammatory aspects of global tissue hypoxia that accompany the inflammation or infection.
Recommendations of the panel
Severe sepsis and septic shock patients should receive early aggressive therapy to restore and maintain oxygen availability to the cells. There should also be generous use of fluids and inotropic agents titrated by appropriate hemodynamic monitoring.
Drotrecogin alfa (activated)
Background
A large number of observational studies have shown that patients with sepsis have severe depletion of protein C [30,31]. A number of studies have also shown the association of protein C depletion with high mortality in sepsis [32,33,34]. Furthermore, baboon studies have demonstrated that treatment with activated protein C prevents death from live Escherichia coli infusions [35,36].
Activated protein C exerts a number of actions. Anticoagulant action includes the inactivation of coagulation factors Va and VIIIa, and the inhibition of the formation of thrombin. Profibrinolytic action allows the activity of tissue plasminogen activator (endogenous tissue plasminogen activator), by inactivating plasminogen activator inhibitor 1 and thrombin activatable fibrinolysis inhibitor. Finally, anti-inflammatory action reduces IL-6 (in vivo) and proinflammatory cytokines (in vitro).
The specific mechanisms by which drotrecogin alfa (activated) exerts its effect on survival in patients with severe sepsis are not completely understood.
The efficacy of drotrecogin alfa (activated) (recombinant human activated protein C) in reducing mortality in patients with severe sepsis was investigated in a large multicenter, blinded, placebo-controlled, randomized, phase III clinical trial, the Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) trial [14]. All patients in the PROWESS trial received standard supportive care in addition to either drotrecogin alfa (activated) or placebo.
Protocol
For a summary of the protocol used in the PROWESS study, see Appendix 3.
Key data
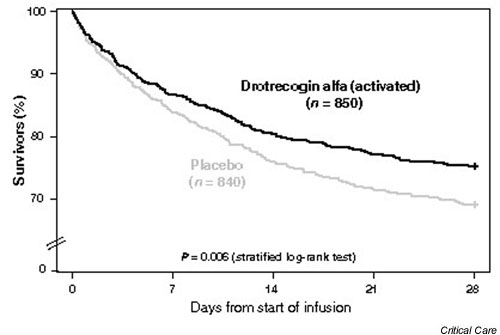
The overall mortality in patients treated with drotrecogin alfa (activated) was 24.7% compared with 30.8% in patients receiving placebo, an absolute risk reduction of 6.1% (P = 0.006) (see Fig. 4). The absolute risk reduction in patients with high risk of death defined by an APACHE II score ≥ 25 was 12.8% (P < 0.001). The absolute risk reduction in patients with high risk of death defined by multiple organ failure was 7.4% (P = 0.006).
Figure 4.
Twenty-eight-day survival in patients treated with drotrecogin alfa (activated) or placebo: all-cause mortality.
No substantial differences in drotrecogin alfa (activated) treatment effects were observed in subgroups defined by gender, ethnic origin, or infectious agent.
Implications and further questions
Can drotrecogin alfa (activated) be used in patients on dialysis for pre-existing renal failure, a category that was specifically excluded in the PROWESS trial?
No pharmacokinetic data were available on drotrecogin alfa (activated) in patients on chronic dialysis when the PROWESS trial began, so such patients were excluded from the trial. Subsequent research has shown that the pharmacokinetics of drotrecogin alfa (activated) are not substantially changed in patients on chronic dialysis.
How relevant is the 48-hour maximum treatment window to clinical practice?
The design of the PROWESS trial allowed a maximum of 48 hours between the onset of first organ dysfunction and the receipt of drotrecogin alfa (activated) (a 24-hour window was allowed for receipt of the drug following the first confirmation of first organ dysfunction, which in turn had to have been present for no more than 24 hours). The treatment effect of drotrecogin alfa (activated) was consistent across all time intervals from meeting the entry criteria to the receipt of the study drug. Treatment with drotrecogin alfa (activated) thus does not appear to be as time critical as interventions such as tissue plasminogen activator in stroke or myocardial infarction. Because most of the experience with drotrecogin alfa (activated) was based on organ failure times less than 48 hours, treatment should not be delayed when an appropriate candidate is identified. The time window employed in the PROWESS trial should allow a full history to be taken and other tests to be performed to determine the bleeding risk.
Does the bleeding risk vary among patient subgroups?
As with all anticoagulants, drotrecogin alfa (activated) is associated with a risk of severe bleeding. During the infusion period in the PROWESS trial, the bleeding rates were 2.4% in the drotrecogin alfa (activated) group versus 1.0% in the placebo group (P = 0.024). The risk of bleeding was fairly constant across most subgroups. However, severe thrombocytopenia (<30,000/mm3) was commonly associated with serious bleeding and intracerebral hemorrhage.
What criteria should be used to identify patients who may benefit from drotrecogin alfa (activated)?
Patients at high risk of death in the PROWESS trial were most likely to benefit from drotrecogin alfa (activated). In the PROWESS trial, the APACHE II score was the most effective predictor of risk of death and likelihood of benefit from drotrecogin alfa (activated), particularly in those patients with an APACHE II score ≥ 25.
In the PROWESS trial, the number of organ dysfunctions was also an important indicator that supported an association between likelihood of benefit from drotrecogin alfa (activated) and risk of death. Two or more organ dysfunctions identify a population that responds well to therapy, and is a practical measurement.
If a patient has one organ dysfunction and looks likely to develop a second, should we delay treatment with drotrecogin alfa (activated)?
The panel believes that acute respiratory failure or hypotension unresponsive to fluid challenge should suggest the use of drotrecogin alfa (activated). However, coagulopathy, a platelet count <80,000/mm3, acidosis, or low urine output alone should not suggest its use.
A very large international study of 11,500 patients will be started in late 2002 to investigate the efficacy of drotrecogin alfa (activated) in patients with a single organ failure and/or APACHE scores <25.
Should we only prescribe drotrecogin alfa (activated) to patients in the ICU or can it be given beforehand?
The decision on whether to administer the drug should ultimately depend on whether the patient meets the selection criteria. A patient presenting in the emergency room with acute respiratory failure or acute cardiovascular decompensation should receive appropriate treatment there.
