Abstract
Chapter summary
The human leukocyte antigen HLA-B27 is strongly associated with development of a group of inflammatory arthritides collectively known as the spondyloarthritides. We have set out to define the natural immunological function of HLA-B27, and then to apply this knowledge to understand its pathogenic role. Human leukocyte antigen class 1 molecules bind antigenic peptides for cell surface presentation to cytotoxic T lymphocytes. HLA-B27 binds and presents peptides from influenza, HIV, Epstein-Barr virus, and other viruses. This leads to vigorous and specific cytotoxic T lymphocyte responses, which play an important role in the body's immune response to these viruses. HLA-B27 thus carries out its natural function highly effectively. Although many theories have been proposed to explain the role of HLA-B27 in the pathogenesis of spondyloarthropathy, we favour those postulating that the pathogenic role of HLA-B27 stems from its natural function. For example, the 'arthritogenic' peptide hypothesis suggests that disease results from the ability of HLA-B27 to bind a unique peptide or a set of antigenic peptides. Additionally, a number of lines of evidence from our laboratory and other laboratories have suggested that HLA-B27 has unusual cell biology. We have recently demonstrated that HLA-B27 is capable of forming disulfide-bonded homodimers. These homodimers are expressed on the cell surface and are ligands for a number of natural killer and related immunoreceptors, expressed on a variety of cell types including natural killer cells, T lymphocytes and B lymphocytes, and members of the monocyte/macrophage lineage. We are currently investigating the possibility that such interactions could be involved in disease pathogenesis.
Keywords: cytotoxic C cell, HLA-B27, peptide, spondyloarthritis
Introduction
This chapter will first describe the natural function of HLA-B27, before presenting possible mechanisms by which HLA-B27 might be involved in disease pathogenesis. We will review the data available from HLA-B27 transgenic animals, from structural studies and from biochemical analysis of HLA-B27 function. A concluding section will identify key lines of current and future research.
Historical background
Possession of the human leukocyte antigen (HLA) class 1 allele HLA-B27 is strongly associated with development of the spondyloarthritides, a group of related diseases including ankylosing spondylitis and reactive arthritis (see Table 1). Ankylosing spondylitis is a common inflammatory rheumatic disease, affecting up to 0.5% of the population. The association of HLA-B27 with ankylosing spondylitis was first described in 1973 [1], and is among the strongest described for a HLA locus. A recent study found that 94% of ankylosing spondylitis patients are HLA-B27-positive, compared with 9.4% of controls, giving an odds ratio of 161 with a 95% confidence interval of 113–230 [2]. HLA-B27 is also less significantly associated with reactive arthritis [3] and with the spondyloarthritis associated with psoriasis and inflammatory bowel disease [4]. These conditions share clinical features including arthritis of the spine and large joints, and involvement of the skin, eye, genital mucosa and heart.
Table 1.
HLA-B27-associated spondyloarthritides
| Disease | HLA-B27 frequency (%) (approximate) |
|---|---|
| Ankylosing spondylitis | 96 |
| Undifferentiated spondyloarthropathy | 70 |
| Reactive arthritis | 30–70 |
| Colitis-associated spondyloarthritis | 33–75 |
| Psoriatic spondyloarthritis | 40–50 |
| Juvenile enthesitis-related arthritis | 70 |
| Iritis | 50 |
| Cardiac conduction defects with aortic incompetence | Up to 88 |
While the pathogenic role of HLA-B27 in the spondyloarthropathies is unknown, numerous theories have been proposed. These theories are reviewed in [5], and many are applicable to the HLA associations with other autoimmune diseases (reviewed in [6]). Some theories suggest that the pathogenic role of HLA-B27 is independent of its immune function; for example, suggesting that HLA-B27 acts as a receptor for a disease-causing microorganism or is even merely a genetic marker for the true gene responsible. We favour theories suggesting that the pathogenic role of HLA-B27 stems from its immunological role. The 'arthritogenic' peptide theory (see later) proposes that HLA acts to present antigens to T cells. Alternatively, it is possible that HLA-B27 itself acts as a source of antigen, providing peptides that can be presented by other HLA molecules.
The finding that the natural role of HLA molecules is peptide binding and presentation to T cells [7,8] led to the suggestion that the spondyloarthropathies result from the ability of HLA-B27 to bind a unique set of peptides [9]. This 'arthritogenic' peptide hypothesis proposes that disease results from an HLA-B27-restricted cytotoxic T-cell response to a peptide or peptides found only in joint and other affected tissues. Such a peptide could be bound and presented by all disease-associated HLA-B27 subtypes (see later), but not by other class I molecules. Pathogenic T cells might be primed in the joint or at other sites such as the genital or gut mucosa. A modification of this original hypothesis could entail a breakdown of self-tolerance by initial HLA-B27-restricted presentation of a peptide or peptides derived from one of the triggering pathogens.
