Abstract
Chapter summary
Integrin receptors transduce bidirectional signals between extracellular adhesion molecules and intracellular cytoskeletal and signalling molecules. The structural basis of integrin signalling is unknown, but the recent publication of the first crystal structure of the extracellular domain of integrin αVβ3 has provided a number of insights. In this review, previous structure–function analyses of integrins that have employed biochemical and molecular biological approaches are placed in the context of the crystal structure, and novel routes to the development of integrin antagonists are discussed.
Keywords: adhesion, cations, extracellular matrix, integrin, structure
Introduction
The integrins are a family of αβ heterodimeric receptors that mediate dynamic linkages between extracellular adhesion molecules and the intracellular actin cytoskeleton. Integrins are expressed by all multicellular animals. In mammals, 18 α-subunit genes and eight β-subunit genes encode polypeptides that combine to form 24 different receptors. Both integrin subunits are noncovalently associated, type I transmembrane proteins with large extracellular domains and short cytoplasmic domains of 700–1100 and 30–50 residues, respectively.
Thousands of studies have investigated the molecular, cellular and organismal basis of integrin function. Gene deletion has demonstrated essential roles for almost all integrins, with the defects suggesting widespread contributions to the maintenance of tissue integrity and the promotion of cellular migration. Integrin–ligand interactions are now considered to provide physical support for cells to maintain cohesion, to permit the generation of traction forces to enable movement, and to organise signalling complexes to modulate differentiation and cell fate.
Animal model studies have also shown integrins to contribute to the progression of many common diseases, and have implicated them as potential therapeutic targets. The use of anti-integrin mAbs and ligand mimetic peptides has validated this suggestion for inflammatory, neoplastic, traumatic and infectious conditions. There is thus intense interest in determining the molecular basis of integrin function to identify approaches for regulating integrin function in disease. The recent publication of an integrin crystal structure promises to aid this process, most obviously by defining the ligand-binding pocket but also by suggesting mechanisms of receptor activation. These topics form the basis of this review.
An integrin crystal structure
The first three-dimensional structure of the extracellular domain of an integrin was published in October 2001, a decade and a half after the family was first defined [1]. The team responsible for this landmark study was led by Amin Arnaout (Massachusetts General Hospital, Boston, MA, USA), and comprised crystallographers at the Massachusetts General Hospital and the Argonne National Laboratory, IL, USA, and protein chemists at Merck KGaA in Darmstadt, Germany. The integrin selected for the work was αVβ3, a promiscuous receptor that binds vitronectin, fibronectin, von Willebrand factor and other extracellular matrix ligands. Both subunits of the heterodimer were expressed as full-length, soluble, glycosylated constructs in insect cells, and were crystallised in the presence of Ca2+.
The overall shape of the crystallised conformer (resolved to 3.1Å) is that of a large 'head' on two 'legs', with the N-termini of both subunits forming the head and the C-termini forming the legs (Fig. 1). Similar images of integrins had been obtained previously from rotary-shadowed and negatively stained specimens analysed by electron microscopy [2,3], and it had been correctly predicted that the legs would be the sites of subunit insertion into the plasma membrane. Furthermore, rotary-shadowed images of the platelet integrin αIIbβ3 bound to its major ligand fibrinogen revealed a highly specific interaction of the head of the integrin with the distal end of the fibrinogen hexamer, suggesting that the head contains the ligand-binding domain [4]. One major difference between the results from these two different structure-determination approaches, however, is the degree of extension of the legs. Both legs are bent in the crystal structure, whereas most electron microscopy images possess straightened legs. The relevance of these differences for receptor activation is discussed later.
Figure 1.
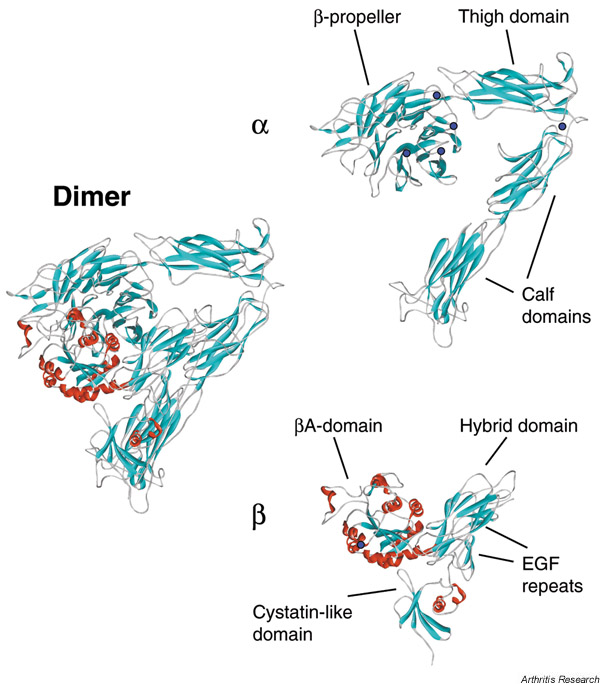
Crystal structure of integrin αVβ3 showing the dimer and individual subunits [1]. The domains that make up each integrin subunit are shown. Secondary structure elements are shown as red α-helices or cyan β-strands/ribbons. Blue circles represent the six cation-binding sites. The plexin–semaphorin–integrin domain and two of the four epidermal growth factor (EGF) repeats in the β-subunit are not visible in the structure.
In the crystal structure, the head of the integrin contains a seven-bladed β-propeller structure from the α-subunit (comprising seven ~60-amino-acid N-terminal repeats) and a von Willebrand factor A-domain from the β-subunit (termed the βA-domain; Fig. 1). The presence of these two folds had been predicted previously [5,6]. The βA-domain is anchored to the upper face of the β-propeller, with an arginine residue in a 310 helical segment of the βA-domain (between βD and α5) linked to a hydrophobic 'cage' in the central shaft of the β-propeller. The remainder of the head composes an immunoglobulin module into which the βA-domain is inserted.
The α-subunit leg of the integrin contains three large β-sandwich domains. Between the so-called 'thigh' domain and the first of two 'calf' domains is a highly flexible 'knee' (or 'genu'), which is the site of the bend in the crystal structure. The more C-terminal domain of the calf domains contains a site that is cleaved post-translationally to yield an N-terminal heavy chain and a C-terminal light chain, although the atomic detail is not visible in the structure.
The β-subunit leg contains a plexin–semaphorin–integrin (PSI) domain, four epidermal growth factor-like repeats and a novel cystatin-like fold. The 50-residue PSI domain was also not visible in the structure, but it has been predicted to possess α-helical character [7]. The β-subunit knee region, formed from the conjunction of the hybrid domain, two epidermal growth factor repeats, and the PSI domain, is also bent.
It has previously been shown that truncated αIIb constructs that ended before N-terminal repeats 5, 6 or 7 all associated with their partner subunit β3 [8], and that limited proteolysis of αIIbβ3 bound to a ligand affinity matrix produced a 55/85 kDa heterodimer containing the N-termini of both subunits that reacted with dimer-specific antibodies [9]. The suggestion from these studies that the head region of the integrin contains the major sites of intersubunit association and dimerisation was therefore confirmed by the crystal structure.