The drawback to treatment in the emergency room is that there may not be sufficient time in which to evaluate the patient's bleeding risks. Delaying treatment for a few hours will enable more tests to be performed and a fuller history to be taken, both of which will provide a better indication of whether drotrecogin alfa (activated) is appropriate.
Do the treatment dose of drotrecogin alfa (activated) and the duration of therapy remain the same under all circumstances?
The dose is always the same (24 μg/kg/hour), regardless of the type of organ failure or the degree of sepsis severity. In addition, the 96-hour window of treatment is always the same so that interruptions of treatment are made up at the end to maintain a total of 96 hours of treatment.
Do patients require any laboratory testing before they receive drotrecogin alfa (activated)?
No laboratory testing was carried out in the PROWESS trial, and subgroup analysis identified no biochemical marker that conclusively indicates treatment. For example, treatment-associated reductions in mortality were observed in patients with normal protein C levels and in those with low protein C levels. Clinical criteria are recommended for the initiation of therapy.
Can drotrecogin alfa (activated) be given to a patient with severe sepsis who is on anticoagulants (e.g. warfarin) or antiplatelet agents (e.g. aspirin or glycoprotein IIb/IIIa inhibitors) for cardiac disease?
Aspirin (650 mg/day) was allowed in the PROWESS trial. Patients on glycoprotein IIb/IIIa inhibitors were excluded because no data were available regarding drug interactions and pharmacokinetics. Use of these types of agents is likely to increase the risk of bleeding with drotrecogin alfa (activated) therapy. The anticipated benefits must therefore be weighed against the potential risks. In the PROWESS trial, efforts were made to correct the international normalized ratio towards normal if it was greater than 3 at any time during infusion of drotrecogin alfa (activated).
Is it acceptable to treat concomitantly with steroids?
Approximately one-third of patients in the PROWESS trial received steroids at the same time as drotrecogin alfa (activated). There was no interaction with steroid use, presumably because the mechanism of action of steroids is so different from that of activated protein C. Hence, steroids should be used if they are needed, and if the patient qualifies for drotrecogin alfa (activated) the two should be used together.
Recommendations of the panel
Drotrecogin alfa (activated) should be considered for use in all adult patients with recent onset severe sepsis or septic shock, and a high risk of death.
Moderate-dose corticosteroids
Background
The value of steroids in the treatment of patients with severe sepsis and septic shock has been fiercely debated for some time. Although a number of well-designed, randomized, controlled trials failed to show any benefits of steroid therapy in terms of improved survival in patients with severe sepsis (reviewed in [37,38]), with mortality increased in many as a result of an increased incidence of nosocomial infections, these trials were primarily investigating the efficacy of short courses of high-dose steroids. The question of whether lower doses of steroids may provide benefit in these patients has only recently been addressed.
There is a relatively strong rationale for considering the use of steroids in patients with refractory septic shock. Relative adrenal insufficiency is common in patients with refractory septic shock (50–75% of patients) [39]. In addition to such relative adrenal insufficiency and the blunted response to corticotrophin, a large body of evidence indicates that sepsis and refractory septic shock are characterized by peripheral tissue resistance to corticosteroids [40,41]. In septic patients, this can be evidenced in a variety of ways. First, global cortisol binding, which carries cortisol from the adrenal glands to the tissues, decreases in patients with severe sepsis [42]. Second, the number and binding affinity of glucocorticosteroid receptors may be reduced in patients with sepsis and severe sepsis [43], leading to a decrease in the conversion of cortisone to its active form, cortisol, particularly by IL-2 levels in the tissues. Finally, data have been published demonstrating that moderate doses of steroids may restore cell sensitivity to vasopressors [44]. This may reduce the intensity of the inflammatory response and decrease organ dysfunction. Low-dose steroid treatment is also well tolerated [40].
This body of evidence prompted the initiation of a phase III randomized, controlled trial performed in 19 centers in France with 300 patients [45]. The aim of the trial was to determine whether moderate-dose corticosteroid therapy affected survival in patients with refractory septic shock and adrenal insufficiency.
Protocol
All patients had to be treated with vasopressor agents and mechanical ventilation. For a summary of the protocol used in this study, see Appendix 4.
Key data
Patients were stratified according to their response to the adrenocorticotrophic hormone (ACTH) test. Nonresponders were defined by an increment in cortisol levels <9 μg/dl or <250 nM/l after challenge with 250 μg cosyntropin. Of the 300 patients included, there were 229 nonresponders to the corticotropin test (placebo, 115 patients; steroids, 114 patients). A significant survival benefit was demonstrated among non-responders receiving moderate-dose corticosteroids. There were 73 deaths in the placebo group (63%) and 60 deaths in the steroid group (53%) (hazard ratio, 0.67; 95% confidence interval, 0.47–0.95; P = 0.023).
Implications and further questions
Should corticosteroid administration be guided by the ACTH test?
No beneficial effects were observed in the subset of patients who were classified as responders. Hence, in this paradigm, the ACTH test serves as a useful prognostic measure. Since a beneficial effect was observed in the total population, however, the need for an ACTH test can be challenged and further studies are required.
If an ACTH test is performed, corticosteroid administration can be started before results are received.
What is the optimal timing for this intervention?
Moderate-dose corticosteroids should be administered to patients with established refractory septic shock.
What is the optimal dose for this intervention?
Hydrocortisone should be given daily at a dose of 200–300 mg. Fludrocortisone should be given daily at a dose of 50 μg.
What is the optimal duration for this intervention?
Moderate doses of steroids should be given for 7 days.
Should hydrocortisone be given as a continuous infusion?
Hydrocortisone can be administered as serial boluses or as a continuous infusion. It may be that rebound phenomena at treatment discontinuation are more frequent when hydrocortisone is given as a continuous infusion. In addition, in the phase III randomized trial, hydrocortisone was given as serial boluses.
Should a mineralocorticoid be systematically added to hydrocortisone?