If the disease association of HLA-B27 is indeed a consequence of its physiological role in peptide presentation, HLA-B27-restricted cytotoxic T lymphocytes (CTL), specific for self-epitopes or bacterial epitopes, should be demonstrable in the involved joints of patients with spondyloarthropathies. Although Yersinia-specific and Salmonella-specific clones have been isolated from two patients with reactive arthritis [10], many groups have found predominant CD4 T-cell responses to triggering bacteria within the joint.
Although an arthritogenic peptide model of disease causation is supported by the epidemiological and functional studies of HLA-B27 subtypes (see later), evidence from patients and from transgenic models (see later) suggests that other factors and mechanisms need to be considered.
Animal models of HLA-B27-associated disease
Rats and mice carrying HLA-B27 as a transgene provide strong evidence that HLA-B27 is directly involved in disease pathogenesis. These animals can develop illnesses similar to the spondyloarthropathies. Rats carrying a high copy number of HLA-B*2705 transgenes develop an illness characterized by peripheral and axial arthritis, gut inflammation, and genital and skin lesions [11]. Interestingly, rats kept in germ-free conditions do not develop the inflammatory intestinal or peripheral joint disease [12]. It appears that this disease can be transferred by foetal liver cells alone, suggesting that antigen presentation by HLA-B27 in peripheral tissues such as joints is not essential for development of disease [13].
Mice transgenic for HLA-B27 do not normally develop disease. However, spontaneous inflammatory arthritis develops in mice transgenic for HLA-B27 but lacking murine beta-2-microglobulin (β2m), following transfer from germ-free to conventional conditions [14]. In the absence β2m, these animals express very low levels of class 1 molecules. Although normally conformed HLA-B27 is not expressed in these mice, HLA-B27 heavy chains (not associated with β2m) can be detected on the cell surface of concanavalin A-treated peripheral blood leukocytes using the monoclonal antibody HC10 [15].
HLA-B27 structure and function
Much is now known of the molecular structure, peptide-binding specificity and cell biology of HLA-B27. Solution of the crystal structure of HLA-B27, crystallized with a mixture of self-peptides [16], showed that short peptides are bound in an extended conformation within a peptide binding groove. A common arginine residue was found at the second position of all bound peptides. The long side chain of this arginine was accommodated in the 'B' or '45' pocket, comprising in HLA-B27 a unique combination of residues: 45E, 67C, 34V, 26G and 24T. Amino acid analysis of self-peptides eluted from HLA-B27 has confirmed the presence of this arginine residue at the second position [17,18]. Arginine at the second position of the bound peptide is thus an anchor residue for HLA-B27.
There are also preferences for particular amino acids at other positions, with these preferences differing between different HLA-B27 subtypes. HLA-B*2705 thus appears to bind peptides with C terminal amino acids that are either aromatic, hydrophobic or positively charged, whereas HLA-B*2702 can probably only accommodate aromatic or hydrophobic residues at this position [18]. Finally, measurement of the ability of different peptides to bind to HLA-B27 has confirmed the importance of the P2 arginine (for example [19,20]), and also confirmed that different subtypes probably bind different but overlapping subsets of peptides [21,22].
Molecular epidemiological studies have confirmed the association of HLA-B*2702, HLA-B*2704, and HLA-B*2705 with spondyloarthritis first described by Breur-Vriesendorp et al. [23,24]. However, molecular epidemiological studies of other subtypes have produced somewhat conflicting results. Thus, although HLA-B*2703 and HLA-B*2706 have been reported as not associated with disease [24,25], spondyloarthropathy patients bearing these subtypes have subsequently been described [26]. This is an important area of research as these subtypes differ principally in their peptide-binding specificities, and these findings, if confirmed, would support arthritogenic peptide models of pathogenesis.
The role of HLA-B27 in immune responses to viral infection
We have shown that individuals infected with influenza A or HIV make vigorous CTL responses to specific viral peptide epitopes that are presented by HLA-B27 [27,28]. Evidence that HLA-B27-restricted CTL play a major role in HIV infection has recently come from long-term studies of the viral sequence. For certain patients, viral 'escape' mutants that no longer bind to HLA-B27 accumulate after a number of years. These patients, but not those retaining the original viral sequence, progressed to develop AIDS [29].
