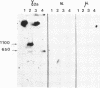
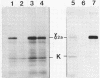
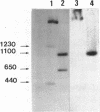
Abstract
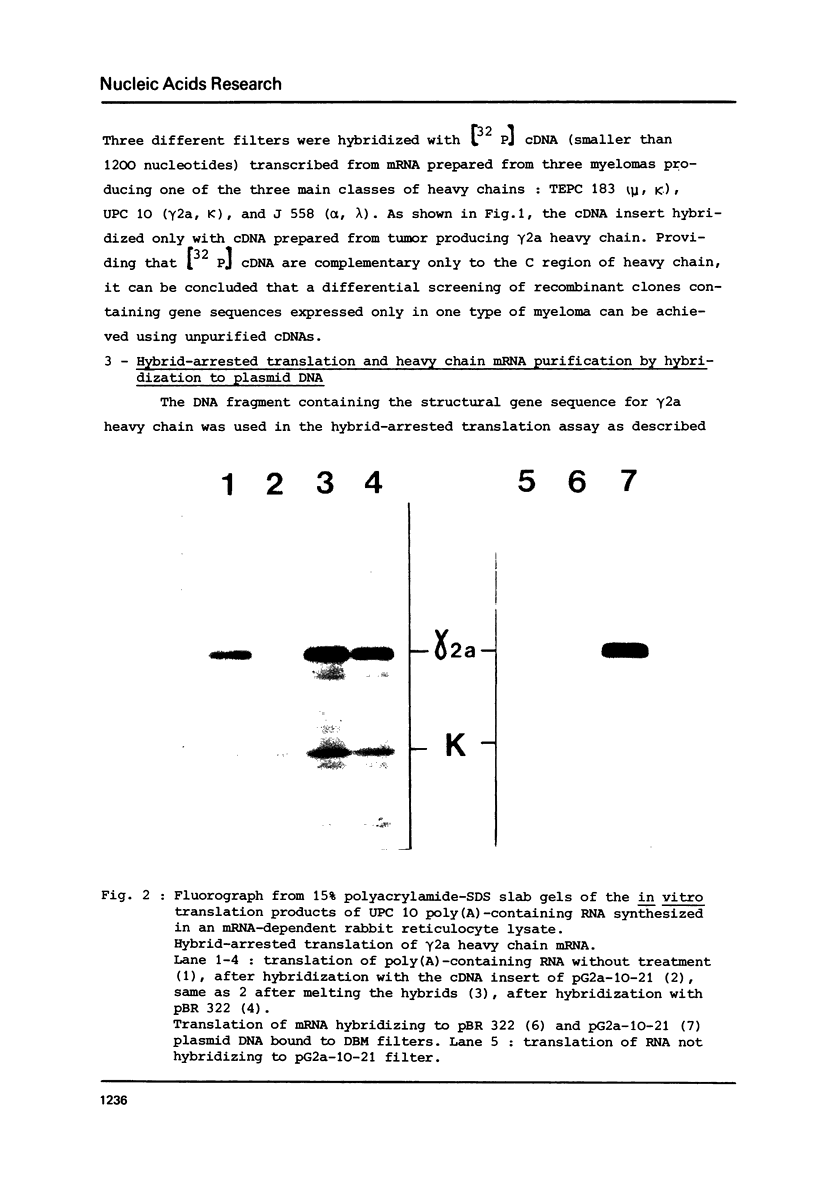
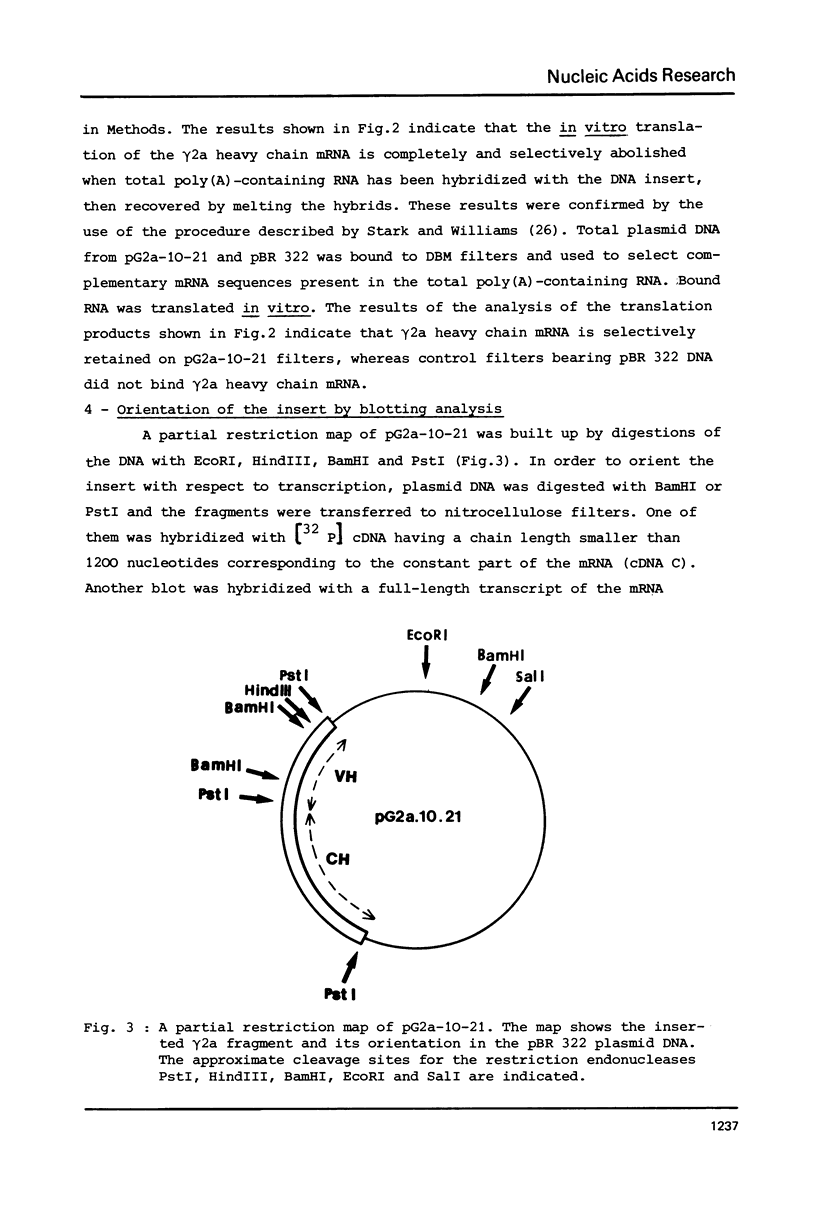
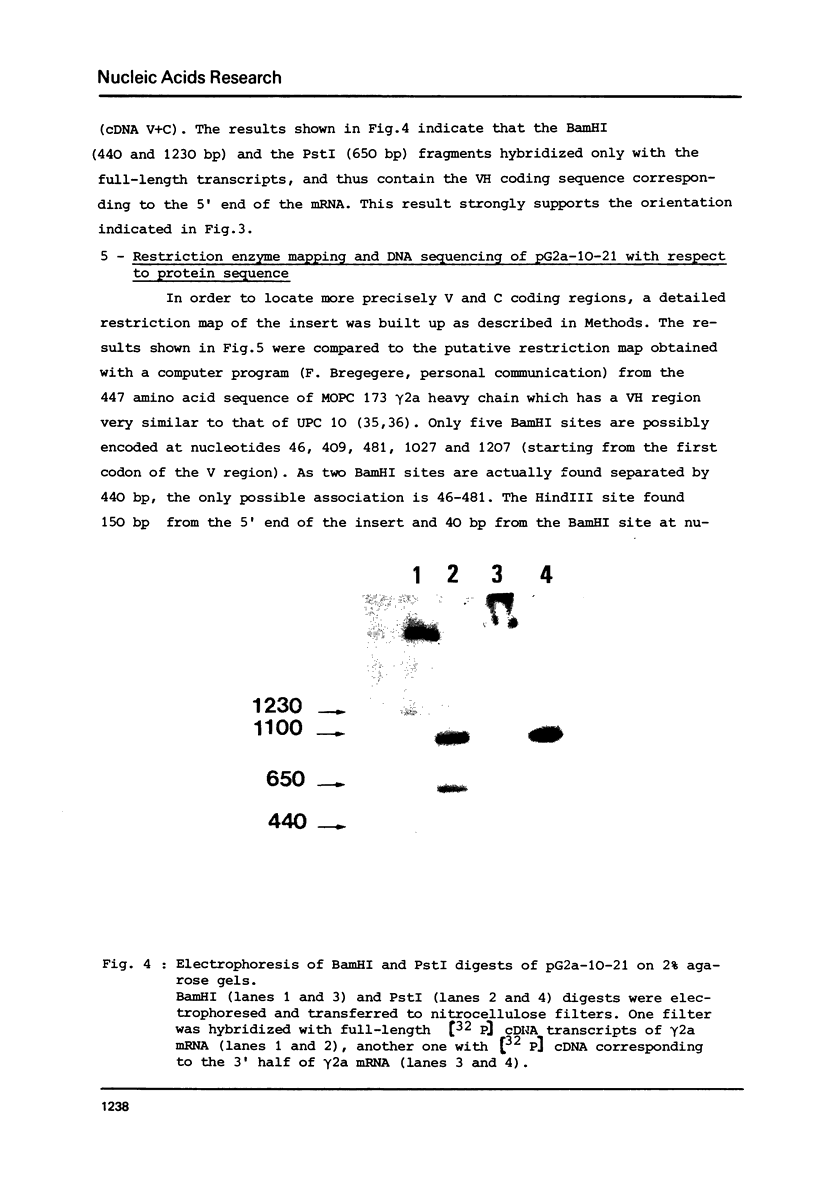
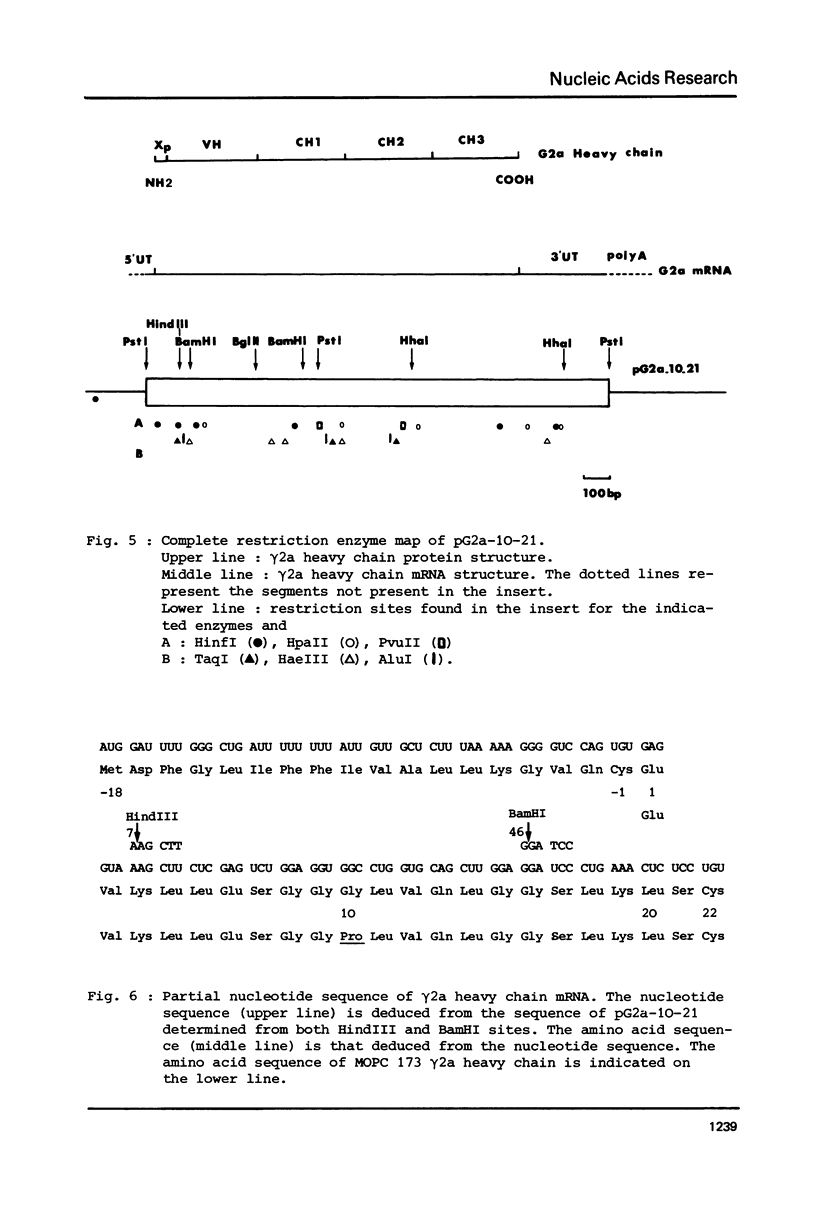
A DNA sequence complementary to the entire coding part of a mouse gamma 2a immunoglobulin heavy chain mRNA isolated from a myeloma producing a levan binding protein (UPC 10), has been cloned in the PstI site of pBR 322. Transformants containing sequences complementary to purified gamma 2a heavy chain mRNA were selected. One transformant, pG2a-10-21, containing a 1750 nucleotide insert, has been characterized by hybrid-arrested translation and purification of gamma 2a heavy chain mRNA on DNA-DBM cellulose filters. Restriction enzyme analysis and partial sequencing demonstrate that the pG2a-10-21 contains the complete structural sequence for the gamma 2a heavy chain and predicts the sequence of a 18 amino acid hydrophobic amino terminal extra piece segment.
Full text
PDF



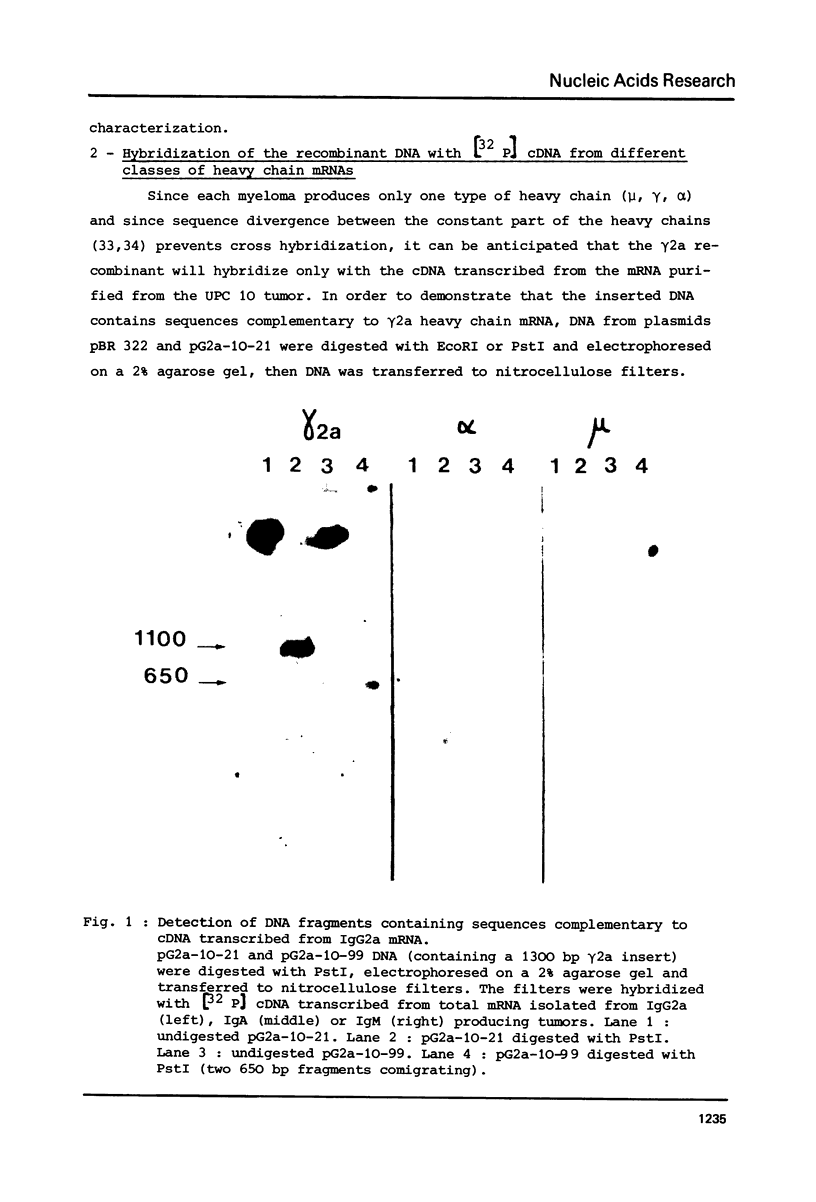


