Abstract
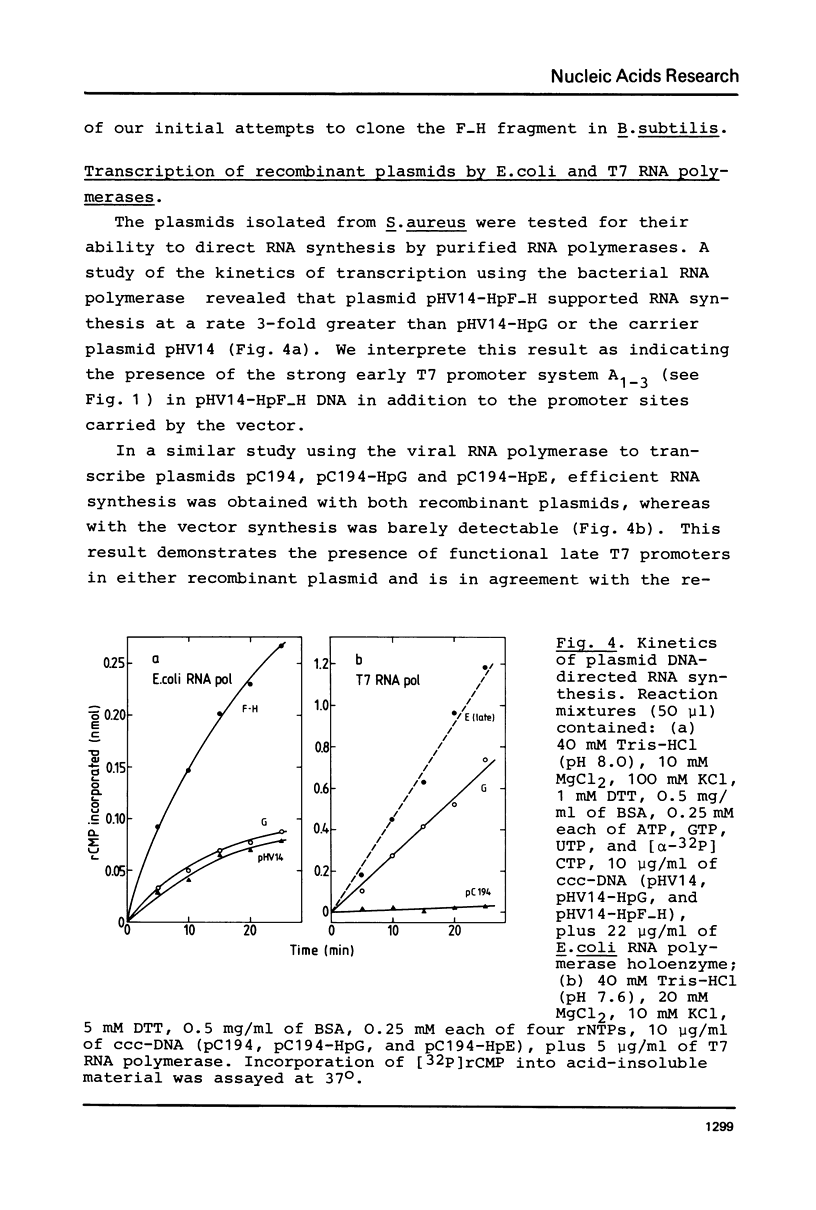
Two full-length contiguous HpaI fragments of the 0 to 18.2% region of T7 H DNA (HpF-H and HpG) were inserted into plasmids pHV14 or pC194 using oligo(dG . dC) connectors or synthetic HindIII adaptors. Amplification of the two early T7 fragments was achieved by transforming lysostaphin-treated S. aureus W57 with the hybrid plasmids. Experimental evidence is presented suggesting that neither of these T7 segments can be cloned in an intact form in E. coli. One of the hybrids, pHV14-HpF-H, proved to be unstable even in B. subtilis 168. The supercoiled recombinant plasmids were tested for their capacity to support RNA synthesis by purified E. coli or T7 RNA polymerases and to serve as templates in a cell-free T7 DNA replication system. The results of these in vitro studies indicate the presence of active "early" promoters in the cloned fragment HpF-H and active "late" promoters, as well as a functional origin of replication in the cloned fragment HpG.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asheshov E. H. The genetics of tetracycline resistance in Staphylococcus aureus. J Gen Microbiol. 1975 May;88(1):132–140. doi: 10.1099/00221287-88-1-132. [DOI] [PubMed] [Google Scholar]
- Bahl C. P., Marians K. J., Wu R. A general method for inserting specific DNA sequences into cloning vehicles. Gene. 1976;1(1):81–92. doi: 10.1016/0378-1119(76)90008-1. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Campbell J. L., Richardson C. C., Studier F. W. Genetic recombination and complementation between bacteriophage T7 and cloned fragments of T7 DNA. Proc Natl Acad Sci U S A. 1978 May;75(5):2276–2280. doi: 10.1073/pnas.75.5.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. L., Tamanoi F., Richardson C. C., Studier F. W. Cloning of the T7 genome in Escherichia coli: use of recombination between cloned sequences and bacteriophage T7 to identify genes involved in recombination and a clone containing the origin of T7 DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):441–448. doi: 10.1101/sqb.1979.043.01.050. [DOI] [PubMed] [Google Scholar]
- Canosi U., Morelli G., Trautner T. A. The relationship between molecular structure and transformation efficiency of some S. aureus plasmids isolated from B. subtilis. Mol Gen Genet. 1978 Nov 9;166(3):259–267. doi: 10.1007/BF00267617. [DOI] [PubMed] [Google Scholar]
- Champoux J. J. Proteins that affect DNA conformation. Annu Rev Biochem. 1978;47:449–479. doi: 10.1146/annurev.bi.47.070178.002313. [DOI] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Dressler D., Wolfson J., Magazin M. Initiation and reinitiation of DNA synthesis during replication of bacteriophage T7. Proc Natl Acad Sci U S A. 1972 Apr;69(4):998–1002. doi: 10.1073/pnas.69.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Buzash-Pollert E., Studier F. W. Mutations of bacteriophage T7 that affect initiation of synthesis of the gene 0.3 protein. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2741–2745. doi: 10.1073/pnas.75.6.