Abstract
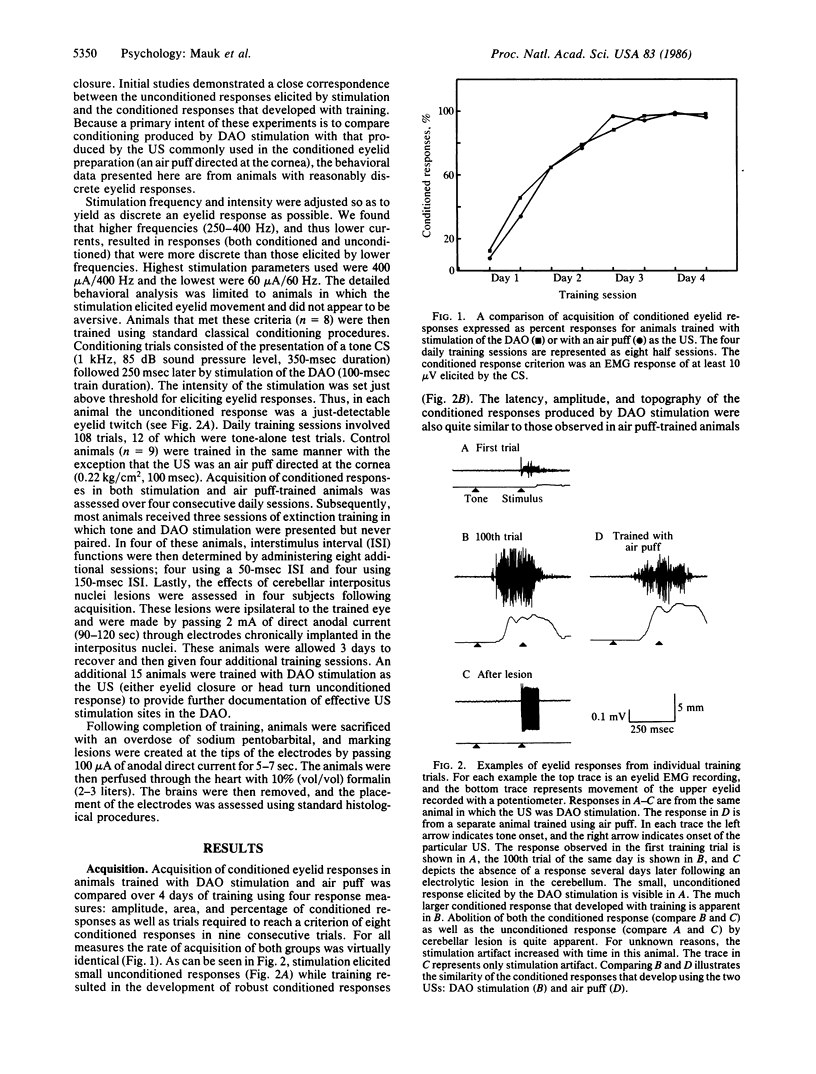
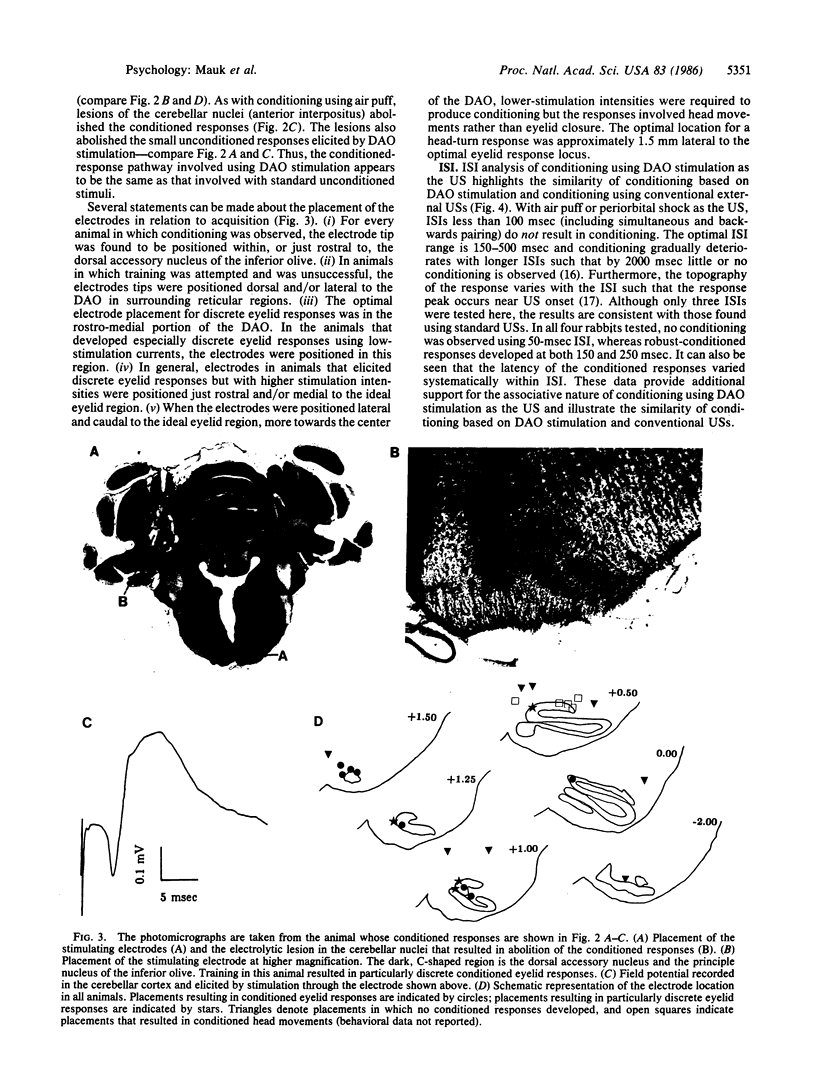
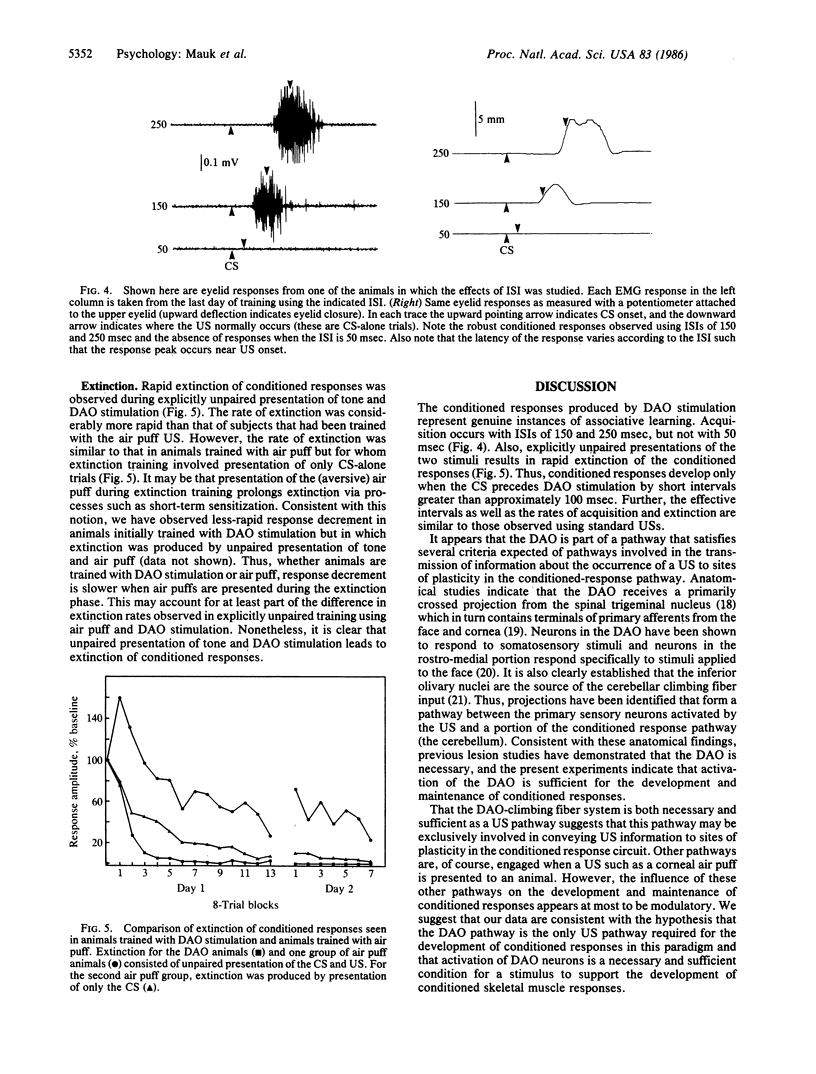
We show that conditioned eyelid responses develop when the unconditioned stimulus is electrical stimulation of the dorsal accessory nucleus of the inferior olive. When compared to conditioning using a standard unconditioned stimulus (air puff), the conditioning produced in this manner appears quite normal: the responses develop at a similar rate, are of comparable magnitude and topography, and demonstrate a steep interstimulus interval function; and response topography varies according to the interstimulus interval. These data indicate that activation of neurons in the dorsal accessory olive is a sufficient condition for a stimulus to be an effective unconditioned stimulus. Previous experiments indicate the dorsal accessory olive is necessary in that lesions have effects functionally equivalent to removal of the unconditioned stimulus. These data indicate that the dorsal accessory olive forms a portion of the pathway conveying information about the occurrence of an unconditioned stimulus to sites of synaptic plasticity responsible for conditioning.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkley K. J., Hand P. J. Projections to the inferior olive of the cat. II. Comparisons of input from the gracile, cuneate and the spinal trigeminal nuclei. J Comp Neurol. 1978 Jul 15;180(2):253–264. doi: 10.1002/cne.901800205. [DOI] [PubMed] [Google Scholar]
- Courville J., Faraco-Cantin F. On the origin of the climbing fibers of the cerebellum. An experimental study in the cat with an autoradiographic tracing method. Neuroscience. 1978;3(9):797–809. doi: 10.1016/0306-4522(78)90032-5. [DOI] [PubMed] [Google Scholar]
