Abstract
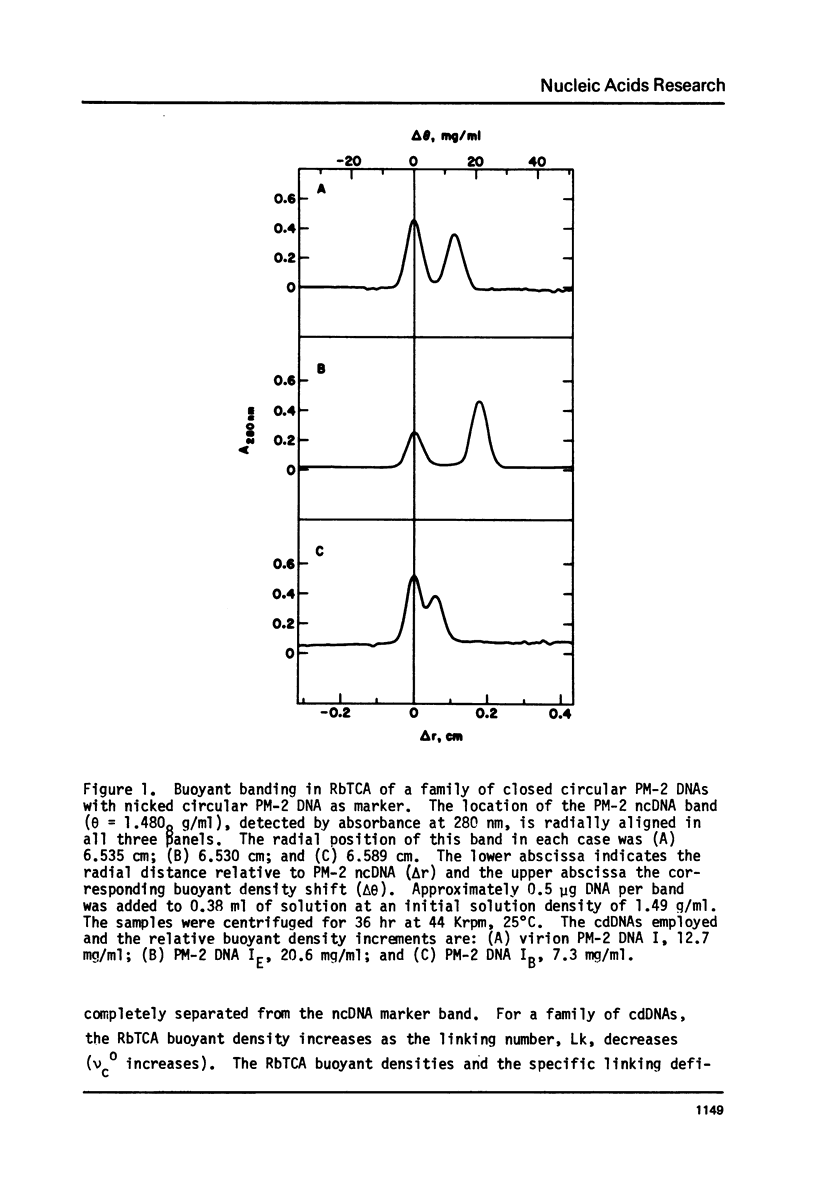
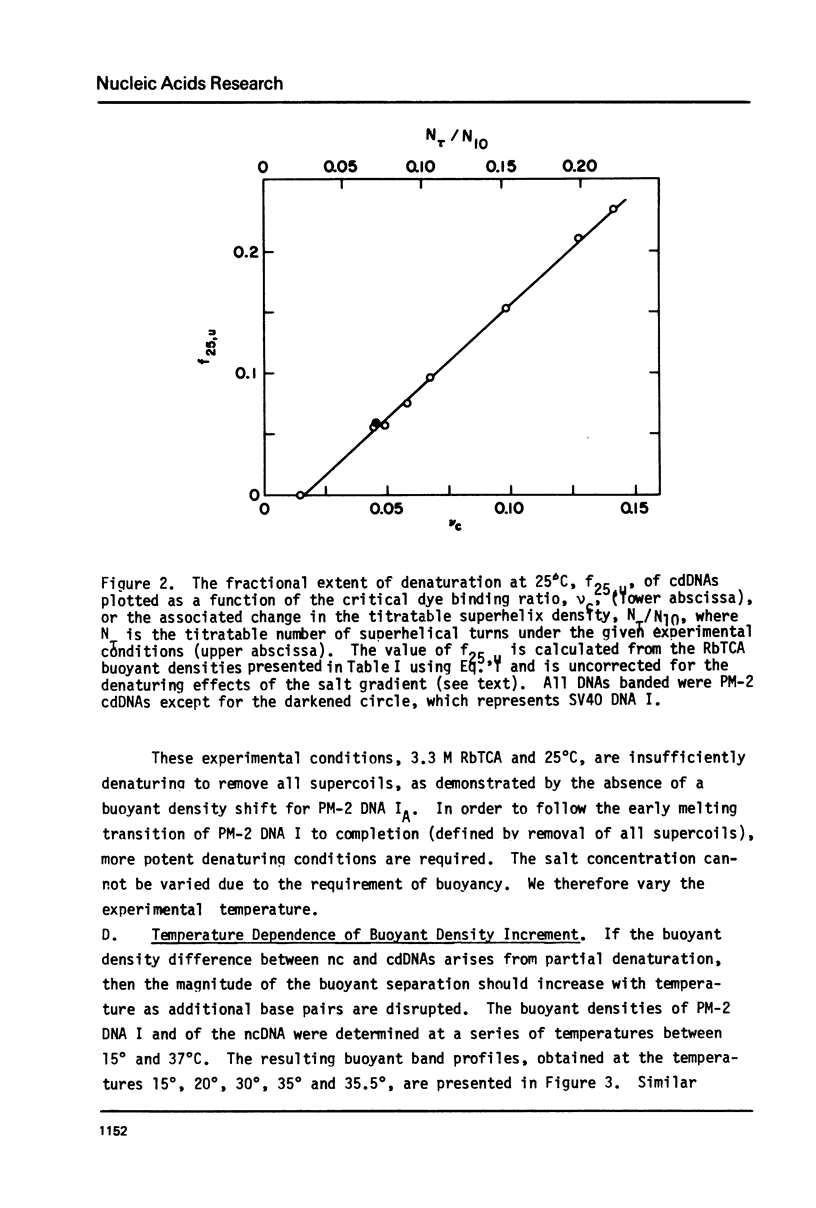
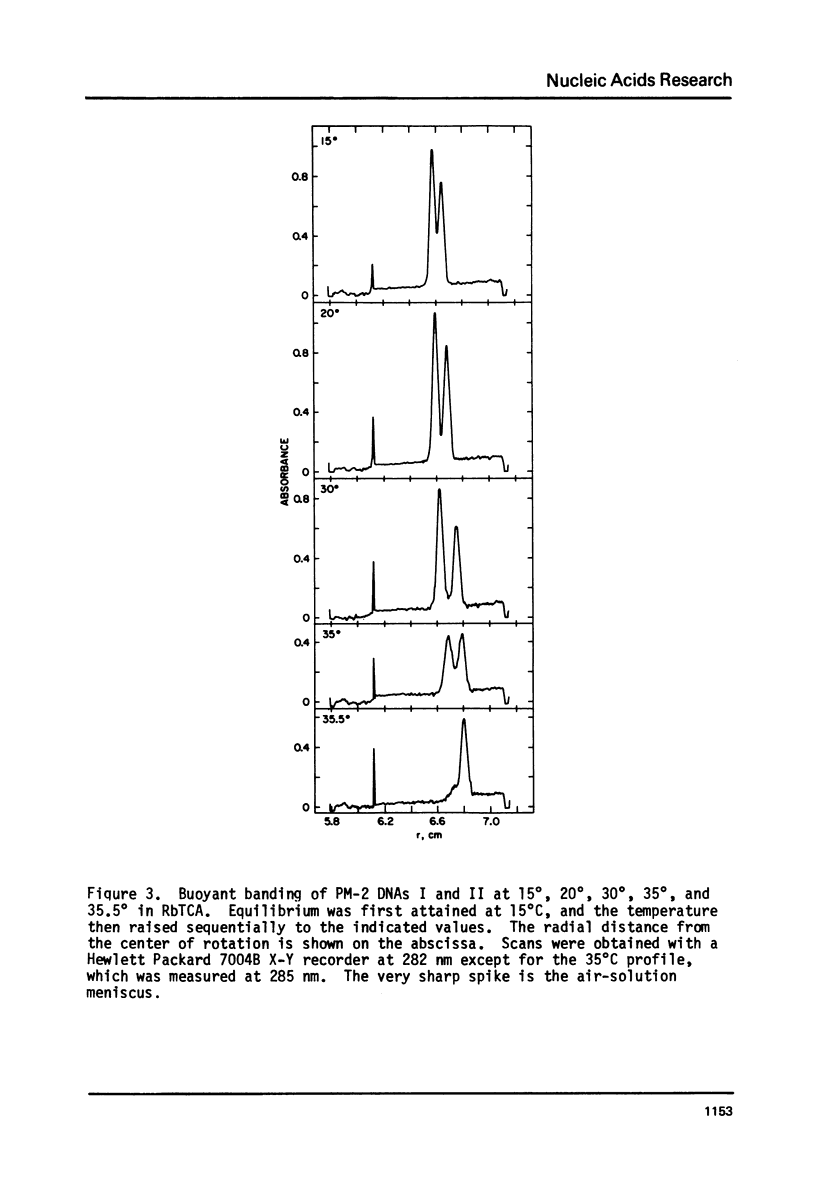
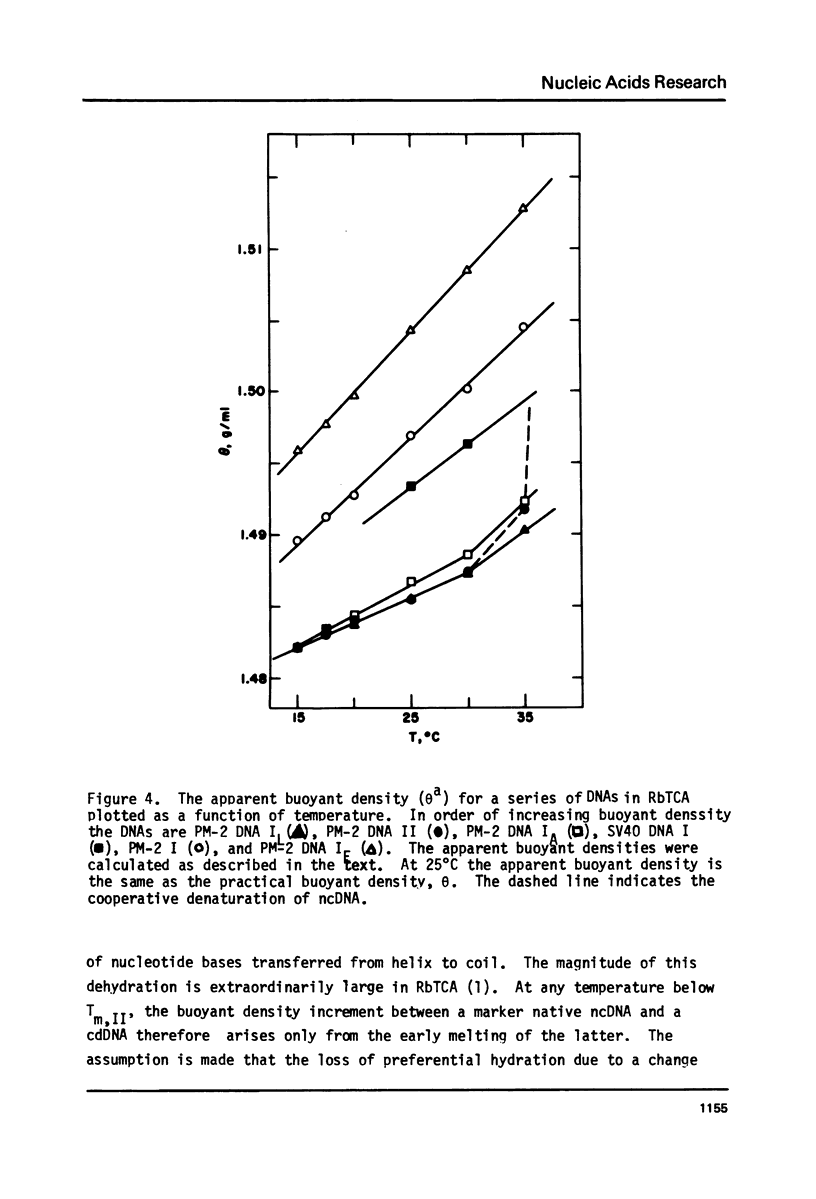
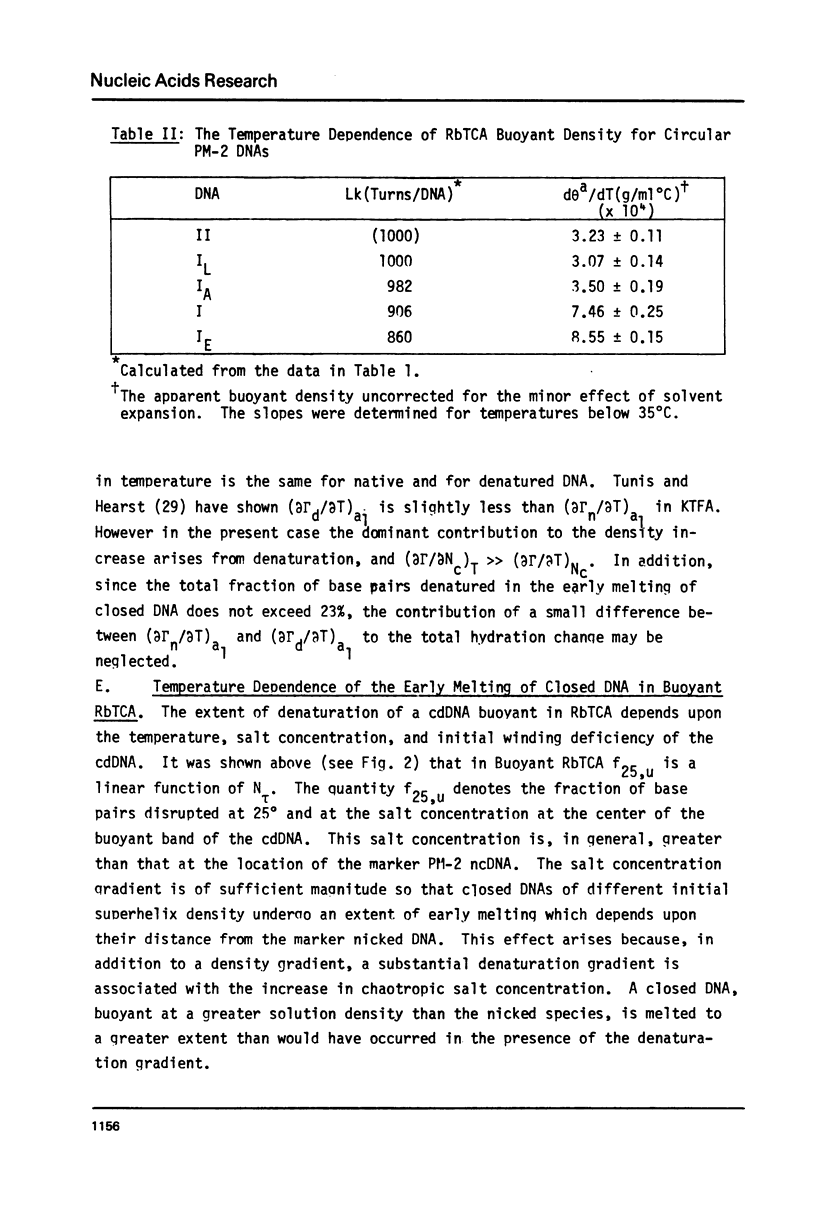
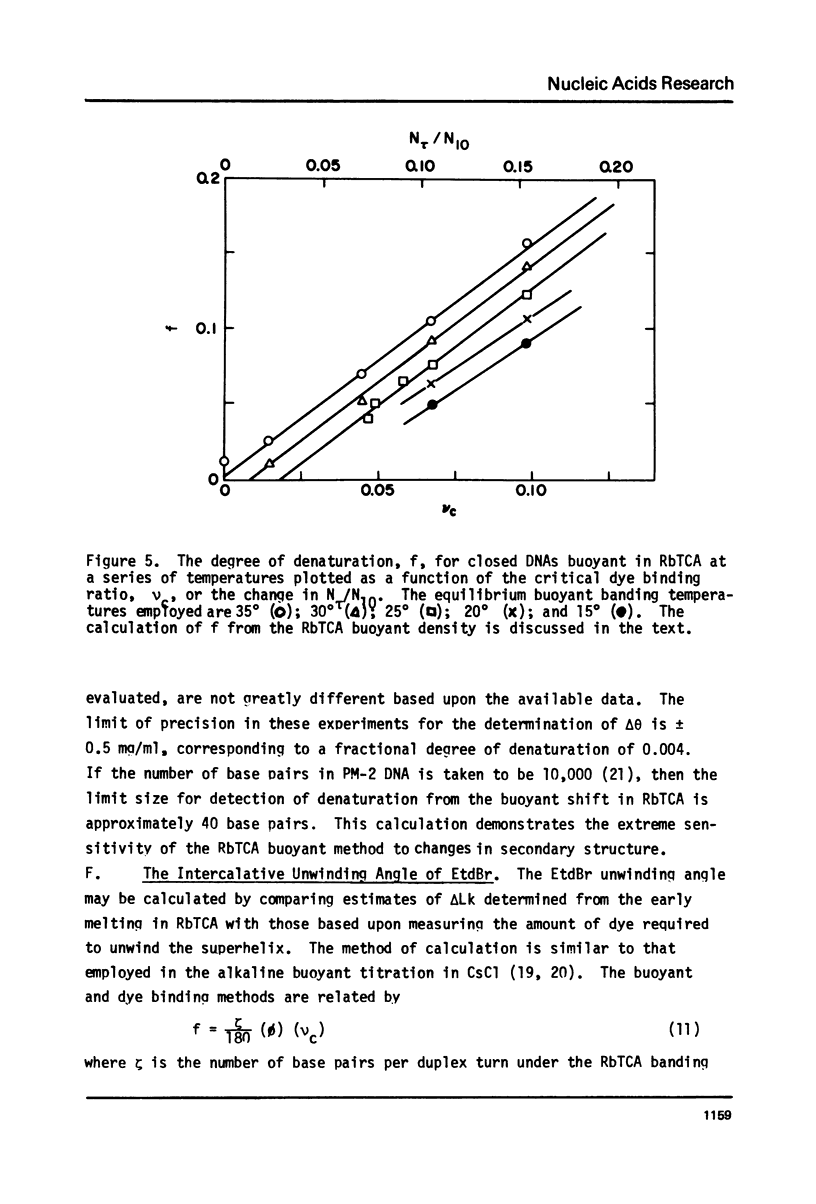
Aqueous RbTCA permits the buoyant banding of both native and denatured DNA at room temperature and neutral pH. A unique property of this solvent is the bouyant resolution of closed circular, underwound DNA (I) from the corresponding nicked (II) species. Conditions are reported here in which PM-2 DNA I is physically resolved from native PM-2 DNA II, the buoyant separation being 1.27 mq/ml in 3.3 M RbTCA at 25 degrees C. The separation between nicked and closed DNAs increases with temperature up to 35.5 degrees C, at which PM-2 DNA II cooperatively melts and subsequently pellets. The isothermal buoyant density of a cloed DNA increases linearly as the linking number (Lk) of the closed DNA decreases. The early melting of closed DNA may be monitored with high precision by buoyant banding in RbTCA, it being possible to detect the disruption of as few as 40 base pairs in PM-2 DNA (10,000 base pairs). The constraint that the linking number be conserved in closed DNA requires that a change in duplex winding be accompanied by a compensating change in supercoiling. We estimate the linking number deficiency of PM-2 DNA I to be 0.094 turns per decibase pair. This result permits the estimation of the EtdBr unwinding angle, phi, by