The phase III randomized trial has shown that the combination of hydrocortisone and fludrocortisone increased survival. In addition, sepsis is more frequently associated with a mineralocorticoid deficiency than a glucocorticoid deficiency. Hence, fludrocortisone should be added to hydrocortisone.
Recommendations of the panel
Administration of moderate-dose corticosteroids should be considered in cases of refractory septic shock, particularly in those with relative adrenal insufficiency. It is recommended that an ACTH test be carried out before starting the intervention.
Tight control of blood sugar
Background
Hyperglycemia, caused by insulin resistance in the liver and muscle, is a common finding in ICU patients. It can be considered an adaptive response, providing glucose for the brain, red cells, and wound healing, and is generally only treated when blood glucose increases to >215 mg/dl (>12 mmol/l).
Previous studies have shown that high levels of insulin-like growth factor binding protein 1 (a very good marker of lack of hepatic insulin effect) predict mortality [46,47]. Patients with high insulin-like growth factor binding protein 1 also tend to have the lowest insulin levels, indicating that beta cell function is impaired and, therefore, not enough insulin is being produced. These results indicate that hyperglycemia may not always be adaptive and that it should be treated to avoid the onset of specific complications.
Nevertheless, conventional wisdom in the ICU has been that hyperglycemia is beneficial and that hypoglycemia should be avoided.
The hypothesis that hyperglycemia (>110 mg/dl, >6.1 mmol/l) predisposes to specific ICU complications, prolonged intensive care dependency and death was tested in a prospective, randomized, controlled trial [48].
Protocol
For a summary of the protocol used in this study, see Appendix 5.
Key data
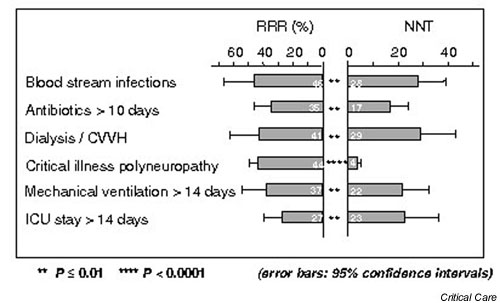
Thirty-five of the 765 patients (4.6%) in the intensive insulin group died in the ICU, compared with 63 patients (8.0%) in the conventional therapy group. For further mortality data on both the length of hospital stay and the cause of death, see Tables 5 and 6. For morbidity data, see Figure 5.
Table 5.
Mortality results [46]
| Insulin treatment (%) | |||
|---|---|---|---|
| Mortality | Conventional (n = 783) | Intensive (n = 765) | P value |
| Intensive care unit deaths (n = 1548) | 8.0 | 4.6 | 0.005* |
| 5-Day mortality rate | 1.8 | 1.7 | 0.9 |
| Long-stayers (n = 451) | 20.2 | 10.6 | 0.005 |
| In-hospital deaths (n = 1548) | 10.9 | 7.2 | 0.01 |
| Long-stayers (n = 451) | 26.3 | 16.8 | 0.01 |
*After correction for multiple interim analyses, adjusted P = 0.036.
Table 6.
Mortality: total number of patients from various causes of death [46]
| Insulin treatment | |||
|---|---|---|---|
| Cause of death | Conventional (n = 783) | Intensive (n = 765) | P value |
| Total number | 0.02 | ||
| Acute cardiovascular collapse | 7 | 10 | |
| Severe brain damage | 5 | 3 | |
| Multiple organ failure, no sepsis focus | 18 | 14 | |
| Multiple organ failure, with sepsis focus | 33 | 8 | |
Figure 5.
Most important effects on morbidity [46]. CVVH, continuous venovenous hemofiltration; ICU, intensive care unit; NNT, number needed to treat; RRR, relative risk reduction.
Implications
Tight control of blood sugar, as outlined in Appendix 5, requires a strict protocol for insulin administration and repeated determination of blood sugar.
Further questions
Since the study was mainly in surgical patients, would this intervention be suitable for medical patients in the ICU?
This is yet to be proven, and is the subject of an ongoing study. Because medical patients tend to stay in the ICU longer than surgical patients, the results from this study indicate that this intervention would be even more favorable to medical ICU patients. However, one needs to be careful with application of the algorithm in certain disease states, especially severe hepatic dysfunction and renal failure.
Does the glucose intake only include intravenous glucose?
No, all carbohydrates are included. See Appendix 5 for guidelines on feeding.
Why was this level of blood glucose chosen?
The level was chosen because it is in the physiologic range for healthy people.
Is it insulin that is important or simply better control of blood sugar?
As well as its effect on glycemia, insulin has been shown to inhibit TNF-α and macrophage inhibitory factor (when infused concomitantly with glucose). This has led to some doubts as to whether the effect in this study was due to normalization of blood glucose levels. However, multivariate analysis of all the risk factors for mortality, including severity of illness on admission, indicated that blood glucose determines the outcome; there was a 75% increase in risk of death per 50 mg/dl increase in blood glucose.
Would glucose control with another drug have the same effect?
It is not yet possible to determine this. Although it was blood glucose levels that were measured, the effects of insulin may in fact be on free fatty acids, as they change in parallel with blood glucose. One of the key mechanisms may be prevention of hypertriglyceridemia and high concentrations of free fatty acids.
Recommendations of the panel
It is strongly advisable to tightly control blood sugar close to physiologic levels, especially in surgical patients. Implementation of this recommendation requires a well-defined ICU protocol.
Optimal outcomes through appropriate patient identification and appropriate timing of therapy
The interventions discussed in the present article have been applied in different patient populations and at different times in the course of the disease (see Table 7).
Table 7.