- Courville J. Rubrobulbar fibres to the facial nucleus and the lateral reticular nucleus (nucleus of the lateral funiculus). An experimental study in the cat with silver impregnation methods. Brain Res. 1966 May-Jun;1(4):317–337. doi: 10.1016/0006-8993(66)90125-9. [DOI] [PubMed] [Google Scholar]
- Davis M., Gendelman D. S., Tischler M. D., Gendelman P. M. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci. 1982 Jun;2(6):791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C. An instruction-selection theory of learning in the cerebellar cortex. Brain Res. 1977 May 27;127(2):327–352. doi: 10.1016/0006-8993(77)90550-9. [DOI] [PubMed] [Google Scholar]
- Ekerot C. F., Kano M. Long-term depression of parallel fibre synapses following stimulation of climbing fibres. Brain Res. 1985 Sep 9;342(2):357–360. doi: 10.1016/0006-8993(85)91136-9. [DOI] [PubMed] [Google Scholar]
- Gellman R., Houk J. C., Gibson A. R. Somatosensory properties of the inferior olive of the cat. J Comp Neurol. 1983 Apr 1;215(2):228–243. doi: 10.1002/cne.902150210. [DOI] [PubMed] [Google Scholar]
- Ito M., Orlov I., Shimoyama I. Reduction of the cerebellar stimulus effect on rat Deiters neurons after chemical destruction of the inferior olive. Exp Brain Res. 1978 Sep 15;33(1):143–145. doi: 10.1007/BF00238802. [DOI] [PubMed] [Google Scholar]
- Ito M., Sakurai M., Tongroach P. Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J Physiol. 1982 Mar;324:113–134. doi: 10.1113/jphysiol.1982.sp014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning: modulation of transmitter release. Science. 1982 Oct 29;218(4571):433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Lincoln J. S., McCormick D. A., Thompson R. F. Ipsilateral cerebellar lesions prevent learning of the classically conditioned nictitating membrane/eyelid response. Brain Res. 1982 Jun 17;242(1):190–193. doi: 10.1016/0006-8993(82)90510-8. [DOI] [PubMed] [Google Scholar]