Similarly, the spatial relationship between different subdomains of integrin subunits had been partially determined by protein chemistry. For example, cyanogen bromide cleavage of αIIbβ3, followed by amino acid and N-terminal sequence analysis of the isolated fragments, permitted localisation of all S–S bonds, including a long-range bond joining the N-terminus to the region just after the hybrid domain (C5–C435) [10,11]. Although this bond is not visible in the crystal structure, it is clear that the PSI domain is positioned close enough to the junction between the hybrid domain and the first epidermal growth factor repeat to form the link. The close association of the N-terminal regions of both integrin subunits explains their mutual dependence for folding as assessed using conformation-dependent mAbs as probes [12,13]. mAbs that recognise only the α-subunit β-propeller or the βA-domain recognise heterodimeric integrin, while mAbs that are directed against the αA-domain or the legs of either subunit react with their respective monomer.
Integrins actually fall into two subfamilies based on the presence or absence of a 200-amino-acid module in the α-subunit. This module, which is present in nine α-subunits, shares sequence homology with a von Willebrand factor A-domain, and is inserted between the second and third N-terminal repeats. Although αV lacks an A-domain, the crystal structure does suggest a potential location for the inserted αA-domain, at the side and the top of the β-propeller (see Fig. 3 later).
Figure 3.
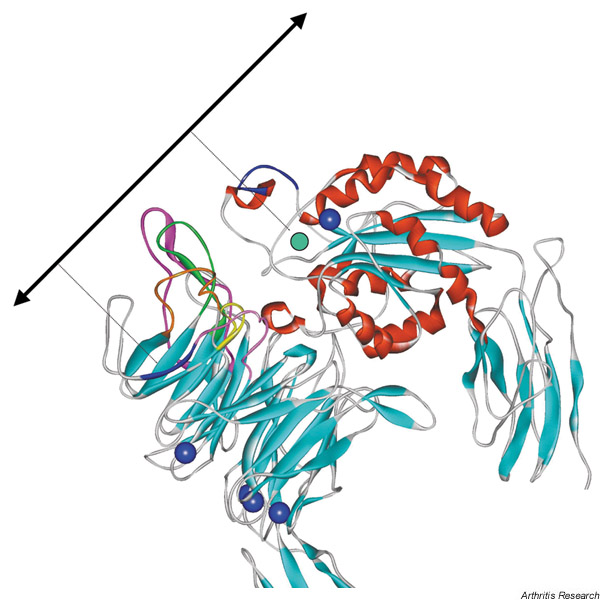
Enlarged view of the potential ligand-binding pocket of integrin αVβ3. The loops on the top of the α-subunit β-propeller implicated in ligand binding are coloured: purple, the 4–1 loop connecting repeats 1–2; orange, the 4–1 loop connecting repeats 2–3; pink, the 4–-1 loop connecting repeats 3–4; green, the 2–3 loop in repeat 2; yellow, the 2–3 loop in repeat 3. The potential site for binding the fibronectin synergy sequence in α5β1, the β-strand 4 in repeat 3, is coloured blue (left side of β-propeller). The CYDMKTTC peptide sequence determining ligand specificity in β3 is coloured blue (top of βA-domain). Cations in the αVβ3 crystal structure are shown as blue spheres. The potential site of the metal ion-dependent adhesion site (MIDAS) cation is shown as a green circle. The site of insertion of an αA-domain would be in the orange loop of the β-propeller. The solid double arrow shows the possible orientation of ligand relative to the integrin, with dashed lines indicating speculative contacts with the MIDAS cation and β-strand 4 in repeat 3.
Prior to the αVβ3 structure, the only region of an integrin for which tertiary structure information was available was the αA-domain, as these domains fold independently and can be expressed in recombinant form. Crystal structures from four α-subunits (α1, α2, αL and αM) have been solved, the first being the αM A-domain [14]. The protein was found to adopt a classical αβ Rossmann fold in which the core of the module was made up of five parallel β-strands and one antiparallel β-strand, decorated peripherally by a series of seven α-helices (Fig. 2). A Mg2+ ion was located at one end of the module, where it was coordinated in an octahedral geometry by residues from three different loops and from a glutamate side chain from another A-domain molecule adjacent in the crystal lattice. This latter interaction was suggested to mimic a ligand–receptor complex [14]. Further crystal forms of the αM and αL A-domains were then reported in which water completed the metal coordination sphere and there was no equivalent of the glutamate ligand [15]. This raised doubts about the relevance of the cation-dependent difference in the A-domain conformation, as discussed later.
Figure 2.
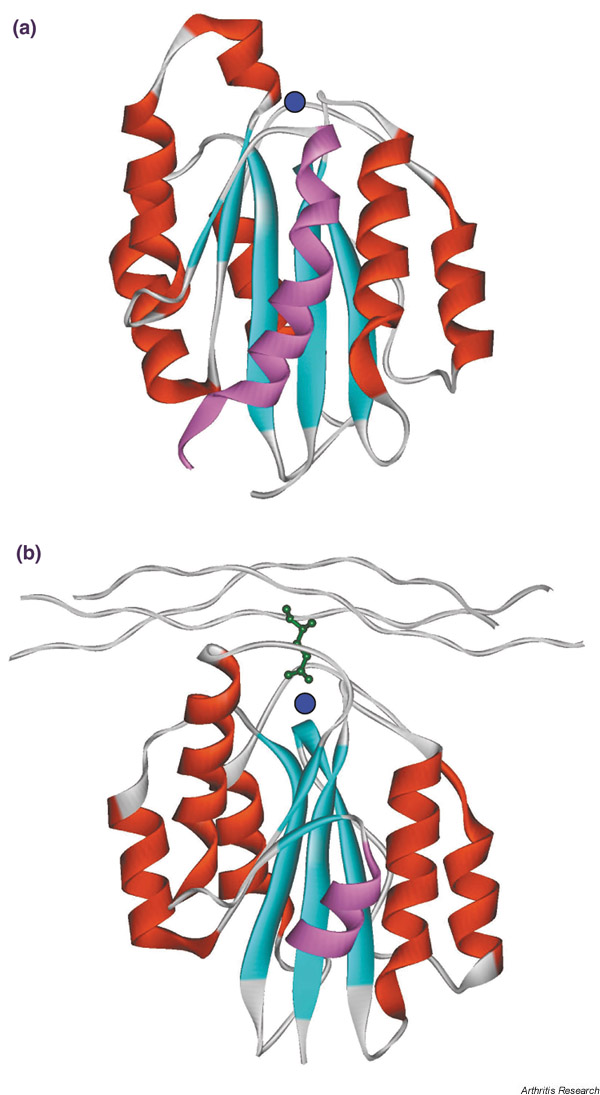
Comparison of the crystal structure of the α2 A-domain either (a) free or (b) complexed with a collagenous peptide [41,47]. Secondary structure elements are shown as red α-helices or cyan β-strands/ribbons. Spheres represent the divalent cation coordinated by the metal ion-dependent adhesion site (MIDAS) motif. The α7 helix is shown in pink. Note the difference in position of α7 in the two structures and the fact that the construct used in (b) contained a truncated α7 helix. The collagen glutamate residue that coordinates the MIDAS cation is shown in green. The MIDAS cation is shown as a blue circle.