comparison with alternative determinations of the linking number deficiency which depend upom the value of phi. The result obtained here is that phi = 27.7 degrees +/- 0.5 degrees and is approximately independent of temperature over the range 15 degrees-35 degrees.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Bauer W. Supercoiling in closed circular DNA: dependence upon ion type and concentration. Biochemistry. 1978 Feb 21;17(4):594–601. doi: 10.1021/bi00597a006. [DOI] [PubMed] [Google Scholar]
- Bauer W. R. Premelting unwinding of the deoxyribonucleic acid duplex by aqueous magnesium perchlorate. Biochemistry. 1972 Jul 18;11(15):2915–2920. doi: 10.1021/bi00765a027. [DOI] [PubMed] [Google Scholar]
- Bauer W. R. Structure and reactions of closed duplex DNA. Annu Rev Biophys Bioeng. 1978;7:287–313. doi: 10.1146/annurev.bb.07.060178.001443. [DOI] [PubMed] [Google Scholar]
- Bauer W. R. Structure of DNA in denaturing solvents. I. Bacteriophage PM2 DNA in aqueous sodium perchlorate. J Mol Biol. 1972 Jun 20;67(2):183–198. doi: 10.1016/0022-2836(72)90235-5. [DOI] [PubMed] [Google Scholar]
- Bauer W., Vinograd J. Interaction of closed circular DNA with intercalative dyes. II. The free energy of superhelix formation in SV40 DNA. J Mol Biol. 1970 Feb 14;47(3):419–435. doi: 10.1016/0022-2836(70)90312-8. [DOI] [PubMed] [Google Scholar]
