Abstract
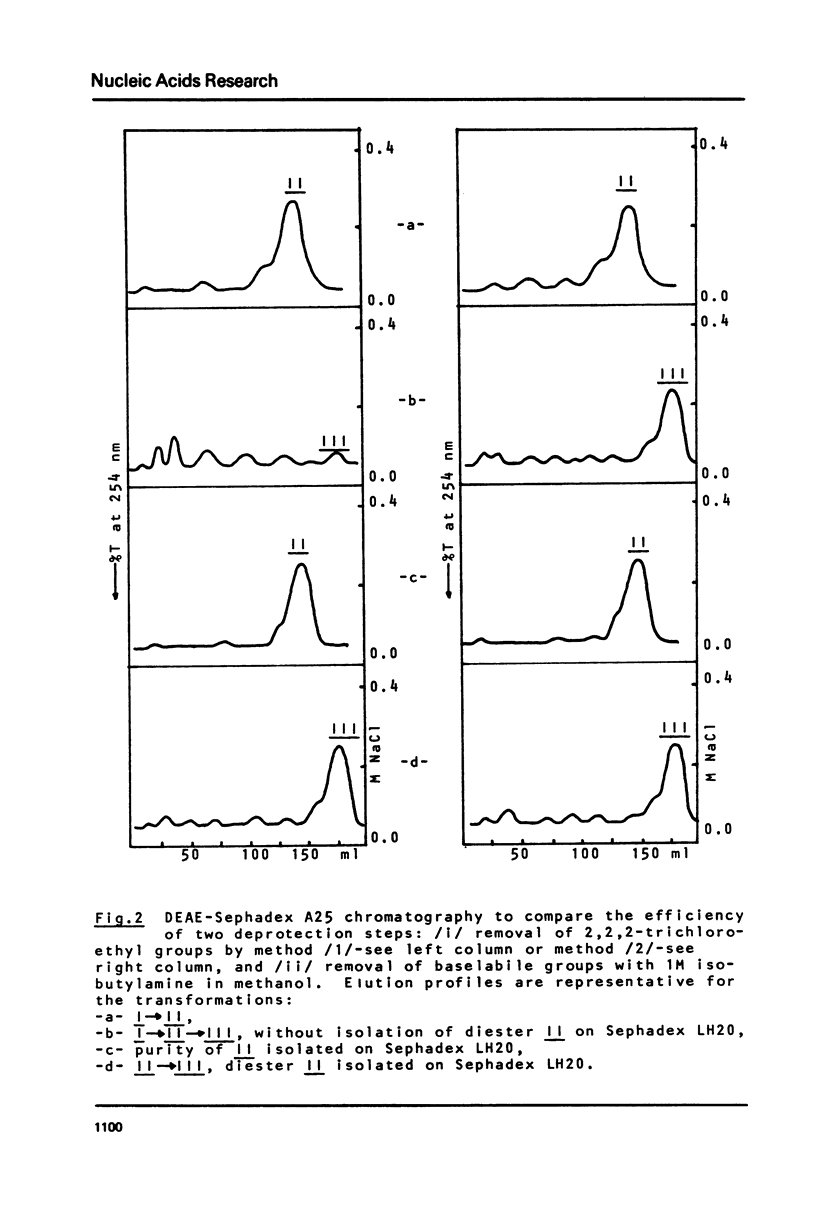
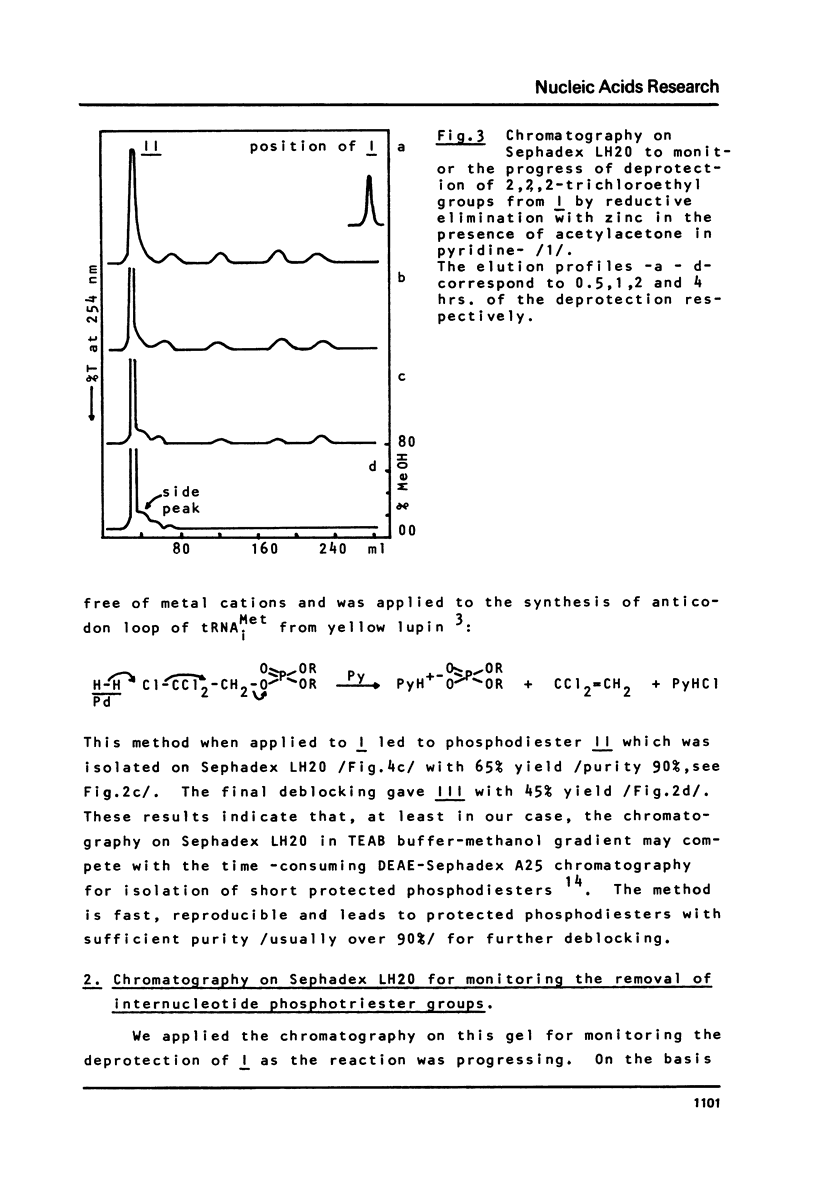
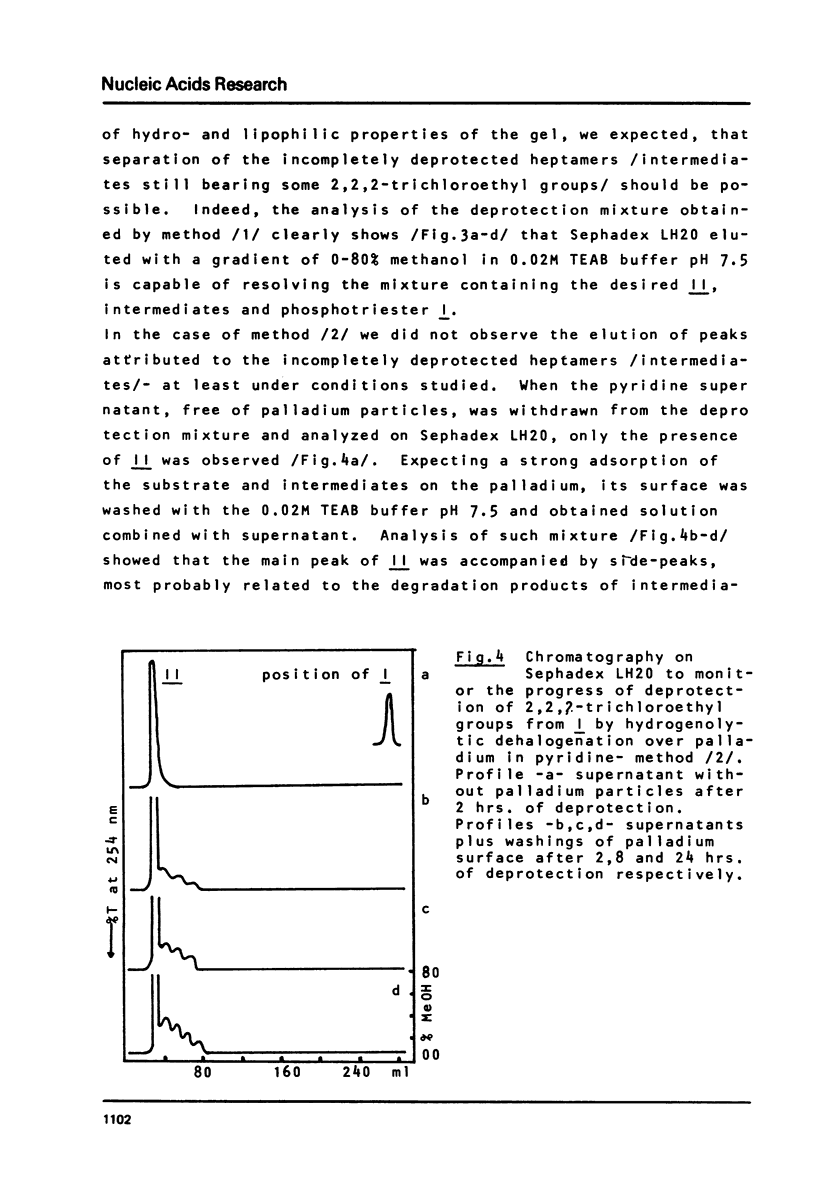
Chromatography on Sephadex LH20, in a linear gradient of methanol in 0.02M TEAB buffer pH 7.5, is proposed as a fast and efficient method for the isolation and purification of protected oligoribonucleotide phosphodiesters obtained by deprotection of internucleotide phosphotriesters, and for the monitoring of the deprotection step itself. Its utility is shown on the example of removal of 2,2,2-trichloroethyl groups from oligoribonucleotide phosphotriester I of sequence CCCAUAA by two methods: /1/ reductive elimination with zinc in the presence of acetylacetone modified as presented here, and /2/ hydrogenolytic dehalogenation over palladium in pyridine. This method of chromatography on Sephadex LH20 is used as a key purification step during the removal of 2,2,2-trichloroethyl groups from I by method /1/ and allows to raise the yield of III during fianl deprotection step from 5 to 65%.