Abstract
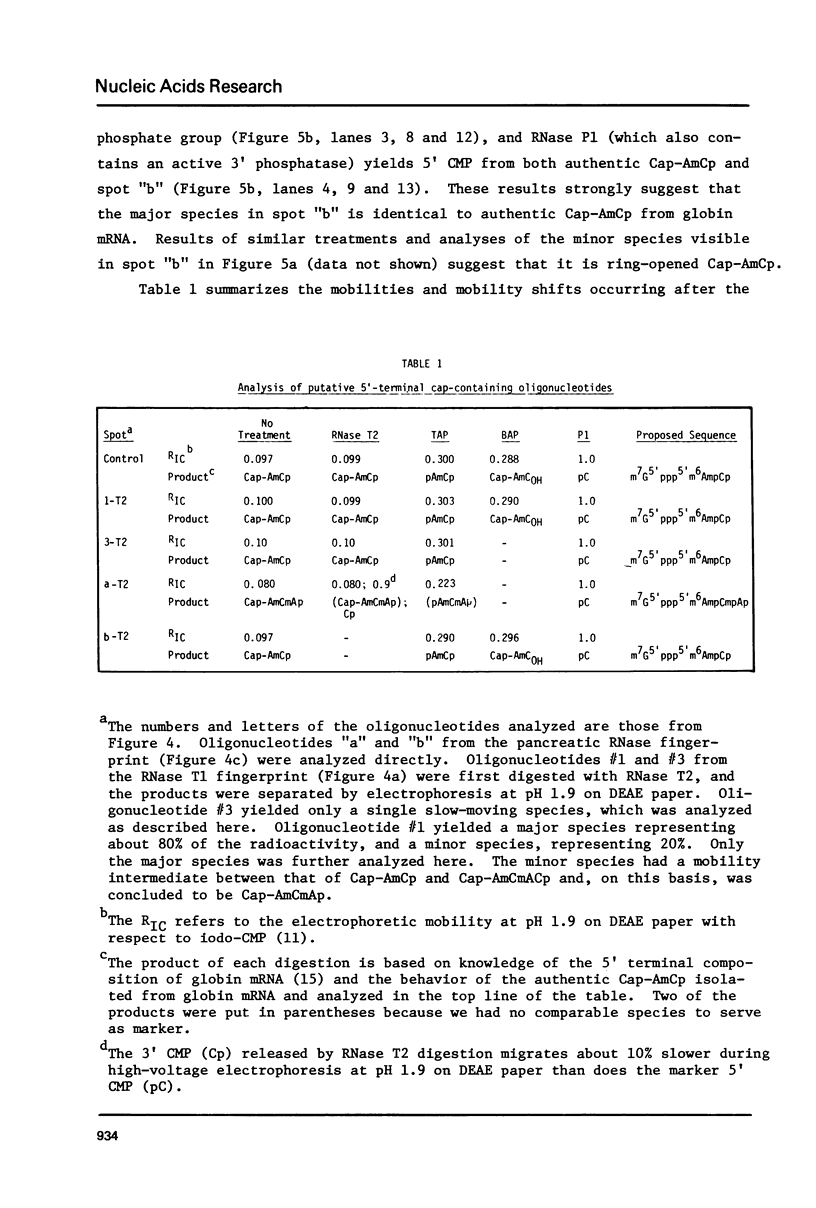
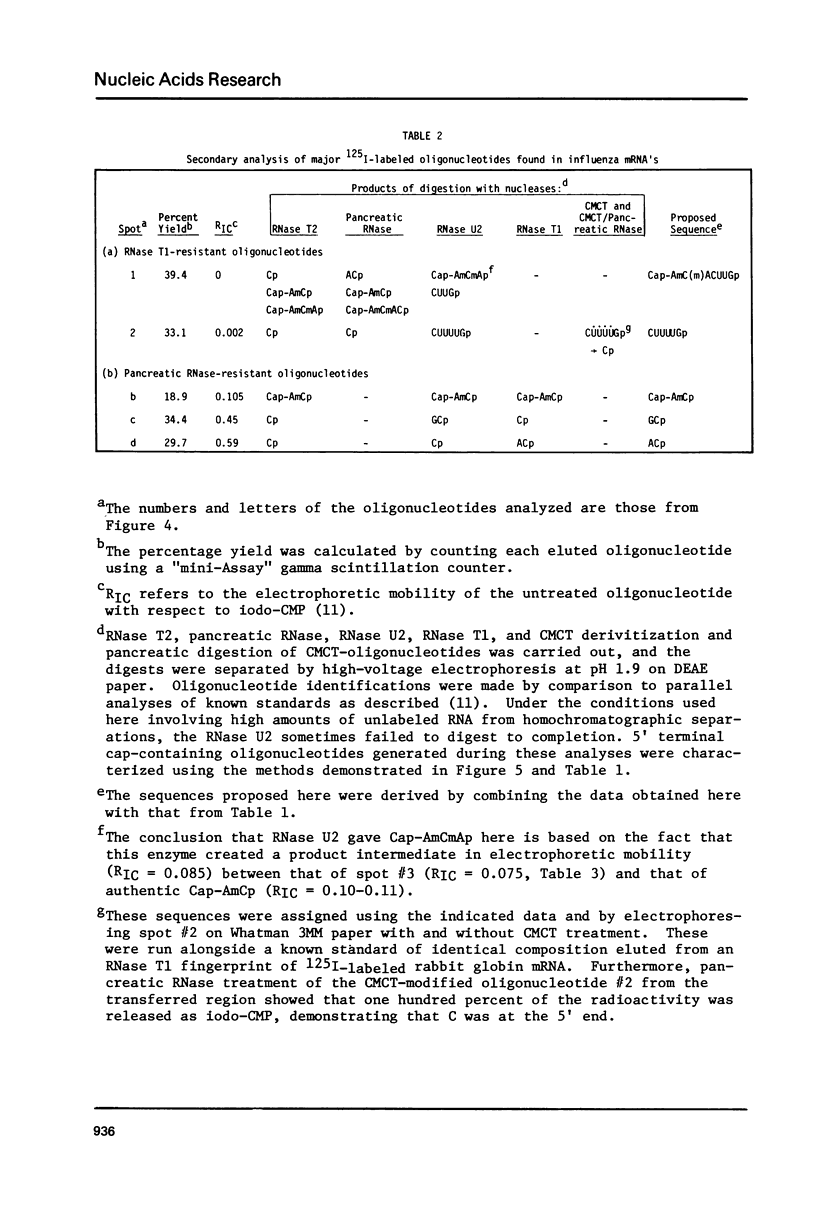
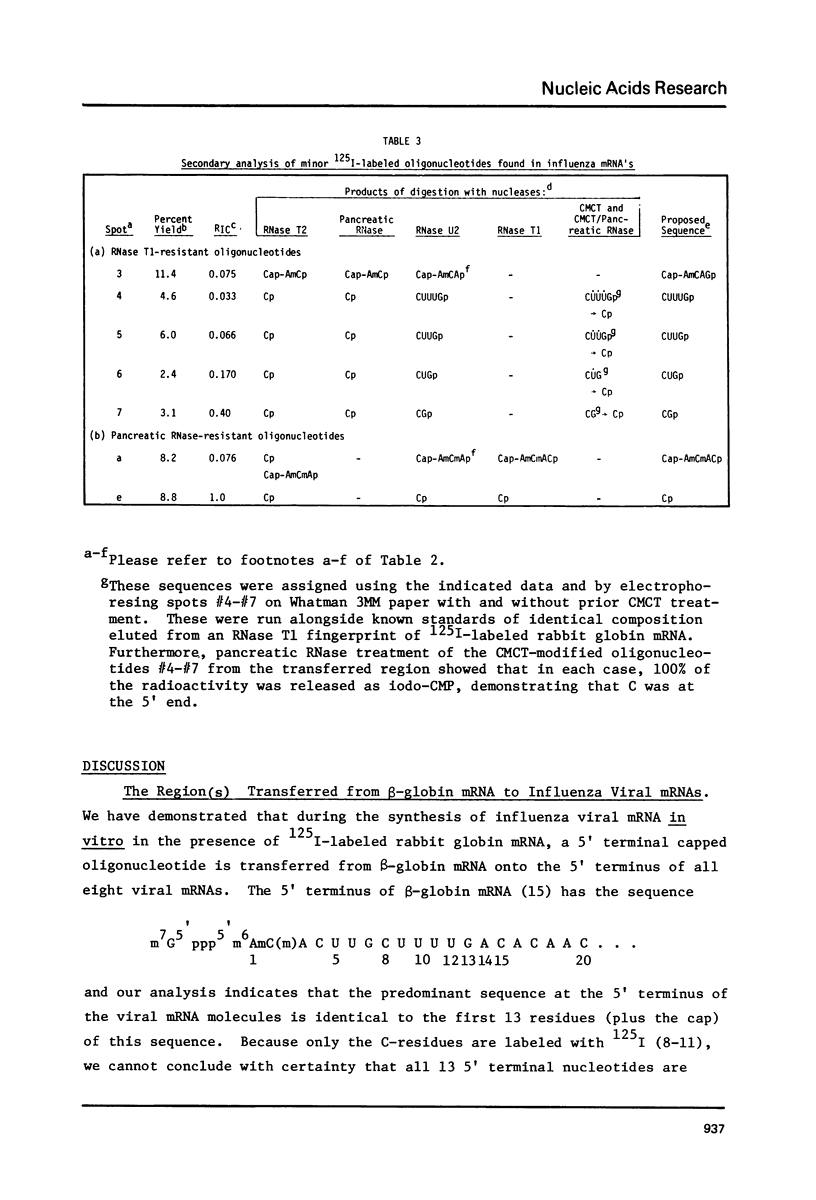
Capped eukaryotic mRNAs strongly stimulate influenza viral RNA transcription in vitro and donate their cap and also additional nucleotides to the viral transcripts (1). To identify which bases of a given primer mRNA are transferred, we synthesized influenza viral mRNA using a primer rabbit globin mRNA (enriched in beta-globin mRNA) which had been labeled in vitro to high specific activity with 125I. We show that during transcription the same 125I-labeled oligonucleotides were transferred to the 5' termini of each of the eight viral mRNA segments. The predominant sequence, representing 75 percent of the transferred oligonucleotides, was identical to the first 13 nucleotides at the 5' end of beta-globin mRNA (m7G5'ppp5'm6AmC(m)ACUUGCUUUUG). Because only the C-residues are labeled with 125I, these results indicate that either the first 12, 13 or 14 5' terminal bases of beta-globin mRNA were transferred to the viral mRNAs. 125I-labeled oligonucleotides recovered from the viral mRNA in minor yields indicated that shorter 5' terminal pieces of beta-globin mRNA were sometimes transferred and that G was probably the first base inserted by transcription.
Full text
PDF






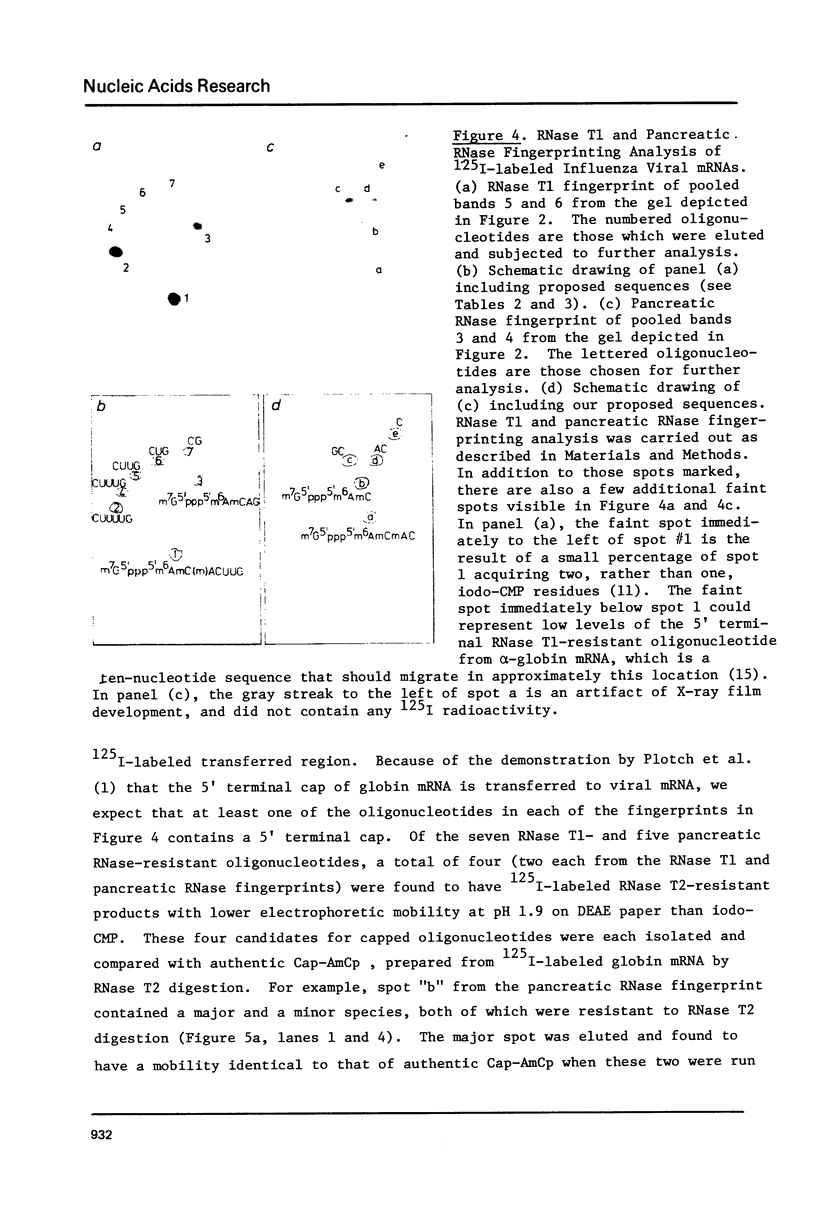







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson J. RNA processing and the intervening sequence problem. Annu Rev Biochem. 1979;48:1035–1069. doi: 10.1146/annurev.bi.48.070179.005131. [DOI] [PubMed] [Google Scholar]