Summary of the five interventions and recommendations of the panel on clinical application of each
| Intervention | Patient population studied | Timing of intervention | Recommendations of the panel |
|---|---|---|---|
| Low tidal volume | ALI/ARDS patients fulfilling blood gas criteria and with bilateral infiltrates | Evidence of ALI/ARDS. Earlier application warranted in patients with sepsis likely to develop ALI/ARDS | Tidal volume should be limited to ~6 ml/kg in patients with ALI/ARDS requiring mechanical ventilation |
| Early goal-directed therapy | Emergency room patients with two out of the four SIRS criteria and systolic blood pressure ≤ 90 mmHg or lactate ≥ 4 mmol/l | Pre entry into the ICU | Severe sepsis and septic shock patients should receive early aggressive hemodynamic therapy, and fluids and inotropic agents where indicated |
| Drotrecogin alfa (activated) | Severe sepsis patients as defined by three or more SIRS criteria plus at least one acute organ dysfunction | Within 48 hours of diagnosis of the most recent organ dysfunction | Patients with severe sepsis and high risk of death (e.g. APACHE II score ≥ 25, or two or more organ dysfunctions) |
| Moderate-dose corticosteroids | Refractory septic shock | As soon as refractory septic shock develops | Administer to refractory septic shock patients, particularly those with relative adrenal insufficiency, after an ACTH test has been carried out |
| Tight control of blood sugar | Mostly surgery patients with SIRS or sepsis | ICU admission | Tightly control blood sugar close to physiologic levels |
ACTH, adrenocorticotrophic hormone; ALI, acute lung injury; APACHE II, Acute Physiology and Chronic Health Evaluation II; ARDS, acute respiratory distress syndrome; ICU, intensive care unit; SIRS, systemic inflammatory response syndrome.
It is essential for physicians to understand that these therapies are not mutually exclusive. Optimal patient management may require a combination of approaches: mechanical ventilation to preserve lung function, hemodynamic support to maintain adequate ScvO2, intensive insulin therapy to normalize blood sugar, steroids to provide adequate immunosuppression, and drotrecogin alfa (activated) to prevent the systemic coagulopathy characteristic of severe sepsis and, hence, to preserve organ function. A sound understanding of the indications and contraindications of these interventions will guide appropriate intervention.
Similarly, the timing of therapy needs to be closely monitored. Education in the signs and symptoms of sepsis and severe sepsis should prompt early initiation of therapy. Many of the interventions discussed in this article were tested at specific points in the evolution of sepsis, yet in clinical practice their use may be required at a different stage of disease. For example, a large trial of drotrecogin alfa (activated) will determine the efficacy of the drug in patients with a single organ failure or APACHE II score <25.
How can use of these new interventions be encouraged among the general ICU community?
Despite the wealth of data to support the approaches discussed, it is clear that uptake of these interventions into clinical practice has been slow. Although there may be practical reasons for this, it would appear in many cases to involve either unfamiliarity with the data or a reluctance, or at least inertia, to change established practices (witness the necessity of proving that hypoglycemia is beneficial in ICU patients despite no good evidence to the contrary).
The ICU has changed in the past 30 years; there are more tools to use and more interventions to implement. Despite application of new methods, however, outcomes have changed very little and certainly not in proportion to the changes that were expected based on the results from clinical trials. Efficient integration of new interventions into the wider ICU population is clearly essential.
The panel believes that optimal use of existing therapies and the integration of proven new therapies will reduce mortality rates. Further positive results from new trials with improved trial designs should encourage intensivists to incorporate new interventions into their practice.
Protocols
Protocols are essential to ensure efficient integration of new therapies and to improve outcomes on the wards. Morris predicted in a recent paper that an increase in compliance with evidence-based recommendations through the use of protocols would decrease error and would enhance patient safety [49]. However, a complete treatment protocol is only effective when each ward (inside and outside of the ICU) has the trained staff to implement it, and when a skilled intensive care physician is available to lead the team. Training and education of staff is essential.
Education
An initiative discussed recently (March 2002) in Brussels at a roundtable conference sponsored by the European Society of Intensive Care Medicine and the Society of Critical Care Medicine was to expand the scope of ICU care to the pre-ICU setting, and even up to 1 year post-ICU, so as to develop a better understanding of the issues surrounding ICU care. A commonly used term is that there are 'doctors without borders'. Similarly, there should be ICU physicians without borders. There is a belief among many physicians that a patient only becomes an ICU patient on arrival at the unit, yet there are many critically ill patients in non-ICU settings. Disease definitions need to be broadened to such an extent that they are no longer by location.
Conclusion
All five of the interventions discussed in this article have generated convincing evidence for their use, and they hold out hope for reducing mortality in patients with sepsis, severe sepsis and septic shock. Yet, despite compelling data, the application of these interventions has yet to become routine practice in most ICUs.
It is our hope that this article will enable physicians to understand how best to apply these therapies in clinical practice; from appropriate patient selection and timing of therapy, to combining different approaches for optimal patient management.
A willingness to embrace new interventions, coupled with the development and implementation of rigorous protocols to ensure appropriate use, will improve outcomes and lead to a substantial reduction in mortality in these patients.
Competing interests
JLV, EA, GB and ER are consultants to Eli Lilly and Company. All authors received an honorarium/grant for participating in this meeting.
Appendix 1: low tidal volume
Patient selection
Inclusion criteria
• Partial pressure of arterial oxygen/FiO2 ≤ 300 mmHg.
• Bilateral infiltrates consistent with pulmonary edema on frontal chest radiograph.
• No clinical evidence of left atrial hypertension, pulmonary artery wedge pressure ≤ 18 mmHg if measured.
• Positive pressure ventilation via endotracheal tube.
Exclusion criteria
• 36 hours had elapsed since they met the first three inclusion criteria.
• Younger than 18 years old.
• Participated in other trials within 30 days before the first three criteria were met.
• Pregnant.
• Increased intracranial pressure.
• Neuromuscular disease that could impair spontaneous breathing.
• Sickle cell disease.
• Severe chronic respiratory disease.
• Weighed more than 1 kg per centimeter of height.
• Burns over more than 30% of their body surface area.
• Other conditions with an estimated 6-month mortality rate greater than 50%.
• Undergone bone marrow or lung transplantation.
• Chronic liver disease (as defined by Child–Pugh class C).
• Attending physician refused or was unwilling to agree to the use of full life support.
Appendix 1 Table 1.