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- Burke R. L., Bauer W. R. The properties of native and denatured DNA in buoyant rubidium trichloroacetate at neutral pH. Nucleic Acids Res. 1977 Jun;4(6):1891–1909. doi: 10.1093/nar/4.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. L., Bauer W. Measurement of superhelix densities in buoyant dye/CsCl. The use of a standard other than native SV40 DNA. J Biol Chem. 1977 Jan 10;252(1):291–292. [PubMed] [Google Scholar]
- Crick F. H. Linking numbers and nucleosomes. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2639–2643. doi: 10.1073/pnas.73.8.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. H., Wang J. C., Bauer W. R. Is DNA really a double helix? J Mol Biol. 1979 Apr 15;129(3):449–457. doi: 10.1016/0022-2836(79)90506-0. [DOI] [PubMed] [Google Scholar]
- Depew D. E., Wang J. C. Conformational fluctuations of DNA helix. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4275–4279. doi: 10.1073/pnas.72.11.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller F. B. The writhing number of a space curve. Proc Natl Acad Sci U S A. 1971 Apr;68(4):815–819. doi: 10.1073/pnas.68.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray H. B., Jr, Upholt W. B., Vinograd J. A buoyant method for the determination of the superhelix density of closed circular DNA. J Mol Biol. 1971 Nov 28;62(1):1–19. doi: 10.1016/0022-2836(71)90127-6. [DOI] [PubMed] [Google Scholar]