- Llinás R., Walton K., Hillman D. E., Sotelo C. Inferior olive: its role in motor learing. Science. 1975 Dec 19;190(4220):1230–1231. doi: 10.1126/science.128123. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969 Jun;202(2):437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Clark G. A., Lavond D. G., Thompson R. F. Initial localization of the memory trace for a basic form of learning. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2731–2735. doi: 10.1073/pnas.79.8.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Steinmetz J. E., Thompson R. F. Lesions of the inferior olivary complex cause extinction of the classically conditioned eyeblink response. Brain Res. 1985 Dec 16;359(1-2):120–130. doi: 10.1016/0006-8993(85)91419-2. [DOI] [PubMed] [Google Scholar]
- Panneton W. M., Martin G. F. Brainstem projections to the facial nucleus of the opossum. A study using axonal transport techniques. Brain Res. 1983 May 9;267(1):19–33. doi: 10.1016/0006-8993(83)91036-3. [DOI] [PubMed] [Google Scholar]
- Rosenfield M. E., Dovydaitis A., Moore J. W. Brachium conjuntivum and rubrobulbar tract: brain stem projections of red nucleus essential for the conditioned nictitating membrane response. Physiol Behav. 1985 May;34(5):751–759. doi: 10.1016/0031-9384(85)90374-9. [DOI] [PubMed] [Google Scholar]
- Smith M. C. CS-US interval and US intensity in classical conditioning of the rabbit's nictitating membrane response. J Comp Physiol Psychol. 1968 Dec;66(3):679–687. doi: 10.1037/h0026550. [DOI] [PubMed] [Google Scholar]
- Smith M. C., Coleman S. R., Gormezano I. Classical conditioning of the rabbit's nictitating membrane response at backward, simultaneous, and forward CS-US intervals. J Comp Physiol Psychol. 1969 Oct;69(2):226–231. doi: 10.1037/h0028212. [DOI] [PubMed] [Google Scholar]
- Yeo C. H., Hardiman M. J., Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. I. Lesions of the cerebellar nuclei. Exp Brain Res. 1985;60(1):87–98. doi: 10.1007/BF00237022. [DOI] [PubMed] [Google Scholar]