Abstract
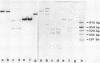
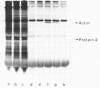
Two overlapping DNA fragments from yeast Saccharomyces cerevisiae containing the actin gene have been inserted into pBR322 and cloned in E.coli. Clones were identified by hybridization to complementary RNA from a plasmid containing a copy of Dictyostelium actin mRNA. One recombinant plasmid obtained (pYA102) contains a 3.93-kb Hindlll fragment, the other (pYA208) a 5.1-kb Pstl fragment, both share a common 2.2-kb fragment harboring part of the actin gene. Cloned yeast actin DNA was identified by R-loop formation and translation of the hybridized actin mRNA and by DNA sequence analysis. Cytoplasmic actin mRNA has been estimated to be about 1250 nucleotides long. There is only one type of the actin gene in S.cerevisiae.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender W., Davidson N., Kindle K. L., Taylor W. C., Silverman M., Firtel R. A. The structure of M6, a recombinant plasmid containing Dictyostelium DNA homologous to actin messenger RNA. Cell. 1978 Nov;15(3):779–788. doi: 10.1016/0092-8674(78)90263-5. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Cami B., Kourilsky P. Screening of cloned recombinant DNA in bacteria by in situ colony hybridization. Nucleic Acids Res. 1978 Jul;5(7):2381–2390. doi: 10.1093/nar/5.7.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Garrels J. I., Gibson W. Identification and characterization of multiple forms of actin. Cell. 1976 Dec;9(4 Pt 2):793–805. doi: 10.1016/0092-8674(76)90142-2. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Macromolecule synthesis in temperature-sensitive mutants of yeast. J Bacteriol. 1967 May;93(5):1662–1670. doi: 10.1128/jb.93.5.1662-1670.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hereford L. M., Rosbash M. Regulation of a set of abundant mRNA sequences. Cell. 1977 Mar;10(3):463–467. doi: 10.1016/0092-8674(77)90033-2. [DOI] [PubMed] [Google Scholar]
- Kindle K. L., Firtel R. A. Identification and analysis of Dictyostelium actin genes, a family of moderately repeated genes. Cell. 1978 Nov;15(3):763–778. doi: 10.1016/0092-8674(78)90262-3. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Biochemistry of actomyosin-dependent cell motility (a review). Proc Natl Acad Sci U S A. 1978 Feb;75(2):588–599. doi: 10.1073/pnas.75.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koteliansky V. E., Glukhova M. A., Bejanian M. V., Surguchov A. P., Smirnov V. N. Isolation and characterization of actin-like protein from yeast Saccharomyces cerevisiae. FEBS Lett. 1979 Jun 1;102(1):55–58. doi: 10.1016/0014-5793(79)80927-8. [DOI] [PubMed] [Google Scholar]
- Lu R. C., Elzinga M. Partial amino acid sequence of brain actin and its homology with muscle actin. Biochemistry. 1977 Dec 27;16(26):5801–5806. doi: 10.1021/bi00645a025. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown M., Taylor W. C., Kindle K. L., Firtel R. A., Bender W., Davidson N. Multiple, heterogeneous actin genes in Dictyostelium. Cell. 1978 Nov;15(3):789–800. doi: 10.1016/0092-8674(78)90264-7. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Philippsen P., Thomas M., Kramer R. A., Davis R. W. Unique arrangement of coding sequences for 5 S, 5.8 S, 18 S and 25 S ribosomal RNA in Saccharomyces cerevisiae as determined by R-loop and hybridization analysis. J Mol Biol. 1978 Aug 15;123(3):387–404. doi: 10.1016/0022-2836(78)90086-4. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. At least six different actins are expressed in a higher mammal: an analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J Mol Biol. 1978 Dec 25;126(4):783–802. doi: 10.1016/0022-2836(78)90020-7. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. Mammalian cytoplasmic actins are the products of at least two genes and differ in primary structure in at least 25 identified positions from skeletal muscle actins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1106–1110. doi: 10.1073/pnas.75.3.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. The amino acid sequence of Physarum actin. Nature. 1978 Dec 14;276(5689):720–721. doi: 10.1038/276720a0. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. The complete amino acid sequence of actins from bovine aorta, bovine heart, bovine fast skeletal muscle, and rabbit slow skeletal muscle. A protein-chemical analysis of muscle actin differentiation. Differentiation. 1979;14(3):123–133. doi: 10.1111/j.1432-0436.1979.tb01021.x. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen R. G., Butler-Browne G. S., Gros F. Protein synthesis and actin heterogeneity in calf muscle cells in culture. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2018–2022. doi: 10.1073/pnas.73.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolford J. L., Jr, Rosbash M. The use of R-looping for structural gene identification and mRNA purification. Nucleic Acids Res. 1979 Jun 11;6(7):2483–2497. doi: 10.1093/nar/6.7.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechel K., Weber K. Actins from mammals, bird, fish and slime mold characterized by isoelectric focusing in polyacrylamide gels. Eur J Biochem. 1978 Aug 15;89(1):105–112. doi: 10.1111/j.1432-1033.1978.tb20901.x. [DOI] [PubMed] [Google Scholar]