- HEARST J. E., IFFT J. B., VINOGRAD J. The effects of pressure on the buoyant behavior of deoxyribonucleic acid and tobacco mosaic virus in a density gradient at equilibrium in the ultracentrifuge. Proc Natl Acad Sci U S A. 1961 Jul 15;47:1015–1025. doi: 10.1073/pnas.47.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEARST J. E. The specific volume of various cationic forms of deoxyribonucleic acid. J Mol Biol. 1962 May;4:415–417. doi: 10.1016/s0022-2836(62)80024-2. [DOI] [PubMed] [Google Scholar]
- HEARST J. E., VINOGRAD J. The net hydration of deoxyribonucleic acid. Proc Natl Acad Sci U S A. 1961 Jun 15;47:825–830. doi: 10.1073/pnas.47.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearst J. E., Vinograd J. A THREE-COMPONENT THEORY OF SEDIMENTATION EQUILIBRIUM IN A DENSITY GRADIENT. Proc Natl Acad Sci U S A. 1961 Jul;47(7):999–1004. doi: 10.1073/pnas.47.7.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T. S., Wang J. C. Thermodynamic properties of superhelical DNAs. Biochemistry. 1975 Feb 11;14(3):527–535. doi: 10.1021/bi00674a011. [DOI] [PubMed] [Google Scholar]
- Jain S. C., Tsai C. C., Sobell H. M. Visualization of drug-nucleic acid interactions at atomic resolution. II. Structure of an ethidium/dinucleoside monophosphate crystalline complex, ethidium:5-iodocytidylyl (3'-5') guanosine. J Mol Biol. 1977 Aug 15;114(3):317–331. doi: 10.1016/0022-2836(77)90253-4. [DOI] [PubMed] [Google Scholar]