National Institutes of Health Acute Respiratory Distress Syndrome Network lower tidal volume ventilation for acute lung injury/acute respiratory distress syndrome protocol summary
| Variable | Protocol |
|---|---|
| Ventilator mode | Volume assist control |
| Tidal volume | ≤ 6 ml/kg predicted body weight (subsequent adjustments were made to maintain plateau pressure ≤ 30 cmH2O) |
| Plateau pressure | ≤ 30 cmH2O |
| Ventilation set rate/pH goal | 6–35 breaths/min, adjusted to achieve arterial pH 7.3–7.45 if possible |
| Inspiratory flow, I:E | Adjust flow to achieve I:E of 1:1–1:3 |
| Oxygenation goal | PaO2 of 55 ≤ 80 mmHg, or SpO2 of 88% ≤ 95% |
| FiO2/PEEP (mmHg) combinations | 0.3/5, 0.4/5, 0.4/8, 0.5/8, 0.5/10, 0.6/10, 0.7/10, 0.7/12, 0.7/14, 0.8/14, 0.9/14, 0.9/16, 0.9/18, 1.0/18, 1.0/22, 1.0/24 |
| Weaning | Attempts to wean by pressure support required when FiO2/PEEP ≤ 0.40/8 |
FiO2, fraction of inspired oxygen; I:E, inspiration:expiration; PaO2, partial pressure of arterial oxygen; PEEP, positive end-expiratory pressure; SpO2, oxygen saturation via pulse oximetry.
Appendix 2: early goal-directed therapy
Patient selection
Inclusion criteria
• Fulfillment of at least two out of the four systemic inflammatory response syndrome criteria and systolic blood pressure ≤ 90 mmHg after a 20–30 ml/kg volume challenge over 30 min, or
• Fulfillment of at least two of the four systemic inflammatory response syndrome criteria and lactate ≥ 4 mmol/l.
Exclusion criteria
• Younger than 18 years old.
• Pregnant.
• Presence of an acute cerebral vascular event.
• Acute coronary syndrome.
• Acute pulmonary edema.
• Status asthmaticus.
• Cardiac dysrhythmias.
• Contraindication to central venous catheterization.
• Active gastrointestinal hemorrhage.
• Seizure.
• Drug overdose.
• Burn injury.
• Trauma.
• Requirement for immediate surgery.
• Uncured cancer (during chemotherapy).
• Immunosuppression (due to organ transplantation or systemic disease).
• Do-not-resuscitate status.
• Advanced directives restricting implementation of the protocol.
Protocol
When the CVP, MAP and ScvO2 are optimized to the levels presented in Appendix 2 Table 1, if ScvO2 <70%, then administer 2.5 μg/kg/min dobutamine. Increase the dose by 2.5 μg/kg/min every 30 min until ScvO2 ≥70% or the dose of dobutamine has reached 20 μg/kg/min.
Appendix 2 Table 1.
Optimized levels for central venous pressure, mean arterial pressure, and central venous oxygen saturation (ScvO2)
| Variable | Protocol |
|---|---|
| Central venous pressure | 8–12 mmHg (achieved by giving a 500 ml bolus of crystalloid every 30 min) |
| Mean arterial pressure | ≥ 65 ≤ 90 mmHg (if < 65 mmHg, give vasopressors; if > 90 mmHg, give vasodilators) |
| ScvO2 | If < 70%, transfuse with red cells to reach an hematocrit of ≥ 30% |
Decrease the dobutamine dose if MAP <65 mmHg or if heart rate >110 beats/min. Decrease the oxygen consumption in patients in whom hemodynamic optimization could not be achieved using mechanical ventilation and sedatives.
Appendix 3: drotrecogin alfa (activated)
Inclusion criteria
Infection criteria
Patients must have a known infection or a suspected infection, as evidenced by one or more of the following:
• White cells in a normally sterile body fluid.
• Perforated viscus.
• Radiographic evidence of pneumonia in association with the production of purulent sputum.
• A syndrome associated with a high risk of infection (e.g. ascending cholangitis).
Modified systemic inflammatory response syndrome criteria
Patients should meet at least three of the following four criteria:
• A core temperature ≥ 38°C (100.4°F) or ≤ 36°C (96.8°F).
• A heart rate ≥ 90 beats/min, except in patients with a medical condition known to increase the heart rate or those receiving treatment that would prevent tachycardia.
• A respiratory rate ≥ 20 breaths/min or a partial pressure of arterial carbon dioxide ≤ 32 mmHg, or the use of mechanical ventilation for an acute respiratory process.
• A white cell count ≥ 12,000/mm3 or ≤ 4000/mm3, or a differential count showing >10% immature neutrophils.
Criteria for dysfunctional organs or systems
Patients should meet at least one of the following five criteria:
• Cardiovascular system dysfunction: arterial systolic blood pressure ≤ 90 mmHg or MAP ≤ 70 mmHg for at least 1 hour despite adequate fluid resuscitation, adequate intravascular volume status or the use of vasopressors in an attempt to maintain a systolic blood pressure ≥ 90 mmHg or a MAP ≥ 70 mmHg.
• Kidney dysfunction: urine output <0.5 ml/kg per hour for 1 hour, despite adequate fluid resuscitation.
• Respiratory system dysfunction: ratio of partial pressure of arterial oxygen to FiO2 ≤ 250 in the presence of other dysfunctional organs or systems, or ratio ≤ 200 if the lung was the only dysfunctional organ.
• Hematologic dysfunction: platelet count <80,000/mm3 or to have decreased by 50% in the 3 days preceding enrollment.
• Unexplained metabolic acidosis: pH ≤ 7.30 or base deficit ≥ 5.0 mmol/l in association with a plasma lactate level >1.5 times the upper limit of the normal value for the reporting laboratory.
Exclusion criteria
• Pregnancy or breastfeeding.
• Aged younger than 18 years or weight >135 kg.
• Platelet count <30,000/mm3.
• Conditions that increase the risk of bleeding:
• surgery requiring general or spinal anesthesia within 12 hours before the infusion, the potential need for such surgery during the infusion, or evidence of active bleeding postoperatively;
• a history of severe head trauma requiring hospitalization, intracranial surgery, or stroke within 3 months before the study, or any history of intracerebral arteriovenous malformation, cerebral aneurysm, or mass lesions of the central nervous system;
• a history of congenital bleeding diatheses; gastrointestinal bleeding within 6 weeks before the study unless corrective surgery had been performed; or
• trauma considered to increase the risk of bleeding.