The remainder of this review addresses two key questions posed by the integrin structure: how do ligands bind to the two subfamilies of integrin receptors, and how is receptor activation achieved in both types of integrin?
Ligand-binding sites
The definition of the ligand-binding pocket of an integrin is important because it will generate insights into the relative contributions of different regions of receptor and ligand to the specific binding event. In addition, it will inform the process of drug design by identifying the receptor residues that participate in contacts with ligand. Ultimately, the solution of the tertiary structure of an integrin–ligand complex is needed to provide this information but, in the current absence of such structures, data from a number of experimental approaches have suggested the sites within integrins that bind ligand. This information can now be mapped onto the αVβ3 structure.
Non-αA-domain-containing integrins
Integrin chimeras
Inter-integrin chimeras have been employed to pinpoint sites determining ligand specificity. Subtle differences in the ligand-binding specificity of the related α5- and αV-subunits were recently exploited to pinpoint the regions of the α-subunits responsible, using a gain-of-function approach. The α5 subunit preferentially recognises the so-called 'synergy' site in type III repeat 9 of its ligand fibronectin, binds strongly to RGD peptides containing a C-terminal tryptophan residue (e.g. RGDGW), and binds specifically to the peptide RRETAWA. The high sequence identity (~50%) between α5 and αV permitted the construction of chimeras bearing hybrid native structures. It was reported that repeats 2 and 3 of α5, when introduced into αV, were sufficient to endow αV with the epitopes of all function-blocking anti-α5 mAbs and with the ligand-binding specificity of α5 [16].
The exchange of putative loops between the two subunits, based on the β-propeller prediction that turned out to be correct, led to the identification of a single residue, α5 W157, which was sufficient to convert αV into a receptor that strongly recognised RGDGW and RRETAWA [17]. This finding suggests that W157 (located in the loop connecting repeats 2 and 3) is close to the RGD-binding site on β1. Additional studies that measured the loss of ligand-binding activity in chimeras, while not as convincing, produced similar conclusions.
Similar analyses of the integrin β-subunit have highlighted the importance of the βA-domain. For example, replacement of β1 C187TSEQNC with β3 CYDMKTTC converted the ligand specificity of αVβ1 to be more similar to αVβ3 (i.e. increased binding of fibrinogen, von Willebrand factor and vitronectin) [18]. This sequence is located in a large disulfide-bonded loop between βB and βC adjacent to the cation-binding site in the βA-domain (Fig. 3).
Cross-linking
The discovery that many integrin ligands employ short, acidic peptide motifs (such as RGD and LDV) as key receptor-binding sequences [19] led to the use of chemical cross-linking as a means of pinpointing ligand-binding sites. In the earliest studies, RGD was found to cross-link primarily to the β3 A-domain residues 109–171 or residues 61–203 [20,21], and subsequently a 1:1 stoichiometric β3 (residues 119–131)–RGD complex was detected by mass spectroscopy [22].
Synthetic peptides mimicking putative ligand-binding sites in integrins have been synthesised and tested for their ability to bind ligand directly and to inhibit ligand binding to native integrin. The most definitive study identified two overlapping peptides encompassing residues 204–229 of the β3 A-domain that blocked the binding of fibrinogen to purified αIIbβ3 [23]. The minimal active peptide was subsequently determined to be RNRDA in the α2–α3 loop [24].
Cross-linking has been performed with more potent peptidic ligands, the higher affinity of which might be expected to improve specificity. An LDV-based small molecule inhibitor of α4β1 (BIO-1494) that contained a single reactive amino group was cross-linked to purified or cell-expressed α4β1 [25]. The site of cross-linking was localised by CNBr peptide mapping to β1 (residues 130–146), a region that contains the putative metal binding site in the βA-domain. Similarly, tagged photo-reactive cyclic RGD-containing ligands for αIIbβ3 cross-linked specifically to β3 in a cation-dependent manner [26]. Enzymatic and chemical digestions of the radiolabelled conjugate identified β3 (residues 99–118) as the RGD contact site. The similarity between the data generated for LDV and RGD ligands supports the notion of a common ligand-binding pocket for both motifs.
Mutagenesis and mAb epitope mapping
A key assumption underlying the use of epitope mapping to identify ligand-binding sites was that function-blocking mAbs act as competitive inhibitors. This now appears not to be the case, with many anti-integrin mAbs having been shown to function via allosteric mechanisms. The first indication of allosteric inhibition by an anti-integrin mAb came from studies of the binding of a fibronectin fragment and GRGDS peptide to α5β1 [27]. Ligand binding caused a dramatic attenuation of the mAb 13 epitope, and the antibody preferentially recognised the unoccupied conformation of the integrin. This suggested that the antibody inhibits ligand binding either by stabilising the unoccupied state of the receptor or by preventing a conformational change necessary for ligand occupancy. Similar results have subsequently been obtained for many other anti-integrin mAbs.
If anti-functional mAbs recognise integrin sites whose structures are perturbed as a consequence of ligand engagement, then specific sites within the ligand must be responsible for triggering the conformational changes. Using a series of recombinant fragments of fibronectin containing mutations in either the RGD or synergy active sites, the topology of ligand engagement to α5β1 was determined [28]. RGD preferentially perturbed anti-β1 mAb recognition of the integrin, while fragments mutated in the synergy site were unable to block binding of an anti-α5 mAb (P1D6) that mapped to L212 of β1 (β-strand 4 of repeat 3; Fig. 3). Further analysis of the links between ligand binding and mAb binding, in conjunction with the αVβ3 crystal structure, should allow the ligand to be positioned relative to the integrin.
Following the identification of regions within the α-subunit β-propeller and the β-subunit A-domain as putative regions for ligand binding, site-directed mutagenesis has been employed as a method to pinpoint residues contacting the ligand (see [29] for a review). In the α-subunit β-propeller, ligand binding is perturbed by mutations in loops that are predicted to lie on the 'top' of the domain, on the opposite face to the EF-hand-like sequences. In particular, the intra-repeat 2–3 loop of repeat 3 and the inter-repeat 4–1 loops joining repeats 1–2, 2–3 and 3–4 contain the key residues in all integrins tested to date (Fig. 3).
Similar results have come from mAb epitope mapping, where again the 4–1 loops joining repeats 1–2, 2–3 and 3–4 tend to contain key residues. In the integrin β-subunits, virtually all inhibitory mAbs map to the βA-domain, and mutation of cation-coordinating residues in the βA-domain abolishes ligand binding in all integrins tested.
Alteration of residues within the α3–α4 loop and α4 helix (which are located on the same face as the divalent cation) also appear to be important for several integrins. In a recent study mapping fibrinogen binding sites in αIIbβ3, most of the critical residues were located at the edge of the upper face of the propeller, and several critical residues are located on the side of the propeller domain, in a region corresponding to the anti-α5 mAb P1D6 epitope [30].
