Abstract
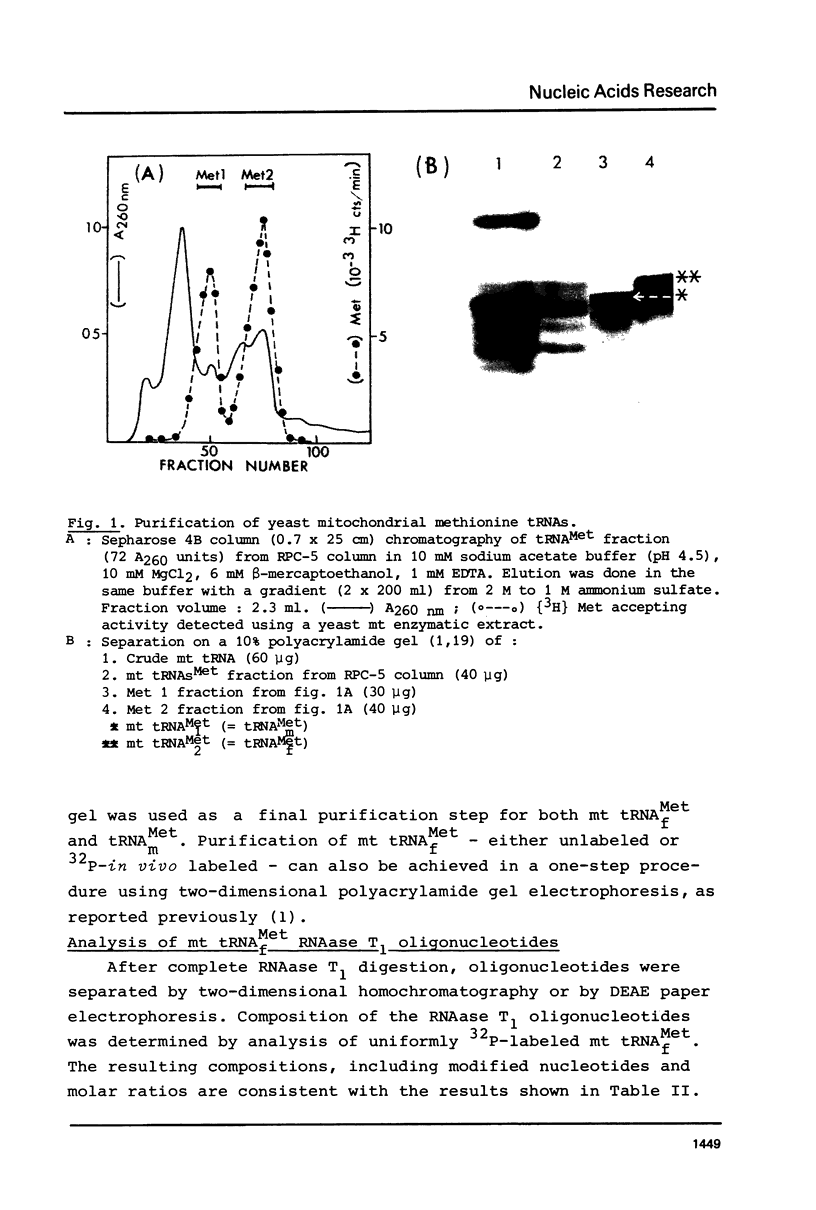
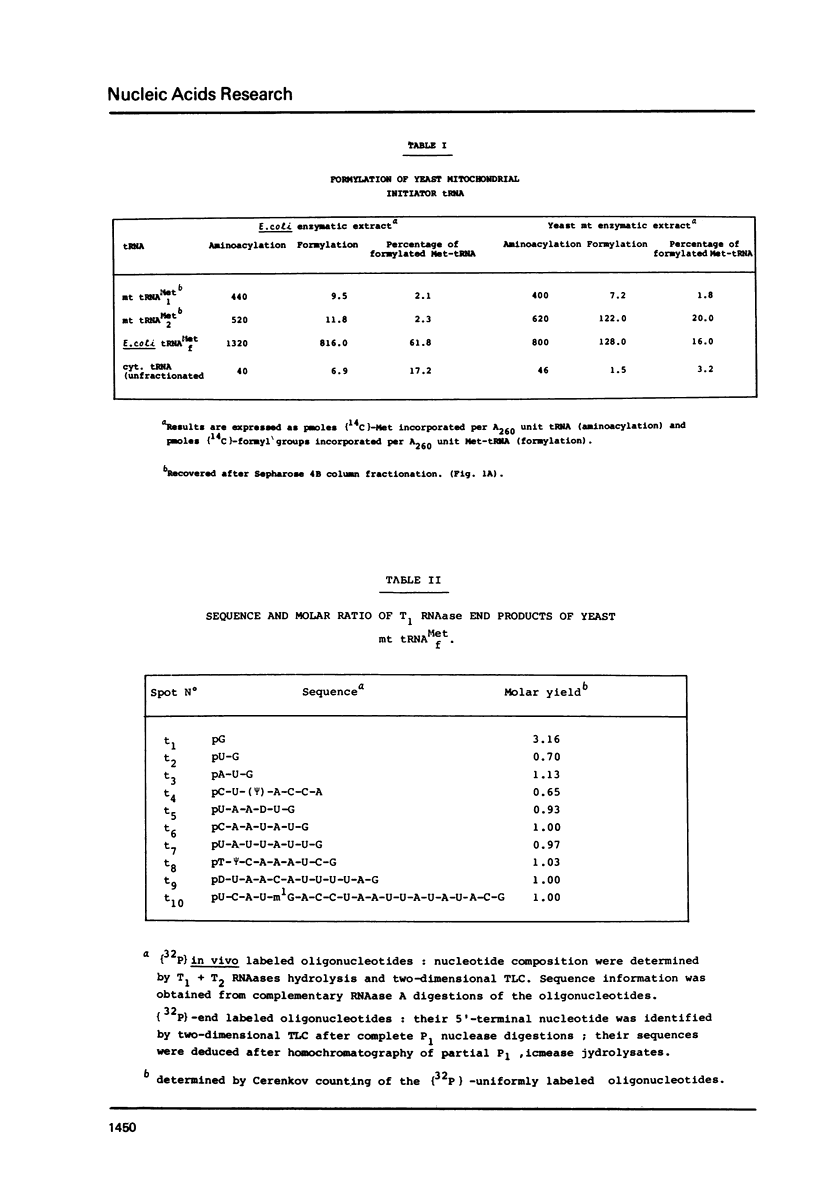
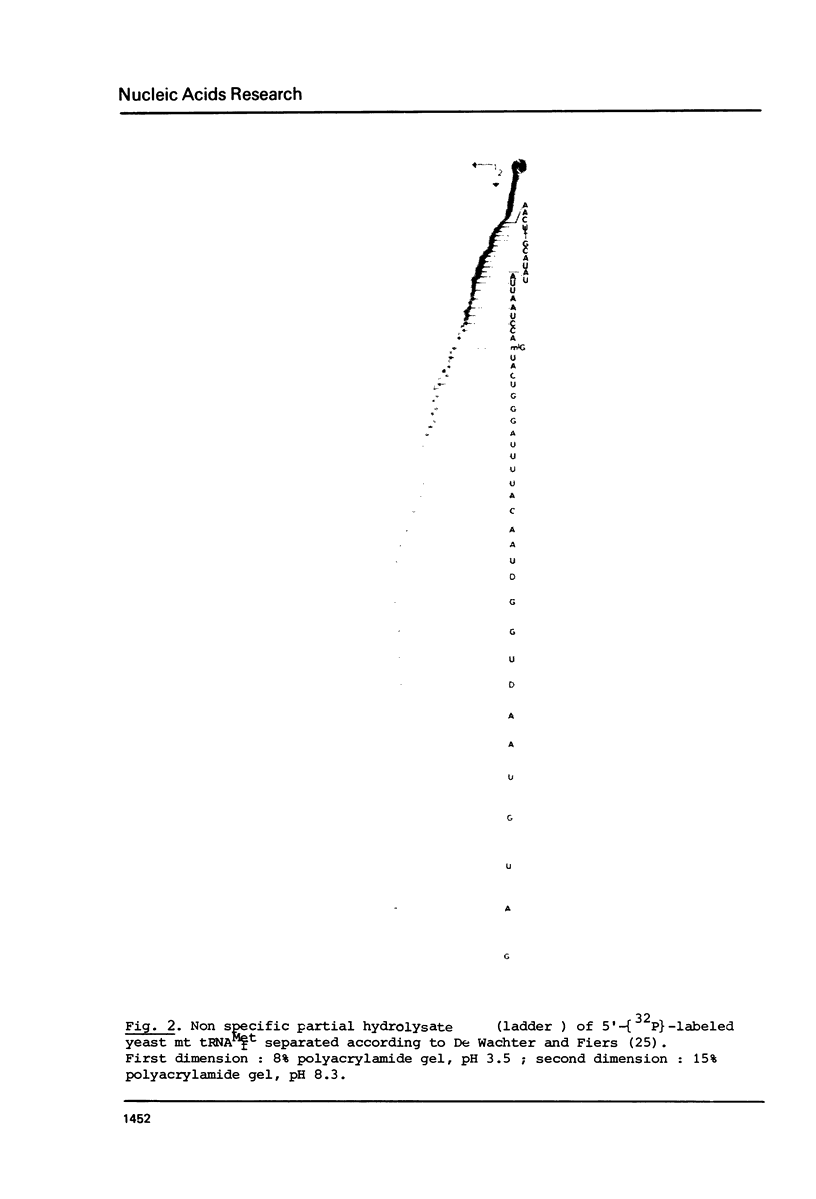
Two methionine tRNAs from yeast mitochondria have been purified. The mitochondrial initiator tRNA has been identified by formylation using a mitochondrial enzyme extract. E. coli transformylase however, does not formylate the yeast mitochondrial initiator tRNA. The sequence was determined using both 32P-in vivo labeled and 32P-end labeled mt tRNAf(Met). This tRNA, unlike N. crassa mitochondrial tRNAf(Met), has two structural features typical of procaryotic initiator tRNAs: (i) it lacks a Watson-Crick base-pair at the end of the acceptor stem and (ii) has a T-psi-C-A sequence in loop IV. However, both yeast and N. crassa mitochondrial initiator tRNAs have a U11:A24 base-pair in the D-stem unlike procaryotic initiator tRNAs which have A11:U24. Interestingly, both mitochondrial initiator tRNAs, as well as bean chloroplast tRNAf(Met), have only two G:C pairs next to the anticodon loop, unlike any other initiator tRNA whatever its origin. In terms of overall sequence homology, yeast mitochondrial tRNA(Met)f differs from both procaryotic or eucaryotic initiator tRNAs, showing the highest homology with N. crassa mitochondrial initiator tRNA.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrell B. G., Bankier A. T., Drouin J. A different genetic code in human mitochondria. Nature. 1979 Nov 8;282(5735):189–194. doi: 10.1038/282189a0. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Osinga K. A., Van der Horst G., Borst P. Nucleotide sequence of the mitochondrial structural genes for cysteine-tRNA and histidine-tRNA of yeast. Nucleic Acids Res. 1979 Jul 25;6(10):3255–3266. doi: 10.1093/nar/6.10.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetow D. E., Wood W. M. The mitochondrial translation system. Subcell Biochem. 