• A known hypercoagualable condition including:
• resistance to activated protein C;
• hereditary deficiency of protein C, protein S, or antithrombin III;
• presence of anticardiolipin antibody, antiphospholipid antibody, lupus anticoagulant, or homocysteinemia; or
• recently documented (within 3 months) or highly suspected deep-vein thrombosis or pulmonary embolism.
• Patient's family or physician, or both, not in favor of aggressive treatment of the patient, or the presence of an advanced directive to withhold life-sustaining treatment.
• Patient not expected to survive 28 days because of an uncorrectable medical condition, such as poorly controlled neoplasm or other end-stage disease.
• Moribund state in which death is perceived to be imminent.
• Human immunodeficiency virus infection in association with a last known CD4 cell count ≤ 50/mm3.
• History of bone marrow, lung, liver, pancreas, or small-bowel transplantation.
• Chronic renal failure requiring hemodialysis or peritoneal dialysis (acute renal failure was not an exclusion criterion).
• Known or suspected portosystemic hypertension, chronic jaundice, cirrhosis, or chronic ascites.
• Acute pancreatitis with no established source of infection.
• Participation in an investigational study within 30 days before treatment.
• Use of any of the following medications or treatment regimens:
• unfractionated heparin to treat an active thrombotic event within 8 hours before the infusion (prophylactic treatment with a dose of unfractionated heparin of up to 15,000 U/day was permitted);
• low molecular weight heparin at a higher dose than recommended for prophylactic use (as specified in the package insert) within 12 hours before the infusion;
• warfarin (if used within 7 days before study entry and if the prothrombin time exceeded the upper limit of the normal range for the institution);
• acetylsalicylic acid at a dose of more than 650 mg/day within 3 days before the study;
• thrombolytic therapy within 3 days before the study (thrombolytic agents permitted for the treatment of thromboses within a catheter);
• glycoprotein IIb/IIIa antagonists within 7 days before study entry;
• antithrombin III at a dose of more than 10,000 U within 12 hours before the study;
• protein C within 24 hours before the study.
Dosage
Drotrecogin alfa (activated) should be given at a dose of 24 μg/kg/hour for 96 hours.
Infusion should be interrupted 1 hour prior to any percutaneous procedure or major surgery, and should be resumed 1 and 12 hours later, respectively, in the absence of bleeding complications.
Appendix 4: moderate-dose corticosteroids
Patient selection
Inclusion criteria
• Aged 18 years or older.
• Documented site(s) of infection.
• Temperature >38.3°C or <35.6°C.
• Heart rate >90 beats/min.
• Systolic blood pressure <90 mmHg (fluids and >5 μg/kg/min dopamine).
• Mechanical ventilation.
• Partial pressure of arterial oxygen/FiO2 <280, urine output <0.5 ml/kg/hour or lactate >2 mmol/l.
• ACTH test.
Exclusion criteria
• Pregnancy
• AMI, pulmonary embolism
• Corticotherapy.
• Contraindication to steroids.
Protocol
There was an 8-hour time window from shock onset to check for eligibility and to perform a short ACTH test (blood samples before and 30 and 60 min after a 250 μg intravenous bolus of tetracosactrin). Patients were then randomly assigned to receive 50 mg hydrocortisone as an intravenous bolus every 6 hours and one 50 μg tablet of fludrocortisone through a nasogastric tube once a day, or their respective placebos. Treatments were given for 7 days, and patients were followed up for 1 year.
Appendix 5: tight control of blood sugar
Patient selection
The study included all mechanically ventilated patients entering the ICU: predominantly surgical patients, with some neurological patients (the ICU in which the trial took place also sees such patients). Medical ICU patients (e.g. those with chronic obstructive pulmonary disease or oncologic or hematological disorders) were not included as they are not treated in the unit where the study was conducted. However, septic patients that were initially surgical but then came back from the ward with sepsis were included.
Only those patients who were moribund or had do-not-resuscitate status at ICU admission were excluded from the trial.
Protocol
Intensive insulin treatment
If blood glucose ≥ 110 mg/dl (≥ 6.1 mmol/l), infuse with insulin to maintain normoglycemia (80–110 mg/dl, 4.4–6.1 mmol/l). Do not exceed 50 IU/hour.
Insulin adjustments
Adjust insulin dose based on measurements of whole-blood glucose in undiluted arterial blood, performed at 1–4 hour intervals, based on the following algorithm: adjust the dose in proportion to the observed change in blood glucose level (if blood glucose decreases by 50% then the insulin dose should be decreased by 50% and checked within the next hour). Appendix 5 Table 1 provides information on the appropriate action depending on the blood glucose level. The numerical instructions provided in Appendix 5 Table 1 are a guide; insulin dosage should always be done with common sense, proportionate to the previous changes in blood glucose observed upon previous changes in dosage.
Appendix 5 Table 1.
Appropriate action depending on blood glucose level
| Test | Blood glucose result (mg/dl) | Action |
|---|---|---|
| A: Measure blood glucose on entry to ICU | >220 | Start insulin at a dose of 2–4 IU/h. Continue as per test B |
| 220–110 | Start insulin at a dose of 1–2 IU/h. Continue as per test B | |
| <110 | Do not start insulin but continue blood glucose monitoring every 4 h. Continue as per test A | |
| B: Measure glucose every 1–2 h until in normal range | >140 | Increase insulin dose by 1–2 IU/h |
| 110–140 | Increase insulin dose by 0.5–1 IU/h | |
| Approaching normal range | Adjust insulin dose by 0.1–0.5 IU/h. Continue as per test C | |
| C: Measure glucose every 4 h | Approaching normal range | Adjust insulin dose by 0.1–0.5 IU/h |
| Normal | Leave insulin dose unchanged | |
| Falling steeply | Reduce insulin dose by half and check every 1–2 h | |
| 60–80 | Reduce insulin dose and check blood glucose within 1 h | |
| 40–60 | Stop insulin infusion, assure adequate baseline glucose intake and check blood glucose within 1 h | |
| <40 | Stop insulin infusion, assure adequate baseline glucose intake, administer glucose per 10 g IV boluses and check blood glucose within 1 h |
Glucose consumption
On admission, patients should receive continuous intravenous glucose (200–300 g over 24 hours). After 24 hours, total parenteral, combined parenteral and enteral, or total enteral feeding should be instituted: 20–30 nonprotein kcal/kg/day with a balanced composition (0.13–0.26 g nitrogen/kg/day and 20–40% nonprotein calories in the form of lipids). Total enteral feeding should be attempted as early as possible.