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkner K. L., Folk W. R. Polynucleotide kinase exchange reaction: quantitave assay for restriction endonuclease-generated 5'-phosphoroyl termini in DNA. J Biol Chem. 1977 May 25;252(10):3176–3184. [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bosma M. J., De Witt C., Hausman S. J. Serological distinciton of heavy chain variable regions (VH subgroups) of mouse immunoglobulins. I. Common VH determinants on the heavy chains of mouse myeloma proteins having different binding sites. J Exp Med. 1977 Oct 1;146(4):104l–1053. [PubMed] [Google Scholar]
- Breathnach R., Mandel J. L., Chambon P. Ovalbumin gene is split in chicken DNA. Nature. 1977 Nov 24;270(5635):314–319. doi: 10.1038/270314a0. [DOI] [PubMed] [Google Scholar]
- Burstein Y., Schechter I. Amino acid sequence of the NH2-terminal extra piece segments of the precursors of mouse immunoglobulin lambda1-type and kappa-type light chains. Proc Natl Acad Sci U S A. 1977 Feb;74(2):716–720. doi: 10.1073/pnas.74.2.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Deoxynucleotide-polymerizing enzymes of calf thymus gland. V. Homogeneous terminal deoxynucleotidyl transferase. J Biol Chem. 1971 Feb 25;246(4):909–916. [PubMed] [Google Scholar]
