Abstract
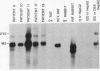
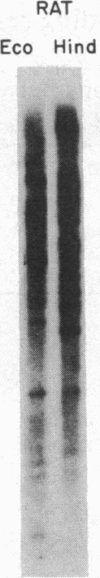
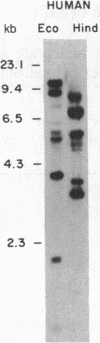
HLp is a human liver cytochrome P-450 that is immunochemically related to the glucocorticoid-inducible liver cytochrome P-450p in the rat and its homologue in the rabbit, P-450 LM3c. To investigate the structure and regulation of HLp, we used a monoclonal antibody that recognizes purified HLp to screen a human liver cDNA library in lambda gt11. We isolated and sequenced two overlapping cDNA clones that span the entire 2011 bases of an mRNA that codes for a protein of 504 amino acids. The predicted amino-terminal amino acid sequence of this protein is identical to the first 20 residues determined from purified HLp. HLp mRNA shares more than 70% sequence homology with related proteins from the rat and rabbit but less than 40% homology with other published cytochrome P-450 genes. Moreover, Southern blot analysis of human and rat genomic DNA revealed 50 and 60 kilobases of DNA, respectively, hybridizable to the HLp cDNAs. Blot analysis of human liver RNA from five patients revealed major (2.2 kilobase) and minor (3.0 kilobase) bands that hybridized to HLp cDNAs. The apparent concentration of these hybridizable mRNAs as well as the amounts of immunoreactive HLp protein in microsomes from the same liver were increased in a dose-dependent relationship in three patients who received dexamethasone, a potent glucocorticoid. Furthermore, in samples of RNA and of microsomes isolated from cultures of a human hepatoma cell line (Hep G2) incubated for 120 hr in medium containing dexamethasone, there was a 6-fold induction of the two mRNA species hybridizable to HLp cDNAs and a 3-fold induction of immunoreactive HLp protein as compared to the values for cultures incubated in steroid-free medium. We conclude that HLp is a human representative of a conserved glucocorticoid-inducible cytochrome P-450 gene family whose mechanism of induction involves accumulation of HLp mRNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aden D. P., Fogel A., Plotkin S., Damjanov I., Knowles B. B. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature. 1979 Dec 6;282(5739):615–616. doi: 10.1038/282615a0. [DOI] [PubMed] [Google Scholar]
- Adesnik M., Atchison M. Genes for cytochrome P-450 and their regulation. CRC Crit Rev Biochem. 1986;19(3):247–305. doi: 10.3109/10409238609084657. [DOI] [PubMed] [Google Scholar]
- Argus M. F., Hoch-Ligeti C., Arcos J. C., Conney A. H. Differential effects of beta-naphthoflavone and pregnenolone-16alpha-carbonitrile on dimethylnitrosamine-induced hepatocarcinogenesis. J Natl Cancer Inst. 1978 Aug;61(2):441–449. [PubMed] [Google Scholar]
- Beaune P., Kremers P., Letawe-Goujon F., Gielen J. E. Monoclonal antibodies against human liver cytochrome P-450. Biochem Pharmacol. 1985 Oct 1;34(19):3547–3552. doi: 10.1016/0006-2952(85)90732-4. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Karam J. H., Rutter W. J. Polymorphic DNA region adjacent to the 5' end of the human insulin gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5759–5763. doi: 10.1073/pnas.78.9.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshourbagy N. A., Barwick J. L., Guzelian P. S. Induction of cytochrome P-450 by pregnenolone-16 alpha-carbonitrile in primary monolayer cultures of adult rat hepatocytes and in a cell-free translation system. J Biol Chem. 1981 Jun 25;256(12):6060–6068. [PubMed] [Google Scholar]
- Gonzalez F. J., Nebert D. W., Hardwick J. P., Kasper C. B. Complete cDNA and protein sequence of a pregnenolone 16 alpha-carbonitrile-induced cytochrome P-450. A representative of a new gene family. J Biol Chem. 1985 Jun 25;260(12):7435–7441. [PubMed] [Google Scholar]
- Gotoh O., Tagashira Y., Iizuka T., Fujii-Kuriyama Y. Structural characteristics of cytochrome P-450. Possible location of the heme-binding cysteine in determined amino-acid sequences. J Biochem. 1983 Mar;93(3):807–817. doi: 10.1093/jb/93.3.807. [DOI] [PubMed] [Google Scholar]