Abbreviations
ACTH = adrenocorticotrophic hormone; ALI = acute lung injury; AMI = acute myocardial infarction; APACHE = Acute Physiology and Chronic Health Evaluation; ARDS = acute respiratory distress syndrome; CVP = central venous pressure; EGDT = early goal-directed therapy; FiO2 = fraction of inspired oxygen; ICU = intensive care unit; IFN = interferon; IL = interleukin; MAP = mean arterial pressure; PEEP = positive end-expiratory pressure; PROWESS = Protein C Worldwide Evaluation in Severe Sepsis; ScvO2 = central venous oxygen saturation; TNF = tumor necrosis factor.
London, UK. 17 June 2002
Acknowledgement
The roundtable discussion was supported by an unrestricted educational grant from Eli Lilly and Company.
References
- Hoyert DL, Arias E, Smith BL, Murphy SL, Kochanek KD. National Vital Statistics Reports [serial online], 21 September 2001. http://www.cdc.gov/nchs/data/nvsr/nvsr49/nvsr49_08.pdf 1 October 2002. [PubMed]
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- The task force on the management of acute myocardial infarction of the European Society of Cardiology. Eur Heart J. 1996;17:43–63. doi: 10.1093/oxfordjournals.eurheartj.a014691. [DOI] [PubMed] [Google Scholar]
- Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–S116. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- Balk RA. Severe sepsis and septic shock. Definitions, epidemiology, and clinical manifestations. Crit Care Clin. 2000;16:179–192. doi: 10.1016/s0749-0704(05)70106-8. [DOI] [PubMed] [Google Scholar]
- Bone RC. Gram-negative sepsis: a dilemma of modern medicine. Clin Microbiol Rev. 1993;6:57–68. doi: 10.1128/cmr.6.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL. Sepsis definitions. Lancet Infect Dis. 2002;2:135. doi: 10.1016/S1473-3099(02)00232-3. [DOI] [PubMed] [Google Scholar]
- Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Crit Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- Milberg JA, Davis DR, Steinberg KP, Hudson LD. Improved survival of patients with Acute Respiratory Distress Syndrome (ARDS): 1983–1993 [Concepts in Emergency and Critical Care]. JAMA. 1995;273:306–309. doi: 10.1001/jama.273.4.306. [DOI] [PubMed] [Google Scholar]
- Ziegler EJ, Fisher CJ, Sprung CL, Straube RC, Sadoff JC, Foulke GE, Wortel CH, Fink MP, Dellinger RP, Teng NN. The HA-1A Sepsis Study Group. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. N Engl J Med. 1991;324:429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]
- Ziegler EJ, McCutchan JA, Fierer J, Glauser MP, Sadoff JC, Douglas H, Braude AI. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982;307:1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]
- Abraham E, Wunderink R, Silverman H, Perl TM, Nasraway S, Levy H, Bone R, Wenzel RP, Balk R, Allred R. The TNFα MAb Sepsis Study Group: monoclonal antibody to human tumor necrosis factor alpha (TNFα MAb): efficacy and safety in patients with the sepsis syndrome. JAMA. 1995;273:934–941. doi: 10.1001/jama.273.12.934. [DOI] [PubMed] [Google Scholar]
- Abraham E, Reynaert M, Lew D, Pingleton S, Butler T, Dugernier T, Margolis B, Kudsk K, Zimmerli W, Anderson P, Reynaert M, Lew D, Lesslauer W, Passe S, Cooper P, Burdeska A, Modi M, Leighton A, Salgo M, Van der Auwera P. Lenercept Study Group. Lenercept (p55-Tumor Necrosis Factor Receptor Fusion Protein, Ro 45–2081, Tenefuse) patients with severe sepsis or early septic shock. A randomized double-blind placebo-controlled multicenter phase III trial with 1342 patients. Crit Care Med. 2001;29:503–510. doi: 10.1097/00003246-200103000-00006. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ Jr. Recombinant Human Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) Study Group. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- Wheeler AP, Bernard GR. Current concepts: treating patients with severe sepsis. N Engl J Med. 1999;340:207–214. doi: 10.1056/NEJM199901213400307. [DOI] [PubMed] [Google Scholar]
- Vincent JL. Need for intensivists in intensive care units [commentary]. Lancet. 2000;356:695–696. doi: 10.1016/S0140-6736(00)02622-2. [DOI] [PubMed] [Google Scholar]
- Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet. 1988;2:349–360. [PubMed] [Google Scholar]
- Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997;99:944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282:54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- Martin GS, Bernard GR. Airway and lung in sepsis. Intensive Care Med. 2001;27:S63–S79. doi: 10.1007/pl00003798. [DOI] [PubMed] [Google Scholar]
- Hayes TA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330:1717–1722. doi: 10.1056/NEJM199406163302404. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Brazzi L, Pelosi P, Latini R, Tognoni G, Pesenti A, Fumagalli R. A trial of goal-oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med. 1995;333:1025–1032. doi: 10.1056/NEJM199510193331601. [DOI] [PubMed] [Google Scholar]
- Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- Task Force of the American College of Critical Care Medicine Society of Critical Care Medicine. Practice parameters for hemodynamic support of sepsis in adult patients in sepsis. Crit Care Med. 1999;27:639–660. doi: 10.1097/00003246-199903000-00049. [DOI] [PubMed] [Google Scholar]
