Abstract
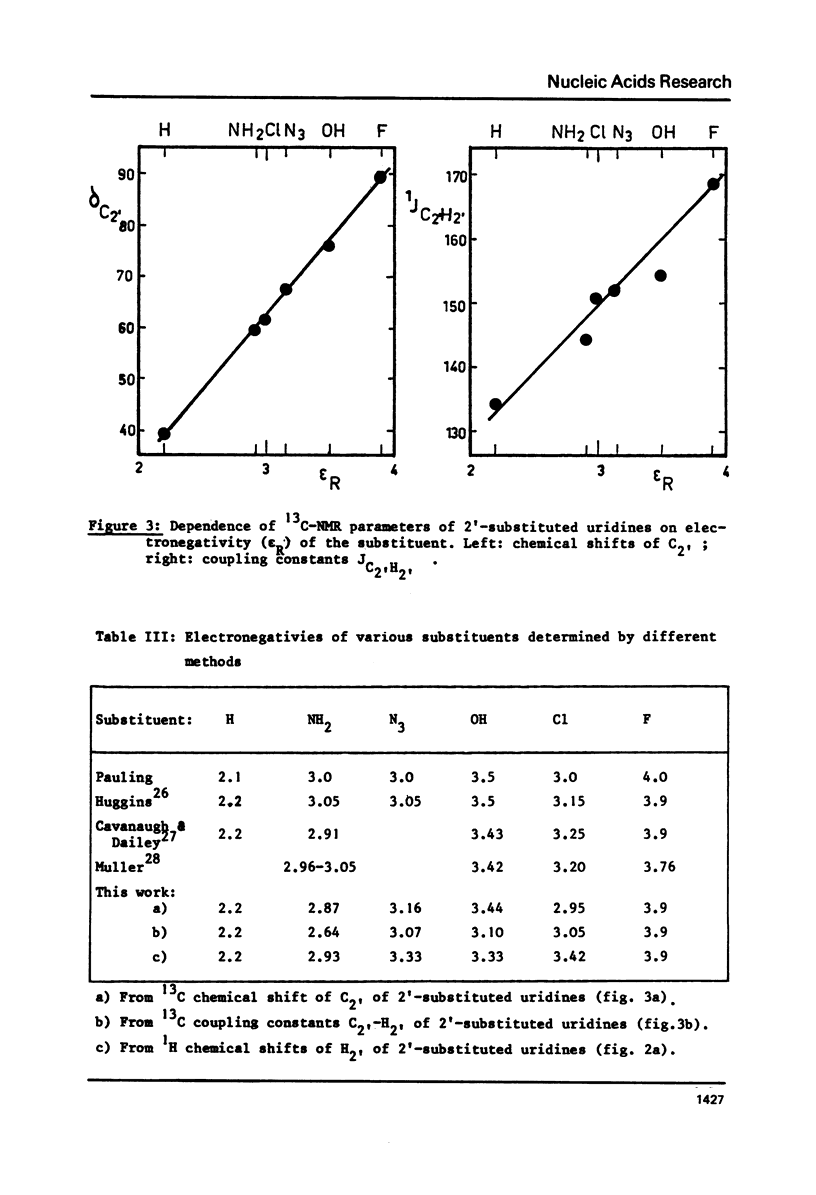
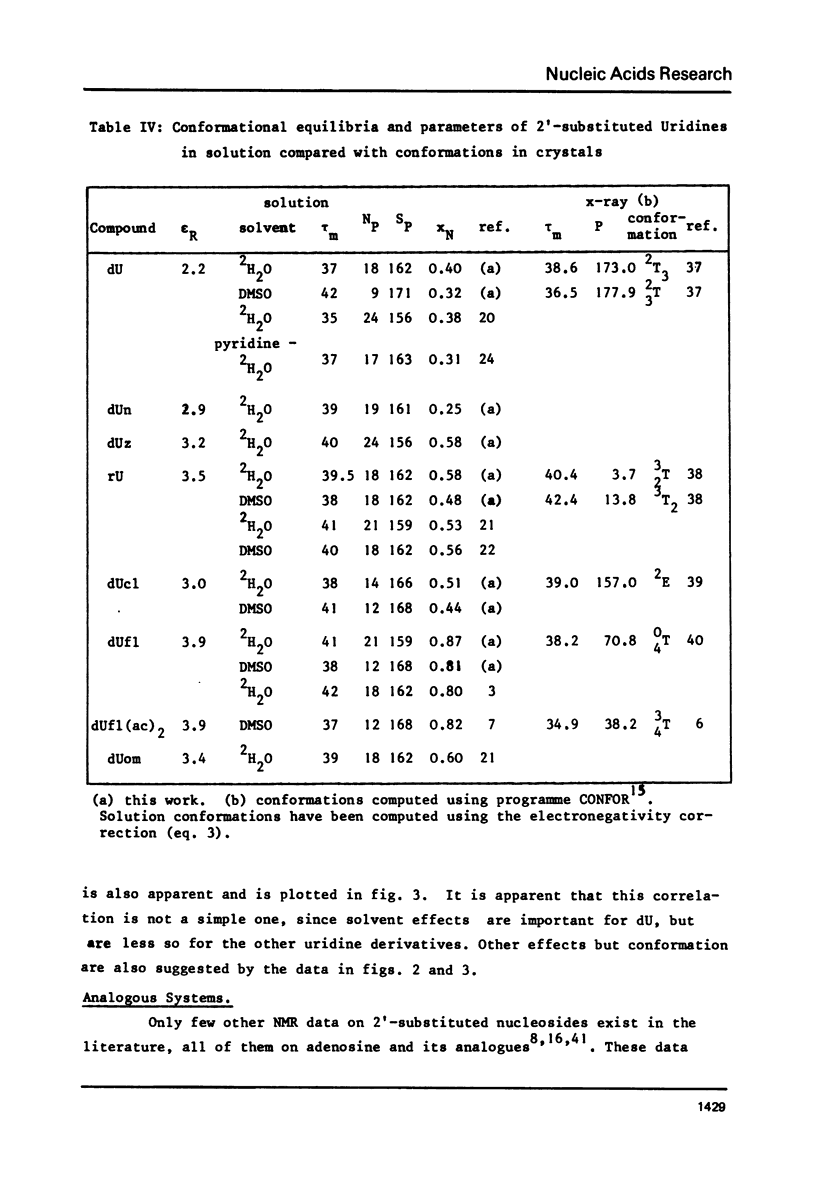
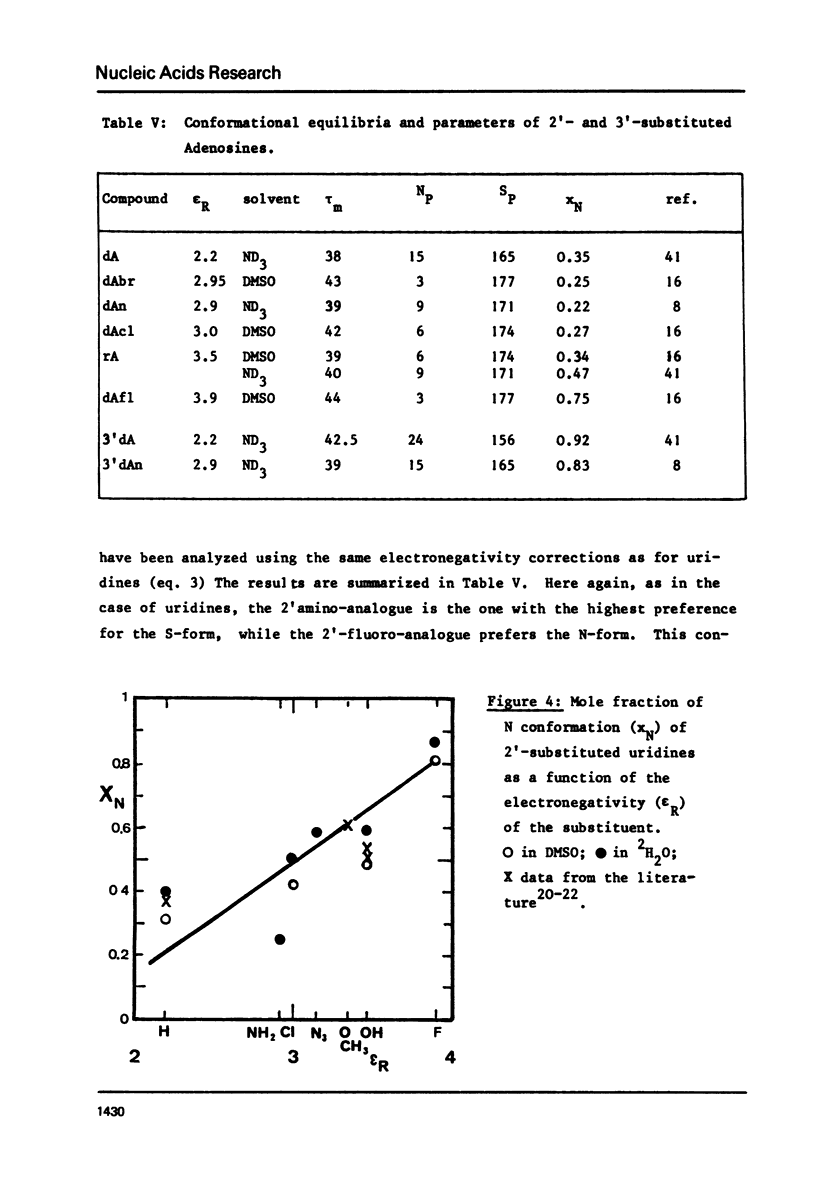
The proton and 13C NMR spectra of uridine, deoxyuridine and four 2' substituted uridines (dUn, dUz, dUcl and dUfl) are reported. A linear relationship between the electronegativity of the 2'-substituent and the carbon-13 chemical shift of C2' is observed. Taking into account the effect of electronegativity by using the correction proposed by Karplus or by Jankowski, the proton-proton coupling constants have been used to compute the conformational equilibria of the six uridines. It is shown that the contribution of the N form (3'-endo -2'-exo) increases with the electronegativity of the 2' substituent. Thus dUfl contains some 85% N form in solution. - Applying similar corrections to published data in the adenosine series, a similar correlation is observed. This observation, that the most polar substituent pulls the pucker to its side, holds also for 3'-substituted compounds, like cordycepin (3'dAdo) and 3'-deoxy-3'-amino-adenosine. It is suggested that the influence of the electronegativity could be the dominating effect of nucleoside conformations and would also hold for arabinosides and xylosides. This effect should therefore also be the principal force which determines the differences between DNA and RNA.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altona C., Sundaralingam M. Conformational analysis of the sugar ring in nucleosides and nucleotides. A new description using the concept of pseudorotation. J Am Chem Soc. 1972 Nov 15;94(23):8205–8212. doi: 10.1021/ja00778a043. [DOI] [PubMed] [Google Scholar]