Taken together, these data support a model in which the RGD or LDV ligand motif interacts with the cation-binding site in the βA-domain and additional contacts are made with the side of the α-subunit β-propeller (Fig. 3). The critical requirement for a carboxyl group in RGD or LDV has led to the suggestion that it participates in a direct coordination with the integrin-bound cation. Clearly, a ligand-integrin co-crystal is needed to determine the validity of this prediction.
αA-domain-containing integrins
In contrast to the aforementioned situation with non-αA-domain-containing integrins, the major ligand-binding site within those integrins that contain an αA-domain is clearly found within the 200-amino-acid polypeptide module that is inserted within the α-subunit β-propeller. A variety of evidence supports this conclusion, including the mapping of antifunctional mAbs to the αA-domain, the ability of the isolated domain to bind ligand, the inhibitory effects of mutations within the αA-domain on ligand binding, and, most recently, the resolution of the tertiary structure of an αA-domain–ligand complex.
Recombinant αA-domains
In solid-phase assays, the αM A-domain was found to bind to its ligands iC3b, intercellular adhesion molecule (ICAM)-1, ICAM-2, and fibrinogen in a divalent cation-dependent manner [31,32]. The αL A-domain similarly bound directly to purified recombinant ICAM-1 and also inhibited αLβ2-dependent T-cell adhesion to ICAM-1 [33]. The A-domains from α1 and α2 were found to bind to a variety of collagen isotypes, including types I and IV, and laminin in a cation-dependent manner [34-36]. Finally, the αD A-domain has been shown to contain a binding site for vascular cell adhesion molecule-1 [37].
αA-domain mutagenesis and chimeras
Inter-integrin chimeras have proven useful to map ligand-binding sites within αA-domains. Since mouse αLβ2 does not bind human ICAM-1, interspecies chimeras were constructed to identify the ligand-binding regions [38]. Replacement of two noncontiguous regions in the A-domain (residues 119–153 and 218–248) abolished binding. Key residues were found to be M140, E146, T243, and S245, which are located around the cation-binding site. In complementary studies, data from a large number of site-directed mutagenesis experiments, the results of which are summarised in [29], have suggested residues that are critical for interaction of αA-domains with ligands. In addition to cation-coordinating residues, which are essential for the function of all αA-domains, sites within the α3–α4 and βD–α5 loops are frequently implicated. Both loops are located on the top, cation-binding face of the αA-domain.
mAb epitope mapping
Recombinant A-domains of α1, α2, αL, αM, αX and αD have now been shown to contain the epitopes for antifunctional anti-α-subunit mAbs, and interspecies and inter-integrin chimeras have been used to localise antifunctional mAb epitopes to αA-domains. For example, three distinct epitopes within residues 126–150 of the αL A-domain, which is a region close to the cation-binding site, were identified using human–mouse point chimeras [39]. Also, the epitope of anti-α1 mAb AJH10 was localised to the loop between the α3 and α4 helices, which again contributes one of the metal coordination sites of the A-domain [40].
Integrin-ligand co-crystal
Many of these predictions were confirmed in an important study where the crystal structure of a complex between the α2 A-domain and a triple-helical collagen peptide containing a critical GFOGER motif was determined [41]. Three loops on the upper surface of the α2 A-domain that coordinate the cation were found to engage the collagen, with a glutamate residue from the collagen completing the coordination sphere of the metal, and an arginine residue from the same strand of the collagen helix bridging to D219 in the α3–α4 loop on top of the A-domain (Fig. 2). Two phenylalanine residues, one in the same collagen strand as the arginine residue and one in the trailing strand, made further contacts. Hydrogen bonds between the collagen main chain and N154, Y157 (βA–α1 loop) and H258 (βD–α5 loop) of the A-domain were seen.
The use of the glutamate residue for cation coordination suggests that the same mechanism may be employed by other integrin ligands to bind to αA-domains, and it also gives a snapshot of how aspartate-containing ligands may bind to βA-domains in those integrins that lack an αA-domain.
Integrin activation
For the interaction of integrins with their ligands to be meaningful for cellular function, the binding event must be able to trigger signal transduction. In part this will be accomplished by ligands inducing conformational changes in integrins that create effector binding sites and/or exposure of sites to modifying enzymes. Following the solution of the αVβ3 crystal structure, it is possible to place information that has accumulated from a plethora of studies on integrin activation into a structural context. The long-term aims of this work are to elucidate the structural link between ligand binding and signalling, and to develop strategies for interfering therapeutically in the activation process.
Conformational changes mediating activation
Gross conformational changes in integrins have been detected by a variety of techniques. For almost all of these studies, αIIbβ3 has served as a prototype. For example, treatment of αIIbβ3 with ligand peptides increased its hydrolysis by thrombin and decreased its sedimentation coefficient [42], and platelet activation caused a change in the spatial separation or orientation of the extracellular domains of the two subunits as measured by fluorescence resonance energy transfer [43].
Much work has been carried out on activation-dependent binding of mAbs to integrins, some of which have been called ligand-induced binding sites (LIBS) [44]. Binding of cyclic mimics of different ligand peptides to αIIbβ3 in intact platelets was recently found to trigger distinct conformational alterations in the receptor, as indicated by the differential exposure of LIBS epitopes [45]. This suggests that different ligands may initiate different functional consequences within the receptor. The changes reported by mAbs can be triggered not only from the extracellular side of the integrin in response to ligand binding, but also from the cytoplasmic side of the plasma membrane, suggesting that conformational regulation is an important feature of bidirectional signalling by integrins [46].
Mechanisms of conformational change causing activation
Activation via the αA-domain
As already described, the first crystal structures of the αA-domain, solved for Mg2+-occupied and Mn2+-occupied forms of the αM domain, revealed different structures [14,15]. A comparative analysis revealed a change in metal coordination, a large (10Å) shift of the C-terminal α7 helix, and the solvent exposure of F302. It was suggested that the movement of the α7 helix in the context of the intact integrin might induce further conformational changes outside of the αA-domain.
Comparison of the tertiary structure of the α2 A-domain–collagen peptide complex with the unoccupied α2 A-domain structure [41,47] revealed similar changes that provide insight into the process of receptor activation (Fig. 2). The central β-sheet did not change appreciably in the two structures, but there were significant changes in cation coordination and helix organisation. A 2.6Å movement of the cation resulted in the formation of a direct bond with T221 (this residue coordinated via a water molecule in the unoccupied structure), a loss of coordination to D254, and a new coordination to E256. This reorganisation of the upper surface of the A-domain reoriented the side chains of Y157 and H258 such that they were able to fit into grooves in the collagen helix. The shifts in the positions of cation-coordinating loops triggered a rearrangement of the α7 and C helices, the former moving away and down by 10Å and the latter unwinding. As a consequence of these movements, Y285 in the C helix moved 17Å and hydrogen bonded with the repositioned α7 helix.
