ABSTRACT
In recent years, major advances have been achieved in the understanding of pulmonary arterial hypertension (PAH) patho-physiology. Associated pulmonary arterial hypertension (APAH) can occur in a variety of other conditions and circumstances including a number of systemic autoimmune diseases. As with PAH in general, clinical symptoms of APAH in systemic autoimmune diseases are unspecific. In addition, there is a long standing association between autoimmunity and APAH. It has been postulated that autoimmunity may play a role in the pathogenesis of APAH. This argument has been based on frequent coexisting clinical and serological rheumatic findings. There is no experimental model of immune mechanism-dependent severe APAH. The loss of self-tolerance could initiate a process which ultimately results in APAH. It is possible that T-cell deficiencies (in either function or number) may contribute to pulmonary vascular injury or disease. These conditions are often associated with autoantibodies as well as defects in the CD4 T-cell compartiment. However, it remains uncertain how autoimmune mechanisms contribute to the pathogenesis of APAH. There are data that show a significant association between APAH and connective tissue diseases (CTD). In this regard, systemic sclerosis, mixed connective tissue disease, systemic lupus erythematosus, dermato/polymyositis and primary Sjögren's syndrome are associated with APAH. The study of APAH in the systemic autoimmune diseases and its relation to basic immunologic disturbances may yet bring effective therapies in the future. APAH can be a severe complication attracting a high excess mortality in autoimmune diseases. The present review will focus on what is known about autoimmune phenomena in APAH patients.
Keywords: associated pulmonary arterial hypertension, connective tissue diseases, pathophysiology
The diagnosis of idiopathic pulmonary arterial hypertension (IPAH) is made when no other risk factor can be identified. PAH is a group of chronic progressive and fatal diseases of the small arterial blood vessels of the pulmonary circulation (1-4).
Pulmonary arterial hypertension is a subset of pulmonary hypertension, which encompasses diseases that affect the pulmonary artery tree and are not due to other causes (lung disease or left heart disease). PAH is hemodynamically defined as a resting mean pulmonary arterial pressure ≥25 mmHg with a normal pulmonary capillary wedge pressure of ≤15 mmHg on right heart catheterisation. In PAH, high blood pressure in the pulmonary arteries damages the lungs and heart. If left untreated, PAH quickly becomes life threatening (5-7).
The association between autoimmunity and associated pulmonary arterial hypertension (APAH) has been appreciated for >40 years, but how autoimmune injury might contribute to the pathogenesis of this disease is not yet known. Most notably, the hypothesis advanced here unites the concept of immunodeficiency and autoimmunity which is frequently observed in conditions associated with PAH (8-13).
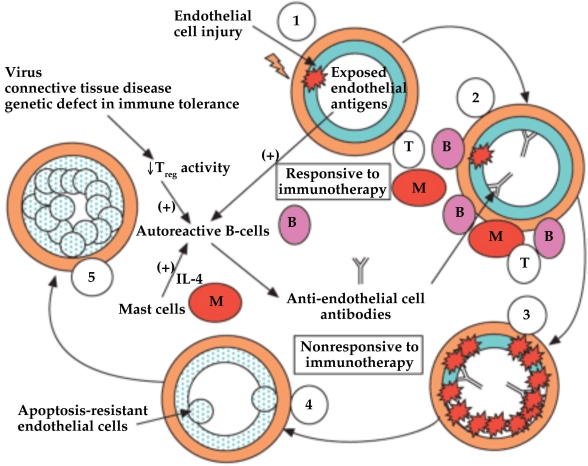
Pathologically, PAH is characterised by a proliferation of endothelial cells and expansion of vascular smooth muscle and adventitial cells in pulmonary arteries. PAH can present with luminal obliteration (vascular lesions) of small precapillary arteries. From a histological point of view, all layers of the pulmonary arterioles-intima, media, and adventitia-are involved, and inflammatory cells are present in PAH. Notably, inflammation in the perivascular space includes accumulation of mast cells and lymphocytes. There are differences in the pathogenesis of IPAH and APAH. Immune disturbances are also believed to contribute to APAH. This is particularly clear in APAH related to CTD. An autoimmune mechanism associated with vascular endothelial damage appears to have played a key pathogenic role in the development of PAH in these patients. The loss of self-tolerance could influence the early development of severe angioproliferative pulmonary hypertension. T-cell deficiency confers increased APAH and vascular remodelling. The absence of T cells enhances the vaso-obliterative remodelling that occurs as a consequence of pulmonary endothelial cell apoptosis and that reconstituting the immune system prevented these effects. APAH patients include an inflammatory infiltrate consisting of macrophages, T cells, B-cells and mast cells. The B cell activation and immune complex deposition may also contribute to the pathogenesis of APAH (13). Mast cells are a rich source of interleukin (IL)-4 which are capable of stimulating auto-reactive B-cells to secrete auto-antibodies, including anti-endothelial cell antibodies. The effector cell of greatest importance may be the dysregulated B-cell that produces auto-antibodies to vascular endothelium because normal regulatory T-cell activity is decreased or absent. APAH is characterised by B-cells, T-cells and mast cells, initiating plexiform lesions, and antibody-complement deposits that are located in the pulmonary arteries (14).