- Keller W. Determination of the number of superhelical turns in simian virus 40 DNA by gel electrophoresis. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4876–4880. doi: 10.1073/pnas.72.12.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller W. Determination of the number of superhelical turns in simian virus 40 DNA by gel electrophoresis. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4876–4880. doi: 10.1073/pnas.72.12.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulleyblank D. E., Morgan A. R. The sense of naturally occurring superhelices and the unwinding angle of intercalated ethidium. J Mol Biol. 1975 Jan 5;91(1):1–13. doi: 10.1016/0022-2836(75)90368-x. [DOI] [PubMed] [Google Scholar]
- Pulleyblank D. E., Shure M., Tang D., Vinograd J., Vosberg H. P. Action of nicking-closing enzyme on supercoiled and nonsupercoiled closed circular DNA: formation of a Boltzmann distribution of topological isomers. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4280–4284. doi: 10.1073/pnas.72.11.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Rodley G. A., Scobie R. S., Bates R. H., Lewitt R. M. A possible conformation for double-stranded polynucleotides. Proc Natl Acad Sci U S A. 1976 Sep;73(9):2959–2963. doi: 10.1073/pnas.73.9.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasisekharan V., Pattabiraman N., Gupta G. Some implications of an alternative structure for DNA. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4092–4096. doi: 10.1073/pnas.75.9.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Shure M., Vinograd J. The number of superhelical turns in native virion SV40 DNA and minicol DNA determined by the band counting method. Cell. 1976 Jun;8(2):215–226. doi: 10.1016/0092-8674(76)90005-2. [DOI] [PubMed] [Google Scholar]