- Air G. M. Nucleotide sequence coding for the "signal peptide" and N terminus of the hemagglutinin from an asian (H2N2) strain of influenza virus. Virology. 1979 Sep;97(2):468–472. doi: 10.1016/0042-6822(79)90358-1. [DOI] [PubMed] [Google Scholar]
- Baralle F. E. Complete nucleotide sequence of the 5' noncoding region of rabbit beta-globin mRNA. Cell. 1977 Apr;10(4):549–558. doi: 10.1016/0092-8674(77)90088-5. [DOI] [PubMed] [Google Scholar]
- Both G. W., Air G. M. Nucleotide sequence coding for the N-terminal region of the matrix protein influenza virus. Eur J Biochem. 1979 May 15;96(2):363–372. doi: 10.1111/j.1432-1033.1979.tb13048.x. [DOI] [PubMed] [Google Scholar]
- Bouloy M., Morgan M. A., Shatkin A. J., Krug R. M. Cap and internal nucleotides of reovirus mRNA primers are incorporated into influenza viral complementary RNA during transcription in vitro. J Virol. 1979 Dec;32(3):895–904. doi: 10.1128/jvi.32.3.895-904.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloy M., Plotch S. J., Krug R. M. Globin mRNAs are primers for the transcription of influenza viral RNA in vitro. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4886–4890. doi: 10.1073/pnas.75.10.