- Altona C., Sundaralingam M. Conformational analysis of the sugar ring in nucleosides and nucleotides. Improved method for the interpretation of proton magnetic resonance coupling constants. J Am Chem Soc. 1973 Apr 4;95(7):2333–2344. doi: 10.1021/ja00788a038. [DOI] [PubMed] [Google Scholar]
- Blandin M., Tran-Dinh-Son, Catlin J. C., Guschlbauer W. Nucleoside conformations. 16. Nuclear magnetic resonance and circular dichroism studies on pyrimidine-2'-fluoro-2'-deoxyribonucleosides. Biochim Biophys Acta. 1974 Sep 13;361(3):249–256. [PubMed] [Google Scholar]
- Bolton P. H., Kearns D. R. Hydrogen bonding of the 2' OH in RNA. Biochim Biophys Acta. 1978 Feb 16;517(2):329–337. doi: 10.1016/0005-2787(78)90199-5. [DOI] [PubMed] [Google Scholar]
- Catlin J. C., Guschlbauer W. Oligonucleotide conformations. III. Comparative optical and thermodynamic studies of uridylyl-3'-5'-nucleosides containing ribose, deoxyribose, or 2'-deoxy-2'-fluororibose in the uridine moiety. Biopolymers. 1975 Jan;14(1):51–71. doi: 10.1002/bip.1975.360140105. [DOI] [PubMed] [Google Scholar]
- Greenberg S., Moffatt J. G. Reactions of 2-acyloxyisobutyryl halides with nucleosides. I. Reactions of model diols and of uridine. J Am Chem Soc. 1973 Jun 13;95(12):4016–4025. doi: 10.1021/ja00793a031. [DOI] [PubMed] [Google Scholar]