1978;5:1–85. doi: 10.1007/978-1-4615-7942-7_1. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin A., Gruhl H., Feldmann H. Mapping of yeast tRNAs by two-dimensional electrophoresis on polyacrylamide gels. FEBS Lett. 1975 Feb 1;50(2):185–189. doi: 10.1016/0014-5793(75)80485-6. [DOI] [PubMed] [Google Scholar]
- Gillum A. M., Hecker L. I., Silberklang M., Schwartzbach S. D., RajBhandary U. L., Barnett W. E. Nucleotide sequence of Neurospora crassa cytoplasmic initiator tRNA. Nucleic Acids Res. 1977 Dec;4(12):4109–4131. doi: 10.1093/nar/4.12.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman J. E., Alzner-Deweerd B., RajBhandary U. L. Interesting and unusual features in the sequence of Neurospora crassa mitochondrial tyrosine transfer RNA. Proc Natl Acad Sci U S A. 1979 Feb;76(2):717–721. doi: 10.1073/pnas.76.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman J. E., Hecker L. I., Schwartzbach S. D., Barnett W. E., Baumstark B., RajBhandary U. L. Structure and function of initiator methionine tRNA from the mitochondria of Neurospora crassa. Cell. 1978 Jan;13(1):83–95. doi: 10.1016/0092-8674(78)90140-x. [DOI] [PubMed] [Google Scholar]
- Hensgens L. A., Grivell L. A., Borst P., Bos J. L. Nucleotide sequence of the mitochondrial structural gene for subunit 9 of yeast ATPase complex. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1663–1667. doi: 10.1073/pnas.76.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes W. M., Hurd R. E., Reid B. R., Rimerman R. A., Hatfield G. W. Separation of transfer ribonucleic acid by sepharose chromatography using reverse salt gradients. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1068–1071. doi: 10.1073/pnas.72.3.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Suddath F. L., Quigley G. J., McPherson A., Sussman J. L., Wang A. H., Seeman N. C., Rich A. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science. 1974 Aug 2;185(4149):435–440. doi: 10.1126/science.185.4149.435. [DOI] [PubMed] [Google Scholar]