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. T., Gelinas R. E., Broker T. R., Roberts R. J. An amazing sequence arrangement at the 5' ends of adenovirus 2 messenger RNA. Cell. 1977 Sep;12(1):1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Dickson E., Diener T. O., Robertson H. D. Potato spindle tuber and citrus exocortis viroids undergo no major sequence changes during replication in two different hosts. Proc Natl Acad Sci U S A. 1978 Feb;75(2):951–954. doi: 10.1073/pnas.75.2.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson E., Pape L. K., Robertson H. D. Approaches to sequence analysis of 125I-labeled RNA. Nucleic Acids Res. 1979 Jan;6(1):91–110. doi: 10.1093/nar/6.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson E., Robertson H. D. Potential regulatory roles for RNA in cellular development. Cancer Res. 1976 Sep;36(9 Pt 2):3387–3393. [PubMed] [Google Scholar]
- Efstratiadis A., Kafatos F. C., Maniatis T. The primary structure of rabbit beta-globin mRNA as determined from cloned DNA. Cell. 1977 Apr;10(4):571–585. doi: 10.1016/0092-8674(77)90090-3. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Broni B. A., Bouloy M. Are the 5' ends of influenza viral mRNAs synthesized in vivo donated by host mRNAs? Cell. 1979 Oct;18(2):329–334. doi: 10.1016/0092-8674(79)90052-7. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Synthesis of influenza virus polypeptides in cells resistant to alpha-amanitin: evidence for the involvement of cellular RNA polymerase II in virus replication. J Virol. 1977 Sep;23(3):816–819. doi: 10.1128/jvi.23.3.816-819.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legon S. Characterization of the ribosome-protected regions of 125I-labelled rabbit globin messenger RNA. J Mol Biol. 1976 Sep 5;106(1):37–53. doi: 10.1016/0022-2836(76)90299-0. [DOI] [PubMed] [Google Scholar]