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S. D. DNA cloning in Bacillus subtilis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1433–1436. doi: 10.1073/pnas.75.3.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S. D. Replication and expression of plasmids from Staphylococcus aureus in Bacillus subtilis. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1680–1682. doi: 10.1073/pnas.74.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Contente S., Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann R., Gomez B. Amber mutants of bacteriophages T3 and T7 defective in phage-directed deoxyribonucleic acid synthesis. J Virol. 1967 Aug;1(4):779–792. doi: 10.1128/jvi.1.4.779-792.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Tomizawa J. I. Involvement of DNA gyrase in bacteriophage T7 DNA replication. Nature. 1977 Nov 3;270(5632):78–80. doi: 10.1038/270078a0. [DOI] [PubMed] [Google Scholar]
- Löfdahl S., Sjöström J. E., Philipson L. Characterization of small plasmids from Staphylococcus aureus. Gene. 1978 Apr;3(2):145–159. [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- McAllister W. T., Barrett C. L. Hybridization mapping of restriction fragments from the early region of bacteriophage T7 DNA. Virology. 1977 Oct 15;82(2):275–287. doi: 10.1016/0042-6822(77)90003-4. [DOI] [PubMed] [Google Scholar]
- McConnell D. J. Control sites in the sequence at the beginning of T7 gene 1. Nucleic Acids Res. 1979 Aug 10;6(11):3491–3503. doi: 10.1093/nar/6.11.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell D. J. The DNA sequence at the T7 C promoter. Nucleic Acids Res. 1979 Feb;6(2):525–544. doi: 10.1093/nar/6.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Minkley E. G., Pribnow D. Transcription of the early region of bacteriophage T7: selective initiation with dinucleotides. J Mol Biol. 1973 Jun 25;77(2):255–277. doi: 10.1016/0022-2836(73)90335-5. [DOI] [PubMed] [Google Scholar]
- Mottes M., Grandi G., Sgaramella V., Canosi U., Morelli G., Trautner T. A. Different specific activities of the monomeric and oligomeric forms of plasmid DNA in transformation of B. subtilis and E. coli. Mol Gen Genet. 1979 Jul 24;174(3):281–286. doi: 10.1007/BF00267800. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Murphy E., Gryczan T. J., Baron E., Edelman I. Penicillinase plasmids of Staphylococcus aureus: restriction-deletion maps. Plasmid. 1979 Jan;2(1):109–129. doi: 10.1016/0147-619x(79)90010-6. [DOI] [PubMed] [Google Scholar]
- Novick R. Plasmid-protein relaxation complexes in Staphylococcus aureus. J Bacteriol. 1976 Sep;127(3):1177–1187. doi: 10.1128/jb.127.3.1177-1187.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley J. L., Strothkamp R. E., Sarris A. H., Coleman J. E. T7 RNA polymerase: promoter structure and polymerase binding. Biochemistry. 1979 Feb 6;18(3):528–537. doi: 10.1021/bi00570a023. [DOI] [PubMed] [Google Scholar]