- Guschlbauer W., Blandin M., Drocourt J. L., Thang M. N. Poly-2'-deoxy-2'-fluoro-cytidylic acid: enzymatic synthesis, spectroscopic characterization and interaction with poly-inosinic acid. Nucleic Acids Res. 1977 Jun;4(6):1933–1943. doi: 10.1093/nar/4.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs J., Sternbach H., Sprinzl M., Eckstein F. Polynucleotides containing 2'-chloro-2'-deoxyribose. Biochemistry. 1972 Nov 7;11(23):4336–4344. doi: 10.1021/bi00773a021. [DOI] [PubMed] [Google Scholar]
- Ikehara M., Fukui T., Kakiuchi N. Polynucleotides. LII. Synthesis and properties of poly(2'-deoxy-2'-fluoroadenylic acid). Nucleic Acids Res. 1978 Jun;5(6):1877–1887. doi: 10.1093/nar/5.6.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janik B., Kotick M. P., Kreiser T. H., Reverman L. F., Sommer R. G., Wilson D. P. Synthesis and properties of poly 2'-fluoro-2'-deoxyuridylic acid. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1153–1160. doi: 10.1016/s0006-291x(72)80095-0. [DOI] [PubMed] [Google Scholar]
- Jones A. J., Grant D. M., Winkley M. W., Robins R. K. Carbon-13 magnetic resonance. XVII. Pyrimidine and purine nucleosides. J Am Chem Soc. 1970 Jul 1;92(13):4079–4087. doi: 10.1021/ja00716a042. [DOI] [PubMed] [Google Scholar]
- Plach H., Westhof E., Lüdemann H. D., Mengel R. Solution conformational analysis of 2'-amino-2''deoxyadenosine, 3'-amino-3'-deoxyadenosine and puromycin by pulsed nuclear-magnetic-resonance methods. Eur J Biochem. 1977 Oct 17;80(1):295–304. doi: 10.1111/j.1432-1033.1977.tb11882.x. [DOI] [PubMed] [Google Scholar]