Using the response to influenza nucleoprotein residues 383–391 as a model, we previously defined the rules for peptide binding to HLA-B27, and identified the key residues for subsequent recognition by the T-cell receptor for antigen (TCR) of cytotoxic T cells [19]. Both healthy and spondyloarthritis patients made good HLA-B27-restricted CTL responses, showing that there is nothing abnormal about the natural function of HLA-B27 in patients with spondyloarthropathy. These findings have also allowed us to predict which residues of a potential arthritogenic peptide could be flexible or conserved if molecular mimicry plays a role in disease pathogenesis.
Finally, we have also shown that the TCR of CTL recognizing the HLA-B27/influenza nucleoprotein peptide combination use a highly conserved repertoire of TCRs [30]. This has lead to studies of the TCR repertoire in patients with spondyloarthropathy, which found evidence of expanded T-cell populations [31], of which some bear identical or almost identical TCRBV chain sequences. One interpretation of these findings is that these oligoclonal expansions are driven by a self-antigen within the joint, presumably presented by HLA-B27 (May et al., unpublished data).
New developments and future prospects in the study of peptide presentation by HLA-B27
The generation of fluorogenic multimeric major histocompatibility complexes (usually tetramers) has recently proven invaluable for phenotypic analysis of viral responses ex vivo[32]. This technique is now also being applied to the study of autoimmune disease, and has recently been reviewed in Arthritis Research[33]. We have made fluorescent HLA-B27/β2m/peptide tetramers for use in studying T-cell recognition of defined complexes, such as with the influenza peptide [34].
Recent advances in genomics and bioinformatics promise to revolutionize our investigation of autoimmune and infectious diseases. For example, 'search' programs have been developed that can identify potential HLA-B27-binding epitopes as well as those likely to be generated by the proteosome. These methods have now been exploited to hunt for potential arthritogenic peptides within the genome of Chlamydia trachomatis, one of the organisms known to trigger reactive arthritis. Peptides have been identified and immune responses detected in both HLA-B27 transgenic mice and in patients with reactive arthritis (following C. trachomatis infection) using both enzyme-linked immunospot (ELISPOT) assays and tetrameric HLA-B27/β2m/peptide complexes [35].
HLA-B27 cell biology and disease
Another distinct, but not necessarily exclusive, possibility is that unique features of the biochemistry or cell biology of HLA-B27 predispose to disease development. A number of lines of evidence suggest that HLA-B27 may not behave like most other class 1 molecules.
An early observation that cell surface HLA-B27 molecules were peptide-receptive lead to the suggestion that disease might result from presentation to T cells of extracellular peptides not normally accessible to the class 1 processing pathway [36]. Unusually long peptides have been isolated bound to HLA-B27 [37]. We have recently shown that HLA-B27 heavy chains can form homodimers in vitro that are dependent on disulfide bonding through their cysteine 67 residues [38]. These homodimers do not contain β2m but are capable of peptide binding, and adopt a different conformation to 'standard' β2m-associated HLA-B27 complexes; for example, reacting with the monoclonal antibody HC10. These 'HC-B27' homodimers can be detected at the cell surface of HLA-B27-transfected cell lines, and are more abundantly expressed when the cell's antigen-presenting function is impaired (Bird et al., unpublished observations).
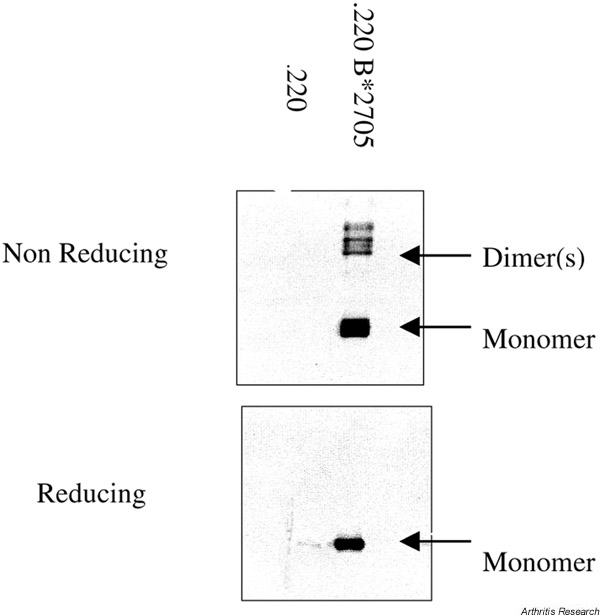
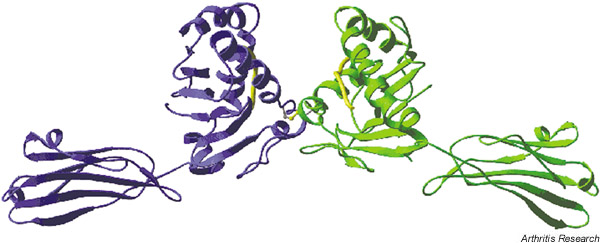
Figure 1 shows an example of HLA-B27 heavy chain homodimer expression in the cell line LBL721.220. A key role for the unpaired cysteine at position 67 of the HLA-B27 alpha 1 helix is suggested by site-directed mutagenesis (Bird et al., unpublished observations). Figure 2 shows a molecular model of a HLA-B27 homodimer. A disulfide bond is shown between position 67 of the two HLA-B27 heavy chains. It is not yet known whether HC-B27 homodimer expression is specific for, or indeed correlates with, spondyloarthropathy, or whether HLA-B27-negative patients with spondyloarthritis express homodimers of other HLA alleles. Interestingly, we have recently observed HLA-B27 homodimer expression at the cell surface of HLA-B27+/β2m knockout mice.