Figure 1. A schematic diagram displaying autoimmunity in the evolution of pulmonary arterial hypertension (14).
Although the occurrence of APAH is a relatively old observation, this new model of disease development can help explain seemingly un related aspects of this condition (15). Pulmonary vascular inflammation has been described in systemic lupus erythematosus (SLE)-associated PAH (16).
Severe APAH is one manifestation of a number of CTD. Survival rates in APAH in particular CTD, such as systemic sclerosis (SSc), are even lower than in IPAH. The occurrence of APAH has been associated with rheumatoid arthritis (RA), SSc, syndrome of calcinosis, Raynaud's phenomenon, esophageal dysmotility, sclerodactyly and telangiectasia (CREST syndrome), SLE, and mixed connective-tissue disease (MCTD). APAH occurs far less frequently in patients with SLE than in those with SSc, but the diagnosis is often delayed due to reduced vigilance in SLE compared with SSc. Whipple's disease should be ruled out as a secondary cause of APAH (4). The reported prevalence for APAH shows a wide range depending on the criteria used for diagnosis, on the methods used to confirm the diagnosis and on the patient population used for the studies. The cardinal sign is shortness of breath on exertion, but patients often do not report this symptom until asked specifically (17).
Additional symptoms include among others fatigue, weakness, angina, syncope and abdominal distension. Many patients with limited scleroderma have mild dyspnea on exertion during their disease course, but this symptom is usually attributed to deconditioning or other aspects of the disease. These symptoms are late manifestations of the disease and hemodynamic as well as morphological changes in the pulmonary vasculature occur long before these manifestations are detectable. However, APAH should be strongly considered, especially in SLE patients presenting with progressive dyspnea in the context of antiphospholipid syndrome (APS) or severe SLE. APS is often associated with APAH. APS is associated with antiphospholipid antibodies (APL) that bind and activate endothelial cells. This antibody engagement ultimately leads to apoptosis of vascular endothelial cells (19-22).
APAH has a highly fatality rate in patients with CTD. Some patients with severe APAH associated with SLE have improved their conditions with immunosuppressive therapy, emphasising the relevance of inflammation and autoimmunity in this subset of patients (23-26).
Antibodies to bone morphogenetic protein receptor (anti-BMPR2) could trigger PAH in patients with MCTD. However, these antibodies failed to be detected in any of the patients. The germline BMPR2 mutations might be responsible for regulatory T-cell defects and predispose to the occurrence of APAH (27-29).