- Legon S., Robertson H. D. The binding of 125I-labelled rabbit globin messenger RNA to reticulocyte ribosomes. J Mol Biol. 1976 Sep 5;106(1):23–36. doi: 10.1016/0022-2836(76)90298-9. [DOI] [PubMed] [Google Scholar]
- Lockhard R. E., Rajbhandary U. L. Nucleotide sequences at the 5'termini of rabbit alpha and beta globin mRNA. Cell. 1976 Dec;9(4 Pt 2):747–760. doi: 10.1016/0092-8674(76)90138-0. [DOI] [PubMed] [Google Scholar]
- Mark G. E., Taylor J. M., Broni B., Krug R. M. Nuclear accumulation of influenza viral RNA transcripts and the effects of cycloheximide, actinomycin D, and alpha-amanitin. J Virol. 1979 Feb;29(2):744–752. doi: 10.1128/jvi.29.2.744-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P. Processing of RNA. Annu Rev Biochem. 1976;45:605–629. doi: 10.1146/annurev.bi.45.070176.003133. [DOI] [PubMed] [Google Scholar]
- Plotch S. J., Bouloy M., Krug R. M. Transfer of 5'-terminal cap of globin mRNA to influenza viral complementary RNA during transcription in vitro. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1618–1622. doi: 10.1073/pnas.76.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotch S. J., Krug R. M. Influenza virion transcriptase: synthesis in vitro of large, polyadenylic acid-containing complementary RNA. J Virol. 1977 Jan;21(1):24–34. doi: 10.1128/jvi.21.1.24-34.