The relevance of the large movement of the α7 helix in both the αM and α2 structures as a consequence of ligand engagement is as yet unclear, but since this sequence is at the C-terminus of the αA-domain, and is therefore connected to the remainder of the subunit, it is tempting to speculate that the conformational change will be propagated through the receptor to initiate signalling. A key question is how the α7 helix contacts the remainder of the integrin, and in particular whether it interacts with the βA-domain to alter its conformation (i.e. whether a ligand-binding event in the αA-domain can have a similar effect to direct ligand binding at the βA-domain).
Activation in non-αA-domain-containing integrins
In contrast to the situation with αA-domain-containing integrins, little is currently known about the intramolecular activation of non-αA-domain-containing integrins. If the ligand-responsive subset of activating mAbs recognises sites that occur naturally in integrins, the location of their epitopes will inform an understanding of the process of receptor activation.
A large number of studies have thus pinpointed activating mAb epitopes by mutagenesis and the use of interspecies integrin chimeras, and these can now be placed in the context of the crystal structure. The overwhelming majority of activating mAbs recognise the β-subunit and, interestingly, while their epitopes are distributed throughout the polypeptide, suggesting large-scale alteration in the conformation of the whole integrin during activation, a number of specific regions appear to be recognised. These regions include the extreme N-terminus of the β-subunit in the PSI domain [48], the βA-domain [49], the hybrid domain [50], and the epidermal growth factor repeats [51,52]. The α1 and α2 helices of the βA-domain, in particular, contain the epitopes for a large number of mAbs, some of which were function blocking and others of which were stimulatory for ligand binding [49]. As these elements are linked to the cation-binding site in the βA-domain, it is conceivable that ligand binding triggers an alteration in their positioning, and that this change is then propagated to the rest of the integrin.
Integrins are relatively large receptors, and a major challenge is to understand how proximal conformational changes in the ligand-binding pocket are passed to the rest of the integrin, and ultimately to the cytoplasmic domains. As there is currently little direct information to inform these issues, any theories will be highly speculative. There is evidence, based on competitive ELISA experiments, that the domains of the α5-subunit and the β1-subunit recognised by mAb JBS5/16 (anti-α5) and mAb 13/12G10 (anti-β1) are spatially close, and that the distance between these two domains increased when α5β1 was occupied by divalent cations [53]. This suggested that divalent cations induced a conformational relaxation in the integrin that resulted in exposure of ligand-binding sites, and that these sites were located near to the interface between the α-subunit and the β-subunit.
The structural homology between the integrin head and heterotrimeric G-proteins would also be consistent with a conformational repositioning of these regions of the receptor [1]. The bending of the legs in the αVβ3 crystal structure, whether a true indication of the native structure of the integrin or a crystallisation artifact, suggests that there are sites in both subunits that exhibit extreme flexibility. While this suggests that the head of the integrin may pivot around the α-subunit thigh–calf junction and the β-subunit hybrid–epidermal growth factor–PSI linkage, it also raises questions about how shape changes induced by ligand binding are able to pass two flexible joints. By constraining the relative position of the membrane-proximal domains of integrin αLβ2 with coiled-coil extensions, it was found that the integrin could be inactivated by bringing its legs close together (with an acid–base coiled coil) and could be activated by keeping them apart (with a base–base extension) [54]. Similarly, proteolytic cleavage of a constrained integrin had the same activating effect [55]. This suggests that a gross repositioning of the legs and cytoplasmic domains underlies integrin activation.
Role of cation-binding sites
The binding of ligands to integrins is universally divalent cation dependent, and occupancy by different cations results in different levels of ligand binding. It is therefore important to consider the location, specificity and functional role of cation-binding sites. Integrin-ligand binding is usually stimulated by Mg2+ and Mn2+, and inhibited by Ca2+, as shown first for α5β1 [56]. In the αVβ3 crystal structure, six Ca2+-binding sites are seen (Fig. 1). Four of these lie in β–loop–β structures on the lower face of the β-propeller, and another site is the knee region of the α-subunit. The top face of the βA-domain contains a potential cation-binding site, known as the metal-ion dependent adhesion site (MIDAS), although this is unoccupied in the crystal structure. A novel site is seen adjacent to the MIDAS, however, which the authors termed 'adjacent to MIDAS' (ADMIDAS).
The functional role of the different cation binding sites is discussed later. Apart from their occupancy in the crystal structure, the most convincing evidence that the EF-handlike sequences in the β-propeller bind calcium derives from the fact that the epitope for Ca2+-dependent mAb CBRM1/20 has been localised to the EF-hand-like sequence in repeat 5 of αM [57].
Crystal structures of αA-domains have proven the presence of a cation-binding site in this module. A characteristic 'DXSXS' motif (D140GSGS in αM) located at the junction of the βA strand and the loop between βA and α1 was found to contribute three of these coordination sites: D140 via a water molecule, and S142 and S144 directly. Two sequentially distant residues, T209 at the C-terminal end of the α3–α4 loop and D242 at the start of the βD–α5 loop, also coordinated, the former directly and the latter via water. Given the sequence and functional homology between the A-domains in both subunits, a similar motif is also highly likely to be found in βA-domains. However, the fact that the crystals were grown in calcium may explain the lack of occupancy.
Until further crystal forms are obtained, it is not clear where Mn2+and Mg2+ bind within integrins. However, there is much biochemical evidence to suggest sites. Using the interaction between α5β1 and fibronectin as a model system, a comprehensive analysis of the effects of Mn2+, Mg2+, and Ca2+ on ligand binding was carried out [58]. Each cation had distinct effects on ligand-binding capacity: Mn2+promoted high levels of ligand binding, Mg2+ promoted low levels of binding, and Ca2+ failed to support binding. Ca2+ strongly inhibited Mn2+-supported ligand binding, but this inhibition was noncompetitive, suggesting that Ca2+ recognises different cation-binding sites to Mn2+. In contrast, Ca2+ acted as a direct competitive inhibitor of Mg2+-supported ligand binding, implying that Ca2+ can displace Mg2+ from the integrin. However, low concentrations of Ca2+ greatly increased the apparent affinity of Mg2+ for its binding site, suggesting the existence of a distinct high-affinity Ca2+-binding site.
In summary, evidence is accumulating to suggest that non-αA-domain-containing integrins generally contain a cation-binding site that is required for ligand binding and that interacts with Mg2+, Mn2+ (to promote binding) or Ca2+ (in which case binding does not occur). In addition, occupancy of a separate calcium-binding site, or class of sites, can enhance ligand binding in an allosteric, synergistic manner. The location of the different sites is not yet defined, although it is likely that the Mg2+/Mn2+/Ca2+-binding site is the MIDAS site in the βA-domain. The role of the ADMIDAS site is currently unclear, although it may be an inhibitory Ca2+-binding site [59].
An interesting feature of some LIBS mAbs is that their epi-topes are also regulated by divalent cations (for example [53,60]). Since cations also regulate ligand binding, and in some cases the pattern of effects by different cations is the same for mAb and ligand binding [53], it appears that some activating mAbs recognise sites that are regulated by natural modulators of integrin function. One possibility is that cation-responsive activating mAbs recognise naturally occurring conformers of integrins.
