Abstract
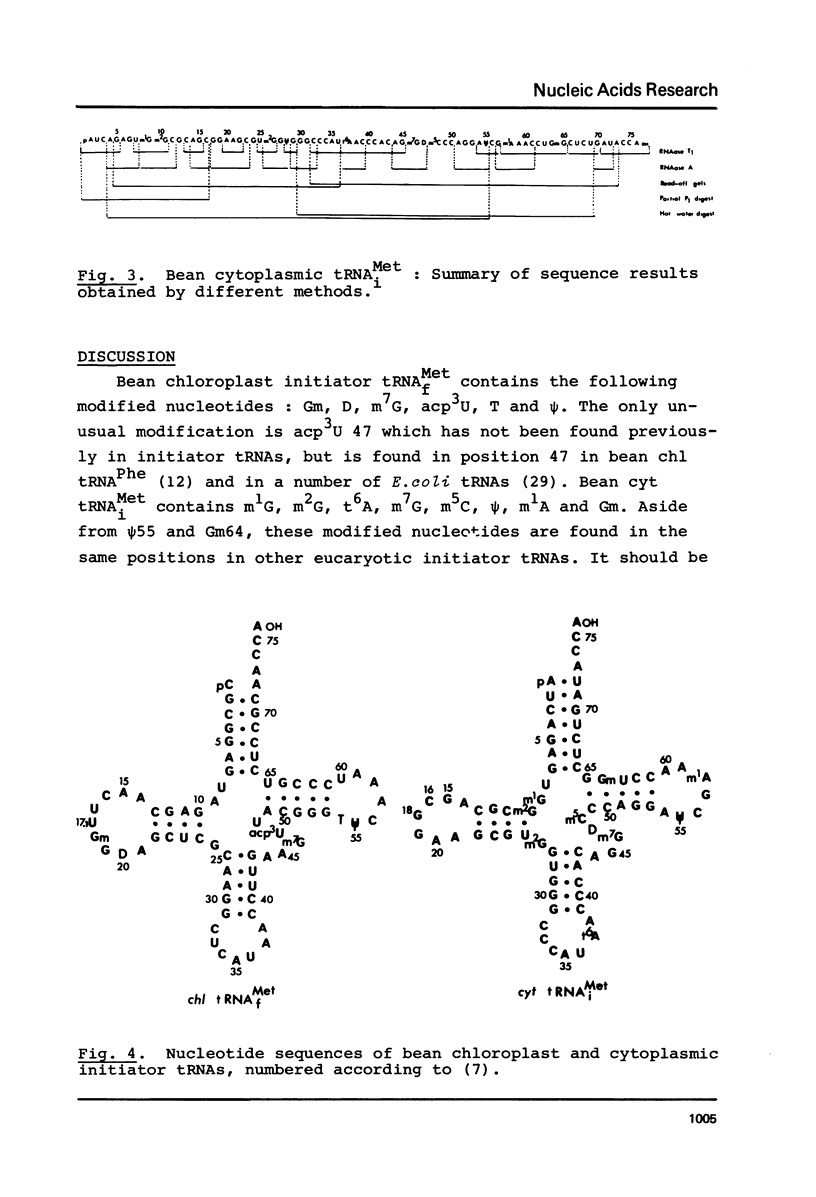
The initiator tRNAsMet from the cytoplasm and chloroplasts of Phaseolus vulgaris have been purified and sequenced. The sequence of bean cytoplasmic initiator tRNAiMet is : pA-U-C-A-G-A-G-U-m1G-m2G-C-G-C-A-G-C-G-G-A-A-G-C-G-U-m2G-G-U-G-G-G2-C-C-C-A-U-t6A-A-C-C-C-A-C-A-G-m7G-D-m5C-C-C-A-G-G-A-psi-C-G-m1A-A-A-C-C-U-Gm-G-C-U-C-U-G-A-U-A-C-C-AOH. The sequence of bean cytoplasmic tRNAiMet is almost identical to that of wheat germ and shows a high degree of homology with other cytoplasmic initiator tRNAs. The sequence of bean chloroplast initiator tRNAfMet is : pC-G-C-G-G-A-G-U-A-G-A-G-C-A-A-C-U-U-Gm-G-D-A-G-C-U-C-G-C-A-A-G-G-C-U-C-A-U-A-A-C-C-U-U-G-A-A-m7G-acp3U-U-A-C-G-G-G-T-psi-C-A-A-A-U-C-C-C-G-U-C-U-C-C-G-C-A-A- C-C-AOH. Bean chloroplast initiator tRNAfMet sequence shows procaryotic characteristics at the 5' end of the acceptor stem and in the TpsiC loop, but also contains some distinctive features.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barciszewski J., Joachimiak A., Rafalski A., Barciszewska M., Twardowski T., Wiewiórowski M. Conservation of the structures of plant tRNAs and aminoacyl-tRNA synthetases. FEBS Lett. 1979 Jun 1;102(1):194–197. doi: 10.1016/0014-5793(79)80958-8. [DOI] [PubMed] [Google Scholar]
- Burkard G., Eclancher B., Weil J. H. Presence of N-formyl-methionyl-transfer RNA in bean chloroplasts. FEBS Lett. 1969 Aug;4(4):285–287. doi: 10.1016/0014-5793(69)80257-7. [DOI] [PubMed] [Google Scholar]
- Chang S. H., Brum C. K., Siberklang M., RajBhandary U. L., Hecker L. I., Barnett W. E. The first nucleotide sequence of an organelle transfer RNA: chloroplastic tRNAphe. Cell. 1976 Dec;9(4 Pt 2):717–723. doi: 10.1016/0092-8674(76)90135-5. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin A., Gruhl H., Feldmann H. Mapping of yeast tRNAs by two-dimensional electrophoresis on polyacrylamide gels. FEBS Lett. 1975 Feb 1;50(2):185–189. doi: 10.1016/0014-5793(75)80485-6. [DOI] [PubMed] [Google Scholar]
- Gauss D. H., Grüter F., Sprinzl M. Compilation of tRNA sequences. Nucleic Acids Res. 1979 Jan;6(1):r1–r19. [PMC free article] [PubMed] [Google Scholar]
- Giegé R., Ebel J. P., Clark B. F. Formylation of mischarged E. coli tRNA Met f . FEBS Lett. 1973 Mar 15;30(3):291–295. doi: 10.1016/0014-5793(73)80672-6. [DOI] [PubMed] [Google Scholar]