- Nguyen HB, Rivers EP, Havstad S, Knoblich B, Ressler JA, Muzzin AM, Tomlanovich MC. Critical care in the emergency department: a physiologic assessment and outcome evaluation. Acad EmergMed. 2000;7:1354–1361. doi: 10.1111/j.1553-2712.2000.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Nelson M, Waldrop RD, Jones J, Randall Z. Critical care provided in an urban emergency department. Am J Emerg Med. 1998;16:56–59. doi: 10.1016/S0735-6757(98)90066-3. [DOI] [PubMed] [Google Scholar]
- Donnino M, Nguyen B, Rivers E. A hemodynamic comparison of early and late phase severe sepsis and septic shock. Chest. 2002;122:5S. doi: 10.1378/chest.122.1.5. [DOI] [Google Scholar]
- Yan SB, Helterbrand JD, Hartman DL, Wright TJ, Bernard GR. Low levels of protein C are associated with poor outcomes in severe sepsis. Chest. 2001;120:915–922. doi: 10.1378/chest.120.3.915. [DOI] [PubMed] [Google Scholar]
- Hesselvik JF, Malm J, Dahlback B, Blomback M. Protein C, protein S, C4b-binding protein in severe infection and septic shock. Thomb Haemost. 1991;65:126–129. [PubMed] [Google Scholar]
- Mesters RM, Helterbrand J, Utterback BG, Yan B, Chao YB, Fernandez JA, Griffin JH, Hartman DL. Prognostic value of protein C concentrations in neutropenic patients at high risk of severe septic complications. Crit Care Med. 2000;28:2209–2216. doi: 10.1097/00003246-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Leclerc F, Hazelzet J, Jude B, Hofhuis W, Hue V, Martinot A, Van der Voort E. Protein C and S deficiency in severe infectious purpura of children: a collaborative study of 40 cases. Intensive Care Med. 1992;18:202–205. doi: 10.1007/BF01709832. [DOI] [PubMed] [Google Scholar]
- Powars D, Larsen R, Johnson J, Hulbert T, Sun T, Patch MJ, Francis R, Chan L. Epidemic meningioccemia and purpura fulminans with induced protein C deficiency. Clin Infect Dis. 1993;17:254–261. doi: 10.1093/clinids/17.2.254. [DOI] [PubMed] [Google Scholar]
- Taylor FB Jr, Chang A, Esmon CT, D'Angelo A, Vigano-D'Angelo S, Blick KE. Protein C prevents the coagulopathic and lethal effects of Escherichia coli infusion in the baboon. J Clin Invest. 1987;79:918–925. doi: 10.1172/JCI112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor Jr FB, Stearns-Kurosawa DJ, Kurosawa S, Ferrell G, Chang AC, Laszik Z, Kosanke S, Peer G, Esmon CT. The endothelial cell protein C receptor aids in host defense against Escherichia coli sepsis. Blood. 2000;95:1680–1686. [PubMed] [Google Scholar]
- Cronin L, Cook DJ, Carlet J, Heyland DK, King D, Lansang MA, Fisher CJ Jr. Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. 1995;23:1430–1439. doi: 10.1097/00003246-199508000-00019. [DOI] [PubMed] [Google Scholar]
- Lefering R, Neugebauer EA. Steroid controversy in sepsis and septic shock: a meta-analysis. Crit Care Med. 1995;23:1294–1303. doi: 10.1097/00003246-199507000-00021. [DOI] [PubMed] [Google Scholar]
- Annane D, Sébille V, Troché G, Raphael JC, Gajdos P, Bellissant E. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA. 2000;283:1038–1045. doi: 10.1001/jama.283.8.1038. [DOI] [PubMed] [Google Scholar]
- Briegel J, Forst H, Haller M, Schelling G, Kilger E, Kuprat G, Hemmer B, Hummel T, Lenhart A, Heyduck M, Stoll C, Peter K. Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blinded, single-center study. Crit Care Med. 1999;27:723–732. doi: 10.1097/00003246-199904000-00025. [DOI] [PubMed] [Google Scholar]
- Meduri GU, Tolley EA, Chrousos GP, Stentz F. Prolonged methylprednisolone treatment suppresses systemic inflammation in patients with unresolving acute respiratory distress syndrome: evidence for inadequate endogenous glucocorticoid secretion and inflammation-induced immune cell resistance to glucocorticoids. Am J Respir Crit Care Med. 2002;165:983–991. doi: 10.1164/ajrccm.165.7.2106014. [DOI] [PubMed] [Google Scholar]
- Beishuizen A, Thijs LG, Vermes I. Patterns of corticosteroid-binding globulin and the free cortisol index during septic shock and multitrauma. Intensive Care Med. 2001;27:1584–1591. doi: 10.1007/s001340101073. [DOI] [PubMed] [Google Scholar]
- Koo DJ, Jackman D, Chaudry IH, Wang P. Adrenal insufficiency during the late stage of polymicrobial sepsis. Crit Care Med. 2001;29:618–622. doi: 10.1097/00003246-200103000-00026. [DOI] [PubMed] [Google Scholar]
- Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A. Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med. 1998;26:645–650. doi: 10.1097/00003246-199804000-00010. [DOI] [PubMed] [Google Scholar]
- Annane D, Sébille V, Charpentier C, Bollaert PE, Francois B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troche G, Chaumet-Riffaut P, Bellissant E. Effect of a treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–971. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G, Wouters P, Weekers F, Mohan S, Baxter RC, Veldhuis JD, Bowers CY, Bouillon R. Reactivation of pituitary hormone release and metabolic improvement by infusion of growth hormone-releasing peptide and thyrotropin-releasing hormone in patients with protracted critical illness. J Clin Endocrinol Metab. 1999;84:1311–1323. doi: 10.1210/jc.84.4.1311. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G, Baxter RC, Weekers F, Wouters P, Bowers CY, Veldhuis JD. A paradoxical gender dissociation within the growth hormone/insulin-like growth factor I axis during protracted critical illness. J Clin Endocrinol Metab. 2000;85:183–192. doi: 10.1210/jc.85.1.183. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- Morris AH. Decision support and safety of clinical environments. Qual Saf Health Care. 2002;11:69–75. doi: 10.1136/qhc.11.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]