Cation effects on integrin conformation have been reported to vary between dimers. Mn2+ or ligand induced 9EG7 or 15/7 binding strongly on α4β1, moderately on α5β1, weakly on α2β1, and undetectably on α3β1 and α6β1 [61]. Ca2+ uniquely supported constitutive expression of the 9EG7 epitope on α4β1. Thus, not all LIBS mAbs will be faithful reporters of occupancy.
Finally, the different properties of activating antibodies imply that care should be taken in using them as probes of integrin function. It is probable that different mAbs will stabilise different integrin conformers and, if so, the consequences for signalling may also differ.
Concluding remarks
Now that the feasibility of generating a crystal structure of an integrin is proven, many other structural questions can be asked. Key targets for future crystallography studies include different integrin conformers representing different activation states, ligand-occupied integrins, and integrins containing an αA-domain.
Although, as already described, the general location of the ligand-binding pocket of an integrin can now be predicted, the atomic detail will require an integrin–ligand co-crystal. Once this is achieved, the process of drug development based on ligand mimetics will be aided. Interestingly, the allosteric inhibition of ligand binding by antifunctional anti-integrin mAbs implies that it may be feasible to synthesise small molecule inhibitors that function in the same way. Such inhibitors may not possess the agonistic properties of ligand mimetics, and may therefore not suffer from mechanism-related side effects.
As yet, the generation of allosteric integrin inhibitors is in its infancy, although a recent report of the tertiary structure of the αL A-domain in complex with lovastatin has provided an insight into such a mode of action. Statins are drugs used clinically for lowering cholesterol levels, but they are also reported to inhibit the interaction of αLβ2 with ICAM-1 [62,63]. Using nuclear magnetic resonance spectroscopy and X-ray crystallography, the inhibitor was shown to bind to the αL A-domain in a crevice between the central β-sheet and the C-terminal α7 helix. This finding suggests that the inhibitor may function by preventing movement of α7 relative to the rest of the domain and preventing subsequent intramolecular conformational changes [64]. It is possible that a similar approach may be used to develop allosteric inhibitors of the βA-domain, and that this may spawn a new generation of anti-adhesive drugs.
Glossary of terms
ADMIDAS = adjacent to metal ion-dependent adhesion site; LIBS = ligand-induced binding site; MIDAS = metal ion-dependent adhesion site; PSI = plexin–semaphorin-integrin.
London, UK. 24-26 June 2002
Acknowledgements
The work discussed within this review was supported by grants from the Wellcome Trust.
References
- Xiong JP, Stehle T, Diefenbach B, Zhang R, Dunker R, Scott DL, Joachimiak A, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin αVβ3. Science. 2001;294:339–345. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell NA, Fitzgerald LA, Steiner B, Erickson HP, Phillips DR. Structure of human platelet membrane glycoproteins IIb and IIIa as determined by electron microscopy. J Biol Chem. 1985;260:1743–1749. [PubMed] [Google Scholar]
- Nermut MV, Green NM, Eason P, Yamada SS, Yamada KM. Electron microscopy and structural model of human fibronectin receptor. EMBO J. 1988;7:4093–4099. doi: 10.1002/j.1460-2075.1988.tb03303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisel JW, Nagaswami C, Vilaire G, Bennett JS. Examination of the platelet membrane glycoprotein IIb–IIIa complex and its interaction with fibrinogen and other ligands by electron microscopy. J Biol Chem. 1992;267:16637–16643. [PubMed] [Google Scholar]
- Springer TA. Folding of the N-terminal, ligand-binding region of integrin α-subunits into a β-propeller domain. Proc Natl Acad Sci USA. 1997;94:65–72. doi: 10.1073/pnas.94.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckwell DS, Humphries MJ. A structure prediction for the ligand-binding region of the integrin β subunit: evidence for the presence of a von Willebrand factor A domain. FEBS Lett. 1997;400:297–303. doi: 10.1016/S0014-5793(96)01368-3. [DOI] [PubMed] [Google Scholar]
- Bork P, Doerks T, Springer TA, Snel B. Domains in plexins: links to integrins and transcription factors. Trends Biochem Sci. 1999;24:261–263. doi: 10.1016/S0968-0004(99)01416-4. [DOI] [PubMed] [Google Scholar]
- Wilcox DA, Paddock CM, Lyman S, Gill JC, Newman PJ. Glanzmann thrombasthenia resulting from a single amino acid substitution between the second and third calcium-binding domains of GPIIb: role of the GPIIb amino terminus in integrin subunit association. J Clin Invest. 1995;95:1553–1560. doi: 10.1172/JCI117828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SCT. Isolation and characterization of a chymotryptic fragment of platelet glycoprotein IIb–IIIa retaining Arg-Gly-Asp binding activity. J Biol Chem. 1992;267:5649–5655. [PubMed] [Google Scholar]
- Calvete JJ, Henschen A, Gonzalez-Rodriguez J. Complete localization of the intrachain disulfide bonds and the N-glycosyla-tion points in the α-subunit of human platelet glycoprotein IIb. Biochem J. 1989;261:561–568. doi: 10.1042/bj2610561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvete JJ, Henschen A, Gonzalez-Rodriguez J. Assignment of disulfide bonds in human platelet GPIIIa. A disulfide pattern for the β-subunits of the integrin family. Biochem J. 1991;274:63–71. doi: 10.1042/bj2740063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Springer TA. Folding of the β-propeller domain of the integrin αL subunit is independent of the I domain and dependent on the β2 subunit. Proc Natl Acad Sci USA. 1997;94:3162–3167. doi: 10.1073/pnas.94.7.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Lu C, Springer TA. Folding of the conserved domain but not of flanking regions in the integrin β2 subunit requires association with the α subunit. Proc Natl Acad Sci USA. 1997;94:3156–3161. doi: 10.1073/pnas.94.7.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JO, Rieu P, Arnaout MA, Liddington R. Crystal structure of the A domain from the α subunit of integrin CR3 (CD11b/CD18). Cell. 1995;80:631–638. doi: 10.1016/0092-8674(95)90517-0. [DOI] [PubMed] [Google Scholar]