- Gillemaut P., Weil J. H. Aminoacylation of Phaseolus vulgaris cytoplasmic, chloroplastic and mitochondrial tRNAsMet and of Escherichia coli tRNAsMet by homologous and heterologous enzymes. Biochim Biophys Acta. 1975 Oct 1;407(2):240–248. doi: 10.1016/0005-2787(75)90288-9. [DOI] [PubMed] [Google Scholar]
- Gillum A. M., Hecker L. I., Silberklang M., Schwartzbach S. D., RajBhandary U. L., Barnett W. E. Nucleotide sequence of Neurospora crassa cytoplasmic initiator tRNA. Nucleic Acids Res. 1977 Dec;4(12):4109–4131. doi: 10.1093/nar/4.12.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum A. M., Urquhart N., Smith M., RajBhandary U. L. Nucleotide sequence of salmon testes and salmon liver cytoplasmic initiator tRNA. Cell. 1975 Nov;6(3):395–405. doi: 10.1016/0092-8674(75)90189-0. [DOI] [PubMed] [Google Scholar]
- Guillemaut P., Keith G. Primary structure of bean chloroplastic tRNAPhe. Comparison with Euglena chloroplastic tRNAPhe. FEBS Lett. 1977 Dec 15;84(2):351–356. doi: 10.1016/0014-5793(77)80723-0. [DOI] [PubMed] [Google Scholar]
- Holmes W. M., Hurd R. E., Reid B. R., Rimerman R. A., Hatfield G. W. Separation of transfer ribonucleic acid by sepharose chromatography using reverse salt gradients. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1068–1071. doi: 10.1073/pnas.72.3.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay E., Bambara R., Padmanabhan R., Wu R. DNA sequence analysis: a general, simple and rapid method for sequencing large oligodeoxyribonucleotide fragments by mapping. Nucleic Acids Res. 1974 Mar;1(3):331–353. doi: 10.1093/nar/1.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino Y., Kato M., Sugisaki H., Nishimura S. Nucleotide sequence of starfish initiator tRNA. Nucleic Acids Res. 1979 Aug 10;6(11):3459–3469. doi: 10.1093/nar/6.11.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockard R. E., Alzner-Deweerd B., Heckman J. E., MacGee J., Tabor M. W., RajBhandary U. L. Sequence analysis of 5'[32P] labeled mRNA and tRNA using polyacrylamide gel electrophoresis. Nucleic Acids Res. 1978 Jan;5(1):37–56. doi: 10.1093/nar/5.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi Z., Maeda M., McCloskey J. A., Nishimura S. 3-(3-Amino-3-carboxypropyl)uridine: a novel modified nucleoside isolated from Escherichia coli phenylalanine transfer ribonucleic acid. Biochemistry. 1974 Jun 4;13(12):2620–2625. doi: 10.1021/bi00709a023. [DOI] [PubMed] [Google Scholar]