- Sobell H. M., Tsai C. C., Jain S. C., Gilbert S. G. Visualization of drug-nucleic acid interactions at atomic resolution. III. Unifying structural concepts in understanding drug-DNA interactions and their broader implications in understanding protein-DNA interactions. J Mol Biol. 1977 Aug 15;114(3):333–365. doi: 10.1016/0022-2836(77)90254-6. [DOI] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Electron microscopy of DNA: determination of absolute molecular weights and linear density. Mol Gen Genet. 1977 Sep 9;154(3):299–303. doi: 10.1007/BF00571286. [DOI] [PubMed] [Google Scholar]
- Tsai C. C., Jain S. C., Sobell H. M. Visualization of drug-nucleic acid interactions at atomic resolution. I. Structure of an ethidium/dinucleoside monophosphate crystalline complex, ethidium:5-iodouridylyl (3'-5') adenosine. J Mol Biol. 1977 Aug 15;114(3):301–315. doi: 10.1016/0022-2836(77)90252-2. [DOI] [PubMed] [Google Scholar]
- Tunis M. J., Hearst J. E. On the hydration of DNA. I. Preferential hydration and stability of DNA in concentrated trifluoroacetate solution. Biopolymers. 1968;6(9):1325–1344. doi: 10.1002/bip.1968.360060908. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., HEARST J. E. Equilibrium sedimentation of macromolecules and viruses in a density gradient. Fortschr Chem Org Naturst. 1962;20:373–422. [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J. Physical and topological properties of circular DNA. J Gen Physiol. 