- Lee JO, Bankston LA, Arnaout MA, Liddington RC. Two conformations of the integrin A-domain (I-domain): a pathway for activation? Structure. 1995;3:1333–1340. doi: 10.1016/s0969-2126(01)00271-4. [DOI] [PubMed] [Google Scholar]
- Mould P, Askari JA, Humphries MJ. Molecular basis of ligand recognition by integrin α5β1: Specificity of ligand binding is determined by amino acid sequences in the second and third NH2-terminal repeats of the α subunit. J Biol Chem. 2000;275:20324–20336. doi: 10.1074/jbc.M000572200. [DOI] [PubMed] [Google Scholar]
- Humphries JD, Askari JA, Zhang XP, Takada Y, Humphries MJ, Mould P. Molecular basis of ligand recognition by integrin α5β1: Specificity of Arg-Gly-Asp binding is determined by Trp157 of the α subunit. J Biol Chem. 2000;275:20337–20345. doi: 10.1074/jbc.M000568200. [DOI] [PubMed] [Google Scholar]
- Takagi J, Kamata T, Meredith J, Puzon-McLaughlin W, Takada Y. Changing ligand specificities of αVβ1 and αVβ3 integrins by swapping a short diverse sequence of the β subunit. J Biol Chem. 1997;272:19794–19800. doi: 10.1074/jbc.272.32.19794. [DOI] [PubMed] [Google Scholar]
- Humphries MJ. The molecular basis and specificity of integrin–ligand interactions. J Cell Sci. 1990;97:585–592. doi: 10.1242/jcs.97.4.585. [DOI] [PubMed] [Google Scholar]
- D'Souza SE, Ginsberg MH, Burke TA, Lam SCT, Plow EF. Localization of an Arg-Gly-Asp recognition site within an integrin adhesion receptor. Science. 1988;242:91–93. doi: 10.1126/science.3262922. [DOI] [PubMed] [Google Scholar]
- Smith JW, Cheresh DA. The Arg-Gly-Asp binding domain of the vitronectin receptor. Photoaffinity cross-linking implicates amino acid residues 61–203 of the β subunit. J Biol Chem. 1988;263:18726–18731. [PubMed] [Google Scholar]
- D'Souza SE, Haas TA, Piotrowicz RS, Byers-Ward V, McGrath DE, Soule HR, Cierniewski C, Plow EF, Smith JW. Ligand and cation binding are dual functions of a discrete segment of the integrin β3 subunit: cation displacement is involved in ligand binding. Cell. 1994;79:659–667. doi: 10.1016/0092-8674(94)90551-7. [DOI] [PubMed] [Google Scholar]
- Charo IF, Nannizzi L, Phillips DR, Hsu MA, Scarborough RM. Inhibition of fibrinogen binding to GP IIb–IIIa by a GP IIIa peptide. J Biol Chem. 1991;266:1415–1421. [PubMed] [Google Scholar]
- Steiner B, Trzeciak A, Pfenninger G, Kouns WC. Peptides derived from a sequence within β3 integrin bind to platelet αIIbβ3 (GPIIb–IIIa) and inhibit ligand binding. J Biol Chem. 1993;268:6870–6873. [PubMed] [Google Scholar]
- Chen LL, Lobb RR, Cuervo JH, Lin K, Adams SP, Pepinsky RB. Identification of ligand binding sites on integrin α4β1 through chemical crosslinking. Biochemistry. 1998;37:8743–8753. doi: 10.1021/bi980311a. [DOI] [PubMed] [Google Scholar]
- Bitan G, Scheibler L, Greenberg Z, Rosenblatt M, Chorev M. Mapping the integrin αVβ3–ligand interface by photoaffinity cross-linking. Biochemistry. 1999;38:3414–3420. doi: 10.1021/bi981946c. [DOI] [PubMed] [Google Scholar]
- Mould AP, Akiyama SK, Humphries MJ. The inhibitory anti-β1 integrin monoclonal antibody 13 recognizes an epitope that is attenuated by ligand occupancy. Evidence for allosteric inhibition of integrin function. J Biol Chem. 1996;271:20365–20374. doi: 10.1074/jbc.271.34.20365. [DOI] [PubMed] [Google Scholar]
- Mould AP, Askari JA, Aota S, Yamada KM, Irie A, Takada Y, Mardon HJ, Humphries MJ. Defining the topology of integrin α5β1–fibronectin interactions using inhibitory anti-α5 and anti-β1 monoclonal antibodies. Evidence that the synergy sequence of fibronectin is recognized by the amino-terminal repeats of the α5 subunit. J Biol Chem. 1997;272:17283–17292. doi: 10.1074/jbc.272.28.17283. [DOI] [PubMed] [Google Scholar]
- Humphries MJ. Integrin structure. Biochem Soc Trans. 2000;28:311–339. [PubMed] [Google Scholar]
- Kamata T, Tieu KK, Springer TA, Takada Y. Amino acid residues in the αIIb subunit that are critical for ligand binding to integrin αIIbβ3 are clustered in the beta-propeller model. J Biol Chem. 2001;276:44275–44283. doi: 10.1074/jbc.M107021200. [DOI] [PubMed] [Google Scholar]
- Ueda T, Rieu P, Brayer J, Arnaout MA. Identification of the complement iC3b binding site in the β2 integrin CR3 (CD11b/CD18). Proc Natl Acad Sci USA. 1994;91:10680–10684. doi: 10.1073/pnas.91.22.10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lee DHS, Plescia J, Lau CY, Altieri DC. Differential ligand binding specificities of recombinant CD11b/CD18 integrin I-domain. J Biol Chem. 1994;269:17075–17079. [PubMed] [Google Scholar]
- Randi AM, Hogg N. I Domain of β2 integrin lymphocyte function-associated antigen-1 contains a binding site for ligand intercellular adhesion molecule-1. J Biol Chem. 1994;269:12395–12398. [PubMed] [Google Scholar]
- Tuckwell D, Calderwood DA, Green LJ. Humphries MJ. Integrin α2 I-domain is a binding site for collagens. J Cell Sci. 1995;108:1629–1637. doi: 10.1242/jcs.108.4.1629. [DOI] [PubMed] [Google Scholar]
- Calderwood DA, Tuckwell DS, Eble J, Kuhn K, Humphries MJ. The integrin α1 A-domain is a ligand binding site for collagens and laminin. J Biol Chem. 1997;272:12311–12317. doi: 10.1074/jbc.272.19.12311. [DOI] [PubMed] [Google Scholar]
- Kamata T, Takada Y. Direct binding of collagen to the I domain of integrin α2β1 (VLA-2, CD49b/CD29) in a divalent cation-independent manner. J Biol Chem. 1994;269:26006–26010. [PubMed] [Google Scholar]
- Van der Vieren M, Crowe DT, Hoekstra D, Vazeux R, Hoffman PA, Grayson MH, Bochner BS, Gallatin WM, Staunton DE. The leukocyte integrin αDβ2 binds VCAM-1: evidence for a binding interface between I domain and VCAM-1. J Immunol. 1999;163:1984–1990. [PubMed] [Google Scholar]
- Huang C, Springer TA. A binding interface on the I domain of lymphocyte function-associated antigen-1 (LFA-1) required for specific interaction with intercellular adhesion molecule 1 (ICAM-1). J Biol Chem. 1995;270:19008–19016. doi: 10.1074/jbc.270.32.19008. [DOI] [PubMed] [Google Scholar]
- Champe M, McIntyre BW, Berman PW. Monoclonal antibodies that block the activity of leukocyte function-associated antigen 1 recognize three discrete epitopes in the inserted domain of CD11a. J Biol Chem. 1995;270:1388–1394. doi: 10.1074/jbc.270.21.12635. [DOI] [PubMed] [Google Scholar]