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamiak R. W., Biala E., Grześkowiak, Kierzek R., Kraszewski A., Markiewicz W. T., Stawiński J., Wiewiórowski Nucleoside 3'-phosphotriesters as key intermediates for the oligoribonucleotide synthesis. IV. New method for removal of 2,2,2-trichloroethyl group and 31P NMR as a new tool for analysis of deblocking of internucleotide phosphate protecting groups. Nucleic Acids Res. 1977 Jul;4(7):2321–2329. doi: 10.1093/nar/4.7.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamiak R. W., Biała E., Grześkowiak K., Kierzek R., Kraszewski A., Markiewicz W. T., Okupniak J., Stawiński J., Wiewiórowski M. The chemical synthesis of the anticodon loop of an eukaryotic initiator tRNA containing the hypermodified nucleoside N6-/N-threonylcarbonyl/-adenosine/t6A/1. Nucleic Acids Res. 1978 Jun;5(6):1889–1905. doi: 10.1093/nar/5.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arentzen R., Reese C. B. The phosphotriester approach to oligonucleotide synthesis: Preparation of oligo- and poly-thymidylic acids. J Chem Soc Perkin 1. 1977;4:445–460. doi: 10.1039/p19770000445. [DOI] [PubMed] [Google Scholar]
- Catlin J. C., Cramer F. Deoxy oligonucleotide synthesis via the triester method. J Org Chem. 1973 Jan 26;38(2):245–250. doi: 10.1021/jo00942a011. [DOI] [PubMed] [Google Scholar]
- Izatt R. M., Christensen J. J., Rytting J. H. Sites and thermodynamic quantities associated with proton and metal ion interaction with ribonucleic acid, deoxyribonucleic acid, and their constituent bases, nucleosides, and nucleotides. Chem Rev. 1971 Oct;71(5):439–481. doi: 10.1021/cr60273a002. [DOI] [PubMed] [Google Scholar]
- Otsuka E., Ubasawa M., Morioka S., Ikehara M. Studies on transfer ribonucleic acids and related compounds. VI. Synthesis of yeast alanine transfer ribonucleic acid 3'-terminal nonanucleotides and 5'-terminal hexanucleotides. J Am Chem Soc. 1973 Jul 11;95(14):4725–4733. doi: 10.1021/ja00795a042. [DOI] [PubMed] [Google Scholar]
- de Rooij J. F., Wille-Hazeleger G., Burgers P. M., van Boom J. H. Neighbouring group participation in the unblocking of phosphotriesters of nucleic acids. Nucleic Acids Res. 1979;6(6):2237–2259. doi: 10.1093/nar/6.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]