- Cisar J., Kabat E. A., Liao J., Potter M. Immunochemical studies on mouse myeloma proteins reactive with dextrans or with fructosans and on human antilevans. J Exp Med. 1974 Jan 1;139(1):159–179. doi: 10.1084/jem.139.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fougereau M., Bourgois A., de Preval C., Rocca-Serra J., Schiff C. The complete sequence of the murine monoclonal immunoglobulin MOPC 173 (IgG2a): genetic implications. Ann Immunol (Paris) 1976 Sep-Oct;127(5):607–631. [PubMed] [Google Scholar]
- Greene P. J., Heyneker H. L., Bolivar F., Rodriguez R. L., Betlach M. C., Covarrubias A. A., Backman K., Russel D. J., Tait R., Boyer H. W. A general method for the purification of restriction enzymes. Nucleic Acids Res. 1978 Jul;5(7):2373–2380. doi: 10.1093/nar/5.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood L., Loh E., Hubert J., Barstad P., Eaton B., Early P., Fuhrman J., Johnson N., Kronenberg M., Schilling J. The structure and genetics of mouse immunoglobulins: an analysis of NZB myeloma proteins and sets of BALB/c myeloma proteins binding particular haptens. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):817–836. doi: 10.1101/sqb.1977.041.01.092. [DOI] [PubMed] [Google Scholar]