- Hardwick J. P., Gonzalez F. J., Kasper C. B. Cloning of DNA complementary to cytochrome P-450 induced by pregnenolone-16 alpha-carbonitrile. Characterization of its mRNA, gene, and induction response. J Biol Chem. 1983 Aug 25;258(16):10182–10186. [PubMed] [Google Scholar]
- Jaiswal A. K., Gonzalez F. J., Nebert D. W. Human dioxin-inducible cytochrome P1-450: complementary DNA and amino acid sequence. Science. 1985 Apr 5;228(4695):80–83. doi: 10.1126/science.3838385. [DOI] [PubMed] [Google Scholar]
- Kawajiri K., Gotoh O., Sogawa K., Tagashira Y., Muramatsu M., Fujii-Kuriyama Y. Coding nucleotide sequence of 3-methylcholanthrene-inducible cytochrome P-450d cDNA from rat liver. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1649–1653. doi: 10.1073/pnas.81.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Gonzalez F. J., Nebert D. W. The murine Ah locus. Comparison of the complete cytochrome P1-450 and P3-450 cDNA nucleotide and amino acid sequences. J Biol Chem. 1984 Sep 10;259(17):10705–10713. [PubMed] [Google Scholar]
- Koop D. R., Coon M. J. Purification and properties of P-450LM3b, a constitutive form of cytochrome P-450, from rabbit liver microsomes. Biochem Biophys Res Commun. 1979 Dec 14;91(3):1075–1081. doi: 10.1016/0006-291x(79)91990-9. [DOI] [PubMed] [Google Scholar]
- Lu A. Y., West S. B. Multiplicity of mammalian microsomal cytochromes P-45. Pharmacol Rev. 1979 Dec;31(4):277–295. [PubMed] [Google Scholar]
- Mizukami Y., Sogawa K., Suwa Y., Muramatsu M., Fujii-Kuriyama Y. Gene structure of a phenobarbital-inducible cytochrome P-450 in rat liver. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3958–3962. doi: 10.1073/pnas.80.13.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips I. R., Shephard E. A., Ashworth A., Rabin B. R. Isolation and sequence of a human cytochrome P-450 cDNA clone. Proc Natl Acad Sci U S A. 1985 Feb;82(4):983–987. doi: 10.1073/pnas.82.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz E. G., Guzelian P. S. Induction of cytochrome P-450 by glucocorticoids in rat liver. II. Evidence that glucocorticoids regulate induction of cytochrome P-450 by a nonclassical receptor mechanism. J Biol Chem. 1984 Feb 10;259(3):2007–2012. [PubMed] [Google Scholar]
- Schuetz E. G., Wrighton S. A., Barwick J. L., Guzelian P. S. Induction of cytochrome P-450 by glucocorticoids in rat liver. I. Evidence that glucocorticoids and pregnenolone 16 alpha-carbonitrile regulate de novo synthesis of a common form of cytochrome P-450 in cultures of adult rat hepatocytes and in the liver in vivo. J Biol Chem. 1984 Feb 10;259(3):1999–2006. [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Schimke R. T. Nucleotide sequence surrounding multiple polyadenylation sites in the mouse dihydrofolate reductase gene. J Biol Chem. 1982 May 10;257(9):5143–5147. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taché Y., Taché J., Mécs I., Du Ruisseau P., Selye H. Regulation of resistance to various toxicants by PCN (pregnenolone-16alpha-carbonitrile) and thyroxine. J Med. 1976;7(6):471–479. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins P. B., Wrighton S. A., Maurel P., Schuetz E. G., Mendez-Picon G., Parker G. A., Guzelian P. S. Identification of an inducible form of cytochrome P-450 in human liver. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6310–6314. doi: 10.1073/pnas.82.18.6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. C., Carlson S. S., White T. J. Biochemical evolution. Annu Rev Biochem. 1977;46:573–639. doi: 10.1146/annurev.bi.46.070177.003041. [DOI] [PubMed] [Google Scholar]
- Wrighton S. A., Schuetz E. G., Watkins P. B., Maurel P., Barwick J., Bailey B. S., Hartle H. T., Young B., Guzelian P. Demonstration in multiple species of inducible hepatic cytochromes P-450 and their mRNAs related to the glucocorticoid-inducible cytochrome P-450 of the rat. Mol Pharmacol. 1985 Sep;28(3):312–321. [PubMed] [Google Scholar]
- Yabusaki Y., Shimizu M., Murakami H., Nakamura K., Oeda K., Ohkawa H. Nucleotide sequence of a full-length cDNA coding for 3-methylcholanthrene-induced rat liver cytochrome P-450MC. Nucleic Acids Res. 1984 Mar 26;12(6):2929–2938. doi: 10.1093/nar/12.6.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]