- Roe B. A. Studies on human tRNA. I. The rapid, large scale isolation and partial fractionation of placenta and liver tRNA. Nucleic Acids Res. 1975 Jan;2(1):21–42. doi: 10.1093/nar/2.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel C. E., D'Ari L., Rabinowitz J. C. Evidence against the folate-mediated formylation of formyl-accepting methionyl transfer ribonucleic acid in Streptococcus faecalis R. J Biol Chem. 1970 Oct 10;245(19):5115–5121. [PubMed] [Google Scholar]
- Samuel C. E., Rabinowitz J. C. Effect of formylation on the chromatographic behavior of methionyl transfer ribonucleic acid. Anal Biochem. 1972 May;47(1):244–252. doi: 10.1016/0003-2697(72)90298-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. The use of nuclease P1 in sequence analysis of end group labeled RNA. Nucleic Acids Res. 1977 Dec;4(12):4091–4108. doi: 10.1093/nar/4.12.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Prochiantz A., Haenni A. L., Rajbhandary U. L. Studies on the sequence of the 3'-terminal region of turnip-yellow-mosaic-virus RNA. Eur J Biochem. 1977 Feb;72(3):465–478. doi: 10.1111/j.1432-1033.1977.tb11270.x. [DOI] [PubMed] [Google Scholar]
- Silverman S., Heckman J., Cowling G. J., Delaney A. D., Dunn R. J., Gillam I. C., Tener G. M., Söll D., RajBhandary U. L. The nucleotide sequence of the initiator tRNA from Drosophila melanogaster. Nucleic Acids Res. 1979 Feb;6(2):421–433. doi: 10.1093/nar/6.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Vassilenko S. A different approach to RNA sequencing. Nature. 1978 Jul 6;274(5666):87–89. doi: 10.1038/274087a0. [DOI] [PubMed] [Google Scholar]
- Wrede P., Woo N. H., Rich A. Initiator tRNAs have a unique anticodon loop conformation. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3289–3293. doi: 10.1073/pnas.76.7.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro-Matsumura S., Takemura S. The primary structure of cytoplasmic initiator transfer ribonucleic acid from Torulopsis utilis. J Biochem. 1979 Aug;86(2):335–346. doi: 10.1093/oxfordjournals.jbchem.a132531. [DOI] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]