- Gotwals PJ, Chi-Rosso G, Ryan ST, Sizing I, Zafari M, Benjamin C, Singh J, Venyaminov SY, Pepinsky RB, Koteliansky V. Divalent cations stabilize the α1β1 integrin I domain. Biochemistry. 1999;38:8280–8288. doi: 10.1021/bi982860m. [DOI] [PubMed] [Google Scholar]
- Emsley J, Knight CG, Farndale RW, Barnes MJ, Liddington RC. Structural basis of collagen recognition by integrin α2β1. Cell. 2000;101:47–56. doi: 10.1016/S0092-8674(00)80622-4. [DOI] [PubMed] [Google Scholar]
- Parise LV, Helgerson SL, Steiner B, Nannizzi L, Phillips DR. Synthetic peptides derived from fibrinogen and fibronectin change the conformation of purified platelet glycoprotein IIb–IIIa. J Biol Chem. 1987;262:12597–12602. [PubMed] [Google Scholar]
- Sims PJ, Ginsberg MH, Plow EF, Shattil SJ. Effect of platelet activation on the conformation of the plasma membrane gly-coprotein IIb–IIIa complex. J Biol Chem. 1991;266:7345–7352. [PubMed] [Google Scholar]
- Ginsberg MH, Frelinger AL, Lam SCT, Forsyth J, McMillan R, Plow EF, Shattil SJ. Analysis of platelet aggregation disorders based on flow cytometric analysis of membrane glycoprotein IIb–IIIa with conformation-specific monoclonal antibodies. Blood. 1990;76:2017–2023. [PubMed] [Google Scholar]
- Cierniewski CS, Byzova T, Papierak M, Haas TA, Niewiarowska J, Zhang L, Cieslak M, Plow EF. Peptide ligands can bind to distinct sites in integrin αIIbβ3 and elicit different functional responses. J Biol Chem. 1999;274:16923–16932. doi: 10.1074/jbc.274.24.16923. [DOI] [PubMed] [Google Scholar]
- Mastrangelo AM, Homan SM, Humphries MJ, LaFlamme SE. Amino acid motifs required for isolated β cytoplasmic domains to regulate 'in trans' β1 integrin conformation and function in cell attachment. J Cell Sci. 1999;112:217–229. doi: 10.1242/jcs.112.2.217. [DOI] [PubMed] [Google Scholar]
- Emsley J, King SL, Bergelson JM, Liddington RC. Crystal structure of the I domain from integrin α2β1. J Biol Chem. 1997;272:28512–28517. doi: 10.1074/jbc.272.45.28512. [DOI] [PubMed] [Google Scholar]
- Honda S, Tomiyama Y, Pelletier AJ, Annis D, Honda Y, Orchekowski R, Ruggeri Z, Kunicki TJ. Topography of ligand-induced binding sites, including a novel cation-sensitive epitope (AP5) at the amino terminus, of the human integrin β3 subunit. J Biol Chem. 1995;270:11947–11954. doi: 10.1074/jbc.270.20.11947. [DOI] [PubMed] [Google Scholar]
- Takada Y, Puzon W. Identification of a regulatory region of integrin β1 subunit using activating and inhibiting antibodies. J Biol Chem. 1993;268:17597–17601. [PubMed] [Google Scholar]
- Puzon-McLaughlin W, Yednock TA, Takada Y. Regulation of conformation and ligand binding function of integrin α5β1 by the β1 cytoplasmic domain. J Biol Chem. 1996;271:16580–16585. doi: 10.1074/jbc.271.28.16580. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Shih DT, Buck CA, Hemler ME. Monoclonal antibody 9EG7 defines a novel β1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J Biol Chem. 1995;270:25570–25577. doi: 10.1074/jbc.270.30.17784. [DOI] [PubMed] [Google Scholar]
- Wilkins JA, Li A, Ni H, Stupack DG, Shen C. Control of β1 inte-grin function. Localization of stimulatory epitopes. J Biol Chem. 1996;271:3046–3051. doi: 10.1074/jbc.271.6.3255. [DOI] [PubMed] [Google Scholar]
- Mould AP, Garratt AN, Puzon-McLaughlin W, Takada Y, Humphries MJ. Regulation of integrin function: evidence that bivalent-cation-induced conformational changes lead to the unmasking of ligand-binding sites within integrin α5β1. Biochem J. 1998;331:821–828. doi: 10.1042/bj3310821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Takagi J, Springer TA. Association of the membrane-proximal regions of the α and β subunit cytoplasmic domains constrains an integrin in the inactive state. J Biol Chem. 2001;276:14642–14648. doi: 10.1074/jbc.M100600200. [DOI] [PubMed] [Google Scholar]
- Takagi J, Erickson HP, Springer TA. C-terminal opening mimics 'inside-out' activation of integrin α5β1. Nat Struct Biol. 2001;8:412–416. doi: 10.1038/87569. [DOI] [PubMed] [Google Scholar]
- Gailit J, Ruoslahti E. Regulation of the fibronectin receptor affinity by divalent cations. J Biol Chem. 1988;263:12927–12932. [PubMed] [Google Scholar]
- Oxvig C, Springer TA. Experimental support for a β-propeller domain in integrin α-subunits and a calcium binding site on its lower surface. Proc Natl Acad Sci USA. 1998;95:4870–4875. doi: 10.1073/pnas.95.9.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould AP, Akiyama SK, Humphries MJ. Regulation of integrin α5β1–fibronectin interactions by divalent cations. Evidence for distinct classes of binding sites for Mn2+, Mg2+, and Ca2+. J Biol Chem. 1995;270:26270–26277. doi: 10.1074/jbc.270.44.26270. [DOI] [PubMed] [Google Scholar]
- Cierniewska-Cieslak A, Cierniewski CS, Blecka K, Papierak M, Michalec L, Zhang L, Haas TA, Plow EF. Identification and characterization of two cation binding sites in the integrin β3 subunit. J Biol Chem. 2002. in press . [DOI] [PubMed]
- Dransfield I, Hogg N. Regulated expression of magnesium binding epitope on leukocyte integrin α subunits. EMBO J. 1989;8:3759–3765. doi: 10.1002/j.1460-2075.1989.tb08552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoni G, Ma L, Blue ML, Hemler ME. Divalent cations and ligands induce conformational changes that are highly divergent among β1 integrins. J Biol Chem. 1998;273:6670–6678. doi: 10.1074/jbc.273.12.6670. [DOI] [PubMed] [Google Scholar]
- Kallen J, Welzenbach K, Ramage P, Geyl D, Kriwacki R, Legge G, Cottens S, Weitz-Schmidt G, Hommel U. Structural basis for LFA-1 inhibition upon lovastatin binding to the CD11a I-domain. J Mol Biol. 1999;292:1–9. doi: 10.1006/jmbi.1999.3047. [DOI] [PubMed] [Google Scholar]
- Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, Cottons S, Takada Y, Hommel U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687–692. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- McDowall A, Leitinger B, Stanley P, Bates PA, Randi AM, Hogg N. The I domain of integrin leukocyte function-associated antigen-1 is involved in a conformational change leading to high affinity binding to ligand intercellular adhesion molecule 1 (ICAM-1). J Biol Chem. 1998;273:27396–27403. doi: 10.1074/jbc.273.42.27396. [DOI] [PubMed] [Google Scholar]