- Panayotatos N., Wells R. D. Cloning and localization of the in vitro functional origin of replication of bacteriophage T7 DNA. J Biol Chem. 1979 Jun 25;254(12):5555–5561. [PubMed] [Google Scholar]
- Panayotatos N., Wells R. D. Identification, cloning and characterization of three late promoters at 14.6, 14.8 and 15.9% of T7 DNA. J Mol Biol. 1979 Nov 25;135(1):91–109. doi: 10.1016/0022-2836(79)90342-5. [DOI] [PubMed] [Google Scholar]
- Panayotatos N., Wells R. D. Recognition and initiation site for four late promoters of phage T7 is a 22-base pair DNA sequence. Nature. 1979 Jul 5;280(5717):35–39. doi: 10.1038/280035a0. [DOI] [PubMed] [Google Scholar]
- Ponta H., Rahmsdorf H. J., Pai S. H., Hirsch-Kauffmann M., Herrlich P., Schweiger M. Control of gene expression in bacteriophage T7: transcriptional controls. Mol Gen Genet. 1974;134(4):281–297. doi: 10.1007/BF00337463. [DOI] [PubMed] [Google Scholar]
- Rosa M. D. Four T7 RNA polymerase promoters contain an identical 23 bp sequence. Cell. 1979 Apr;16(4):815–825. doi: 10.1016/0092-8674(79)90097-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Kramer R. A., Steitz J. A. T7 early messenger RNAs are the direct products of ribonuclease III cleavage. J Mol Biol. 1974 Nov 15;89(4):777–782. doi: 10.1016/0022-2836(74)90052-7. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Jan;3(1):101–116. doi: 10.1093/nar/3.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzinger E., Seiffert D. Studies on bacteriophage T7 DNA synthesis in vitro. I. Resolution of the T7 replication system into its components. Mol Gen Genet. 1975 Dec 1;141(3):213–232. doi: 10.1007/BF00341801. [DOI] [PubMed] [Google Scholar]
- Siebenlist U. Nucleotide sequence of the three major early promoters of bacteriophage T7. Nucleic Acids Res. 1979;6(5):1895–1907. doi: 10.1093/nar/6.5.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. N., Studier F. W. Physical mapping of the early region of bacteriophage T7 DNA. J Mol Biol. 1973 Sep 15;79(2):249–265. doi: 10.1016/0022-2836(73)90004-1. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Relationships among different strains of T7 and among T7-related bacteriophages. Virology. 1979 May;95(1):70–84. doi: 10.1016/0042-6822(79)90402-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensink P. C., Finnegan D. J., Donelson J. E., Hogness D. S. A system for mapping DNA sequences in the chromosomes of Drosophila melanogaster. Cell. 1974 Dec;3(4):315–325. doi: 10.1016/0092-8674(74)90045-2. [DOI] [PubMed] [Google Scholar]
- Wiggs J. L., Bush J. W., Chamberlin M. J. Utilization of promoter and terminator sites on bacteriophage T7 DNA by RNA polymerases from a variety of bacterial orders. Cell. 1979 Jan;16(1):97–109. doi: 10.1016/0092-8674(79)90191-0. [DOI] [PubMed] [Google Scholar]
- Yang H. L., Heller K., Gellert M., Zubay G. Differential sensitivity of gene expression in vitro to inhibitors of DNA gyrase. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3304–3308. doi: 10.1073/pnas.76.7.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]