- Leis J. P., Keller E. B. Protein chain initiation by methionyl-tRNA. Biochem Biophys Res Commun. 1970 Jul 27;40(2):416–421. doi: 10.1016/0006-291x(70)91025-9. [DOI] [PubMed] [Google Scholar]
- Martin N. C., rabinowitz M. Mitochondrial transfer RNAs in yeast: identification of isoaccepting transfer RNAs. Biochemistry. 1978 May 2;17(9):1628–1634. doi: 10.1021/bi00602a008. [DOI] [PubMed] [Google Scholar]
- Martin R. P., Schneller J. M., Stahl A. J., Dirheimer G. Import of nuclear deoxyribonucleic acid coded lysine-accepting transfer ribonucleic acid (anticodon C-U-U) into yeast mitochondria. Biochemistry. 1979 Oct 16;18(21):4600–4605. doi: 10.1021/bi00588a021. [DOI] [PubMed] [Google Scholar]
- Martin R. P., Schneller J. M., Stahl A. J., Dirheimer G. Study of yeast mitochondrial tRNAs by two-dimensional polyacrylamide gel electrophoresis: characterization of isoaccepting species and search for imported cytoplasmic tRNAs. Nucleic Acids Res. 1977 Oct;4(10):3497–3510. doi: 10.1093/nar/4.10.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. P., Sibler A. P., Schneller J. M., Keith G., Stahl A. J., Dirheimer G. Primary structure of yeast mitochondrial DNA-coded phenylalanine-tRNA. Nucleic Acids Res. 1978 Dec;5(12):4579–4592. doi: 10.1093/nar/5.12.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. L., Weiss J. F., Kelmers A. D. Improved separation of transfer RNA's on polychlorotrifuoroethylene-supported reversed-phase chromatography columns. Biochim Biophys Acta. 1971 Feb 11;228(3):770–774. doi: 10.1016/0005-2787(71)90748-9. [DOI] [PubMed] [Google Scholar]