Medical data were reviewed including demographic data, clinical features, laboratory findings (complete blood count, serum chemistry and uric acid (hyperuricemia), immunologic profiles [antinuclear antibody (ANA), anti-SSA antibody, antiribonucleoprotein antibody (anti-RNP) and serum immunoglobulin G (IgG), immunoglobulin A (IgA), immunoglobulin M (IgM), etc.], complement C3 and C4, urinanalysis, chest radiological findings and high-resolution computed tomography, and pulmonary function tests (diffusion capacity of the lung for carbon monoxide). Given the association of autoimmune phenomena with PAH, such as detection of ANA and antiendothelial cell antibodies (AECA), the finding of antibody-complement deposits in the lungs of patients with APAH, and the presence of inflammatory cells around plexiform lesions in APAH, there appears to be a basis for an immune-mediated component in APAH pathogenesis. Pathogenetic autoantibodies targeting endothelial cells are capable of inducing vascular endothelial apoptosis and may initiate the development of APAH. The evaluation of APAH includes assessment for an underlying CTD, including physical examination and serologic testing for ANA. Additional serologic studies may be appropriate if initial testing suggests an underlying autoimmune disorder. Serologic studies are often performed in patients with unexplained PAH to screen for CTD. Positive results of ANA tests are common, usually with a low titer, although high titers without a specific pattern have been reported. Anticentromere antibodies (ACA) are associated with the occurrence of APAH in patients with SSc, but their pathogenic role is not clearly established. Patients with SSc and antifibrillarin antibodies (anti-U3RNP) may develop more severe APAH. The presence of anticardiolipin antibodies (aCL) may be one of the factors that precipitate APAH, especially in those patients with SLE. Immunofluorescent studies in APAH patients have shown Ig and C deposits in the pulmonary arterial walls. Autoantibodies most commonly observed were ANA, anti-ssDNA, antitiroglobulin antibodies (ATg). These results may imply that in a subgroup of patients with PAH, the disease may be ascribed to an immune dysregulation or alternatively that autoantibodies accompany the disease progression as a epiphenomenona. Approximately half of patients with APAH have thyroid autoimmune disease. A possible association between hyperthyroidism and APAH has been reported (30-34).
Table 1. Autoimmune diseases associated with pulmonary arterial hypertension (18).
| Systemic lupus erythematosus |
|---|
| Mixed connective tissue disease |
| Systemic sclerosis (CREST syndrome) |
| Primary Sjögren's syndrome |
| Rheumatoid arthritis |
| Antiphospholipid syndrome |
| Thyroid autoimmune disease |
| Polymyositis |
| Whipple's disease |
It is likely that a better understanding of the exact role of autoimmunity and inflammation will help defining novel therapeutic targets in this devastating condition. By giving new consideration to autoimmunity as a potential cause of disease, it is likely that a better understanding of the exact role of autoimmunity and inflammation will help defining novel therapeutic targets in this condition (35-39). More studies are needed to verify the prevalence of APAH (40, 41).
If autoimmunity is truly important in the pathogenesis of APAH, then at risk patients can potentially be targeted with immunotherapy designed to prevent the establishment and propagation of autoimmune injury. Developing an understanding of how autoimmunity can trigger APAH would be invaluable in forming models of APAH pathogenesis and could promote the design of novel therapeutics which specifically address immune dysregulation. A better understanding of the exact role of autoimmunity and inflammation will help defining novel therapeutic targets in this devastating condition. ❑
CONCLUSIONS
Associated pulmonary arterial hypertension is among the most frequent causes of PAH in clinical practice, as shown by international registries. The data plead in favour of the relevance of immune disturbances in APAH. The diagnosis of APAH requires the clinical awareness of symptoms associated with PAH, the use of screening tools and the confirmation of the diagnosis by independent diagnostic procedures. APAH is a major cause of morbidity and mortality. By identifying a critical immune basis for many forms of APAH, a rational design of therapeutic targets for this group of frequently fatal diseases will be strongly facilitated. ❑