- Schleich T., Blackburn B. J., Lapper R. D., Smith I. C. A nuclear magnetic resonance study of the influence of aqueous sodium perchlorate and temperature on the solution conformations of uracil nucleosides and nucleotides. Biochemistry. 1972 Jan 18;11(2):137–145. doi: 10.1021/bi00752a001. [DOI] [PubMed] [Google Scholar]
- Suck D., Saenger W., Hobbs J. Molecular and crystal structure of 2'-chloro-2'-deoxyuridine. Biochim Biophys Acta. 1972 Jan 31;259(2):157–163. doi: 10.1016/0005-2787(72)90055-x. [DOI] [PubMed] [Google Scholar]
- Suck D., Saenger W., Main P., Germain G., Declercq J. P. X-ray structure of 3',5'-diacetyl-2'-deoxy-2'-fluorouridine: a pyrimidine nucleoside in the syn conformation. Biochim Biophys Acta. 1974 Sep 13;361(3):257–265. doi: 10.1016/0005-2787(74)90369-4. [DOI] [PubMed] [Google Scholar]
- Verheyden J. P., Wagner D., Moffatt J. G. Synthesis of some pyrimidine 2'-amino-2'-deoxynucleosides. J Org Chem. 1971 Jan 29;36(2):250–254. doi: 10.1021/jo00801a002. [DOI] [PubMed] [Google Scholar]
- Westhof E., Plach H., Cuno I., Lüdemann H. D. Proton magnetic resonance studies of 2'-,3'-, and 5'-deoxyadenosine conformations in solution. Nucleic Acids Res. 1977 Apr;4(4):939–953. doi: 10.1093/nar/4.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmudzka B., Janion C., Shugar D. Poly 2'-O-methylcytidylic acid and the role of the 2'-hydroxyl in polynucleotide structure. Biochem Biophys Res Commun. 1969 Dec 4;37(6):895–901. doi: 10.1016/0006-291x(69)90215-0. [DOI] [PubMed] [Google Scholar]