- Jilka R. L., Pestka S. Amino acid sequence of the precursor region of MOPC-315 mouse immunoglobulin heavy chain. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5692–5696. doi: 10.1073/pnas.74.12.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovin T. M., Englund P. T., Bertsch L. L. Enzymatic synthesis of deoxyribonucleic acid. XXVI. Physical and chemical studies of a homogeneous deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):2996–3008. [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lieberman R., Potter M., Humphrey W., Jr, Mushinski E. B., Vrana M. Multiple individual and cross-specific indiotypes on 13 levan-binding myeloma proteins of BALB/c mice. J Exp Med. 1975 Jul 1;142(1):106–119. doi: 10.1084/jem.142.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan J. J., McReynolds L. A., O'Malley B. W. The ovalbumin gene. In vitro enzymatic synthesis and characterization. J Biol Chem. 1976 Dec 10;251(23):7355–7362. [PubMed] [Google Scholar]
- Myers J. C., Spiegelman S. Sodium pyrophosphate inhibition of RNA.DNA hybrid degradation by reverse transcriptase. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5329–5333. doi: 10.1073/pnas.75.11.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old R., Murray K., Boizes G. Recognition sequence of restriction endonuclease III from Hemophilus influenzae. J Mol Biol. 1975 Feb 25;92(2):331–339. doi: 10.1016/0022-2836(75)90232-6. [DOI] [PubMed] [Google Scholar]