References
- 1.Klinger JR. Pulmonary Arterial Hypertension: An Overview. Seminars in Cardiothoracic and Vascular. Anesthesia. 2007;11:96–103. doi: 10.1177/1089253207301447. [DOI] [PubMed] [Google Scholar]
- 2.Humbert M. Update in pulmonary hypertension 2008. Am J Respir Crit Care Med. 2009;179:650–6. doi: 10.1164/rccm.200901-0136UP. [DOI] [PubMed] [Google Scholar]
- 3.Humbert M. Update in pulmonary arterial hypertension 2007. Am J Respir Crit Care Med. 2008;177:574–9. doi: 10.1164/rccm.200801-029UP. [DOI] [PubMed] [Google Scholar]
- 4.Kothari SS. Clinical Classification of Pulmonary Hypertension. J Am Coll Cardiol. 2010;55:267–267. doi: 10.1016/j.jacc.2009.07.070. [DOI] [PubMed] [Google Scholar]
- 5.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:40S–47S. doi: 10.1016/j.jacc.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Humbert M, Khaltaev N, Bousquet J, et al. Pulmonary hypertension: from an orphan disease to a public health problem. Chest. 2007;132:365–367. doi: 10.1378/chest.07-0903. [DOI] [PubMed] [Google Scholar]
- 8.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–30. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 9.Mukerjee D, St George D, Coleiro B, et al. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension application of a registry approach. Ann Rheum Dis. 2003;62:1088–93. doi: 10.1136/ard.62.11.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marasini B, Massarotti M, Cossutta R, et al. Pulmonary hypertension in autoimmune rheumatic diseases. Reumatismo. 2005;57:114–8. doi: 10.4081/reumatismo.2005.114. [DOI] [PubMed] [Google Scholar]
- 11.Bendayan D, Shitrit D, Kramer MR. Pulmonary arterial hypertension associated with autoimmune disease: a single medical center experience. IMAJ. 2006;8:252–4. [PubMed] [Google Scholar]
- 12.Cervera R, Asherson RA. Primary pulmonary arterial hypertension and the systemic autoimmune diseases. IMAJ. 2006;8:277–9. [PubMed] [Google Scholar]
- 13.Mouthon L, Guillevin L, Humbert M. Pulmonary arterial hypertension: an autoimmune disease? Eur Respir J. 2005;26:986–8. doi: 10.1183/09031936.05.00112105. [DOI] [PubMed] [Google Scholar]
- 14.Nicolls M, Taraseviciene-Stewart L, Voelkel N. Autoimmunity in the pathogenesis of pulmonary hypertension. Eur Respir J. 2005;26:1110–8. doi: 10.1183/09031936.05.00045705. [DOI] [PubMed] [Google Scholar]
- 15.Rubin LJ, Badesch DB. Evaluation and management of the patient with pulmonary arterial hypertension. Ann Intern Med. 2005;143:282–92. doi: 10.7326/0003-4819-143-4-200508160-00009. [DOI] [PubMed] [Google Scholar]
- 16.Galie N, Manes A, Farahani KV, et al. Pulmonary arterial hypertension associated to connective tissue diseases. Lupus. 2005:14(9):713–7. doi: 10.1191/0961203305lu2206oa. [DOI] [PubMed] [Google Scholar]
- 17.Kahler CM, Colleselli D. Pulmonary arterial hypertension (PAH) in connective tissue diseases. Rheumatology. 2006;45:11–3. doi: 10.1093/rheumatology/kel291. [DOI] [PubMed] [Google Scholar]
- 18.McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:14S–34S. doi: 10.1378/chest.126.1_suppl.14S. [DOI] [PubMed] [Google Scholar]
- 19.Pietra CG, Capron F, Stewart S, et al. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol. 2004;43:25S–32S. doi: 10.1016/j.jacc.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 20.Taraseviciene-Stewart L, Scerbavicious DK, Burns N, et al. The protective role of T-lymphocytes in pulmonary vascular remodelling. Chest. 2005;128:571S–572S. doi: 10.1378/chest.128.6_suppl.571S-a. [DOI] [PubMed] [Google Scholar]
- 21.Humbert M, Morrell NW, Archer SL, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 22.Mukerjee D, St George D, Knight C, et al. Echocardiography and pulmonary function as screening tests for pulmonary arterial hypertension in systemic sclerosis. Rheumatology. 2004;43:461–6. doi: 10.1093/rheumatology/keh067. [DOI] [PubMed] [Google Scholar]