1966 Jul;49(6):103–125. doi: 10.1085/jgp.49.6.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Watson R. Early and late helix-coil transitions in closed circular DNA. The number of superhelical turns in polyoma DNA. J Mol Biol. 1968 Apr 14;33(1):173–197. doi: 10.1016/0022-2836(68)90287-8. [DOI] [PubMed] [Google Scholar]
- Vosberg H. P., Grossman L. I., Vinograd J. Isolation and partial characterisation of the relaxation protein from nuclei of cultured mouse and human cells. Eur J Biochem. 1975 Jun 16;55(1):79–93. doi: 10.1111/j.1432-1033.1975.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Rich A. Atomic resolution analysis of a 2:1 complex of CpG and acridine orange. Nucleic Acids Res. 1979 Aug 24;6(12):3879–3890. doi: 10.1093/nar/6.12.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. Helical repeat of DNA in solution. Proc Natl Acad Sci U S A. 1979 Jan;76(1):200–203. doi: 10.1073/pnas.76.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. The degree of unwinding of the DNA helix by ethidium. I. Titration of twisted PM2 DNA molecules in alkaline cesium chloride density gradients. J Mol Biol. 1974 Nov 15;89(4):783–801. doi: 10.1016/0022-2836(74)90053-9. [DOI] [PubMed] [Google Scholar]
- Wiesehahn G., Cech T. R., Hearst J. E. A study of DNA denaturation in the ultracentrifuge. Biopolymers. 1976 Aug;15(8):1591–1613. doi: 10.1002/bip.1976.360150813. [DOI] [PubMed] [Google Scholar]