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotch S. J., Krug R. M. Segments of influenza virus complementary RNA synthesized in vitro. J Virol. 1978 Feb;25(2):579–586. doi: 10.1128/jvi.25.2.579-586.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prensky W. The radioiodination of RNA and DNA to high specific activities. Methods Cell Biol. 1976;13:121–152. doi: 10.1016/s0091-679x(08)61800-2. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Dickson E., Model P., Prensky W. Application of fingerprinting techniques to iodinated nucleic acids. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3260–3264. doi: 10.1073/pnas.70.11.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. D., Dickson E. RNA processing and the control of gene expression. Brookhaven Symp Biol. 1975 Jul;(26):240–266. [PubMed] [Google Scholar]
- Robertson J. S. 5' and 3' terminal nucleotide sequences of the RNA genome segments of influenza virus. Nucleic Acids Res. 1979 Aug 24;6(12):3745–3757. doi: 10.1093/nar/6.12.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Hay A. J. Nucleotide sequences at the 5' termini of influenza virus RNAs and their transcripts. Nucleic Acids Res. 1978 Apr;5(4):1207–1219. doi: 10.1093/nar/5.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner L. L., Barry R. D. Participation of DNA-dependent RNA polymerase II in replication of influenza viruses. Nature. 1977 Aug 18;268(5621):650–652. doi: 10.1038/268650a0. [DOI] [PubMed] [Google Scholar]