- Samuel C. E., D'Ari L., Rabinowitz J. C. Evidence against the folate-mediated formylation of formyl-accepting methionyl transfer ribonucleic acid in Streptococcus faecalis R. J Biol Chem. 1970 Oct 10;245(19):5115–5121. [PubMed] [Google Scholar]
- Schneller J. M., Schneller C., Martin R., Stahl A. J. Nuclear origin of specific yeast mitochondrial aminoacyl-tRNA synthetases. Nucleic Acids Res. 1976 May;3(5):1151–1165. doi: 10.1093/nar/3.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman L. H., Pelka H. The structural basis for the resistance of Escherichia coli formylmethionyl transfer ribonucleic acid to cleavage by Escherichia coli peptidyl transfer ribonucleic acid hydrolase. J Biol Chem. 1975 Jan 25;250(2):542–547. [PubMed] [Google Scholar]
- Sibler A. P., Martin R. P., Dirheimer G. The nucleotide sequence of yeast mitochondrial histidine-tRNA. FEBS Lett. 1979 Nov 1;107(1):182–186. doi: 10.1016/0014-5793(79)80491-3. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. The use of nuclease P1 in sequence analysis of end group labeled RNA. Nucleic Acids Res. 1977 Dec;4(12):4091–4108. doi: 10.1093/nar/4.12.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Vassilenko S. A different approach to RNA sequencing. Nature. 1978 Jul 6;274(5666):87–89. doi: 10.1038/274087a0. [DOI] [PubMed] [Google Scholar]
- Sundari R. M., Stringer E. A., Schulman L. H., Maitra U. Interaction of bacterial initiation factor 2 with initiator tRNA. J Biol Chem. 1976 Jun 10;251(11):3338–3345. [PubMed] [Google Scholar]
- Wesolowski M., Fukuhara H. The genetic map of transfer RNA genes of yeast mitochondria: correction and extension. Mol Gen Genet. 1979 Mar 5;170(3):261–275. doi: 10.1007/BF00267059. [DOI] [PubMed] [Google Scholar]
- Wrede P., Woo N. H., Rich A. Initiator tRNAs have a unique anticodon loop conformation. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3289–3293. doi: 10.1073/pnas.76.7.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]