- 23.Mukerjee D, St George D, Coleiro B, et al. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: application of a registry approach. Ann Rheum Dis. 2003;62:1088–93. doi: 10.1136/ard.62.11.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coral-Alvarado P, Quintana G, Garces MF, et al. Potential biomarkers for detecting pulmonary arterial hypertension in patients with systemic sclerosis. Rheumatol Int. 2009;29:1017–24. doi: 10.1007/s00296-008-0829-8. [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa N, Osanai S, Ide H, et al. Severe pulmonary hypertension associated with primary Sjogren's syndrome. Intern Med. 2003;42:1248–52. doi: 10.2169/internalmedicine.42.1248. [DOI] [PubMed] [Google Scholar]
- 26.Prabu A, Kiran P, Yee CS, et al. Prevalence and risk factors for pulmonary artery hypertension in lupus. Rheumatology. 2009;48:1506–11. doi: 10.1093/rheumatology/kep203. [DOI] [PubMed] [Google Scholar]
- 27.Sztrymf B, Coulet F, Girerd B, et al. Clinical outcomes of pulmonary arterial hypertension in carriers of BMPR2 mutation. Am J Respir Crit Care Med. 2008;177:1377–83. doi: 10.1164/rccm.200712-1807OC. [DOI] [PubMed] [Google Scholar]
- 28.Elliott CG, Glissmeyer EW, Havlena GT, et al. Relationship of BMPR2 mutations to vasoreactivity in pulmonary arterial hypertension. Circulation. 2006;113:2509–15. doi: 10.1161/CIRCULATIONAHA.105.601930. [DOI] [PubMed] [Google Scholar]
- 29.Lane KB, Machado RD, Pauciulo MW, et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. The International PPH Consortium. Nat Genet. 2000;26:81–4. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 30.Tamby MC, Chanseaud Y, Humbert M, et al. Antiendothelial cell antibodies in idiopathic and systemic sclerosis associated pulmonary arterial hypertension. Thorax. 2005;60:765–72. doi: 10.1136/thx.2004.029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasaki N, Kurose A, Inoue H, et al. A possible role of anti-endothelial cell antibody in the sera of MCTD patients on pulmonary vascular damage relating to pulmonary hypertension. Ryumachi. 2002;42:885–94. [PubMed] [Google Scholar]
- 32.Satoh T, Kimura K, Okano Y, et al. Lack of circulating autoantibodies to bone morphogenetic protein receptor-II or activin receptor-like kinase I in mixed connective tissue disease patients with pulmonary arterial hypertension. Rheumatology. 2005;44:192–6. doi: 10.1093/rheumatology/keh449. [DOI] [PubMed] [Google Scholar]
- 33.Chu JW, Kao PN, Faul JL, et al. High prevalence of autoimmune thyroid disease in pulmonary arterial hypertension. Chest. 2002;122:1668–73. doi: 10.1378/chest.122.5.1668. [DOI] [PubMed] [Google Scholar]
- 34.Virani SS, Mendoza CE, Ferreira AC, et al. Graves' disease and pulmonary hypertension. Tex Heart Inst J. 2003;30:314–5. [PMC free article] [PubMed] [Google Scholar]
- 35.McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:14S–34S. doi: 10.1378/chest.126.1_suppl.14S. [DOI] [PubMed] [Google Scholar]
- 36.Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum. 2005;52:3792–800. doi: 10.1002/art.21433. [DOI] [PubMed] [Google Scholar]
- 37.Mukerjee D, St George D, Coleiro B, et al. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: application of a registry approach. Ann Rheum Dis. 2003;62:1088–93. doi: 10.1136/ard.62.11.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Launay D, Mouthon L, Hachulla E, et al. Prevalence and characteristics of moderate to severe pulmonary hypertension in systemic sclerosis with and without interstitial lung disease. J Rheumatol. 2007;34:1005–11. [PubMed] [Google Scholar]
- 39.Jaïs X, Launay D, Yaici A, et al. Immunosuppressive therapy in lupus- and mixed connective tissue disease-associated pulmonary arterial hypertension: a retrospective analysis of twenty-three cases. Arthritis Rheum. 2008;58:521–31. doi: 10.1002/art.23303. [DOI] [PubMed] [Google Scholar]
- 40.Launay D, Hachulla E, Hatron PY, et al. Pulmonary arterial hypertension: a rare complication of primary Sjögren syndrome: report of 9 new cases and review of the literature. Medicine. doi: 10.1097/MD.0b013e3181579781. [DOI] [PubMed] [Google Scholar]
- 41.Launay D, Souza R, Guillevin L, et al. Pulmonary arterial hypertension in ANCA-associated vasculitis. Sarcoidosis. Vasc Diffuse Lung Dis. 2006;23:223–8. [PubMed] [Google Scholar]