- Panet A., van de Sande J. H., Loewen P. C., Khorana H. G., Raae A. J., Lillehaug J. R., Kleppe K. Physical characterization and simultaneous purification of bacteriophage T4 induced polynucleotide kinase, polynucleotide ligase, and deoxyribonucleic acid polymerase. Biochemistry. 1973 Dec 4;12(25):5045–5050. doi: 10.1021/bi00749a003. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Kuff E. L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Roskam W. G., Rougeon F. Molecular cloning and nucleotide sequence of the human growth hormone structural gene. Nucleic Acids Res. 1979 Sep 25;7(2):305–320. doi: 10.1093/nar/7.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougeon F., Mach B. Cloning and amplification of rabbit alpha- and beta-globin gene sequences into Escherichia coli plasmids. J Biol Chem. 1977 Apr 10;252(7):2209–2217. [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Leder P. The arrangement and rearrangement of antibody genes. Nature. 1978 Dec 21;276(5690):790–795. doi: 10.1038/276790a0. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Max E. E., Leder P. A kappa-immunoglobulin gene is formed by site-specific recombination without further somatic mutation. Nature. 1979 Aug 2;280(5721):370–375. doi: 10.1038/280370a0. [DOI] [PubMed] [Google Scholar]
- Smith D. F., Searle P. F., Williams J. G. Characterisation of bacterial clones containing DNA sequences derived from Xenopus laevis vitellogenin mRNA. Nucleic Acids Res. 1979 Feb;6(2):487–506. doi: 10.1093/nar/6.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark G. R., Williams J. G. Quantitative analysis of specific labelled RNA'S using DNA covalently linked to diazobenzyloxymethyl-paper. Nucleic Acids Res. 1979 Jan;6(1):195–203. doi: 10.1093/nar/6.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valbuena O., Marcu K. B., Weigert M., Perry R. P. Multiplicity of germline genes specifying a group of related mouse kappa chains with implications for the generation of immunoglobulin diversity. Nature. 1978 Dec 21;276(5690):780–784. doi: 10.1038/276780a0. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]
- Yamawaki-Kataoka Y., Sato K., Shimizu A., Kataoka T., Mano Y., Ono M., Kawakami M., Honjo T. Mutual homology of mouse immunoglobulin gamma-chain gene sequences. Biochemistry. 1979 Feb 6;18(3):490–494. doi: 10.1021/bi00570a018. [DOI] [PubMed] [Google Scholar]