Figure 1.
Disulfide-bonded HLA-B27 heavy chain homodimers are present in HLA-B*2705 transfected LBL721.220 cells. HC-10 western blot shown under non-reducing (upper panel) and reducing (lower panel) conditions. The left-hand lane shows untransfected 721.220 cells.
Figure 2.
Hypothetical molecular model of the HLA-B27 heavy chain homodimer structure. The alpha 1, 2, and 3 domains of two HLA-B27 molecules are shown in ribbon form, bound peptide shown. Orientation: cell surface at bottom of picture.
These and other findings have lead to two novel hypotheses for disease causation. Colbert and colleagues have proposed that homodimer formation is a symptom of HLA-B27 'misfolding' within the endoplasmic reticulum, and that accumulation of misfolded protein results in a potentially proinflammatory intracellular stress response [39]. Alternatively, we have suggested that HLA-B27 heavy chain homodimers may be expressed at the cell surface, where they may act as a proinflammatory target or receptor for humoral or cell-mediated autoimmune responses.
We have recently shown that tetrameric complexes of HLA-B27 heavy chain homodimers bind to certain natural killer (NK) and related receptors, expressed on lymphocytes, NK cells and cells of the monocyte/macrophage lineage. (Kollnberger et al., unpublished data). The functional outcome of the interaction of HLA-B27 with NK receptors and other immunoreceptors is as yet unclear. Although many killer immunoglobulin-like receptors have inhibitory effects, there is accumulating evidence that expression of certain receptors is associated with prolonged survival of memory T cells [40].
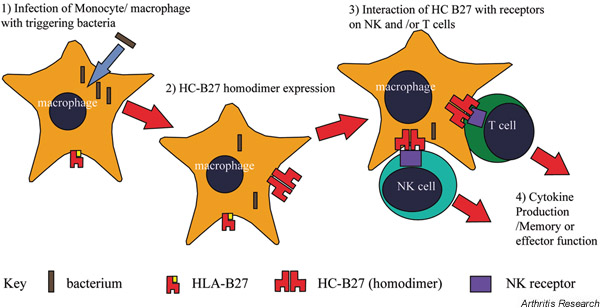
One possible model of disease causation is presented in Figure 3. We first show infection of HLA-B27-expressing cells by an organism capable of triggering spondyloarthropathy. This infection results in interference of the cellular antigen-presenting function and consequent expression of aberrant HLA-B27 homodimers [2]. Notably, other stresses at other sites (e.g. mucosae) could have similar effects. Cell-surface B27 homodimers engage NK or related immunoreceptors expressed on lymphocytes or other cells within the joint, resulting in local cytokine production or enhanced cellular activity [4], and hence perpetuating joint inflammation. Since both CD8 and CD4 T cells can express NK receptors, such a hypothesis could explain the involvement of either cells in disease pathogenesis (expanded populations of both CD4 and CD8 T cells are found in reactive arthritis [31]).
Figure 3.
Hypothetical model for the role of HLA-B27 homodimers in the pathogenesis of spondyloarthritis. NK, natural killer.
An alternative explanation for the involvement of CD4 T cells in spondyloarthropathy has been suggested by recent evidence from Gaston's group showing that HLA-B27 can itself be recognized by CD4 T cells. Different patterns of reactivity have been identified, and it has been suggested that empty or homodimeric forms are being recognized [41]. This is an exciting area for future study.
Concluding remarks
Work from our group and other groups has shown that HLA-B27 appears to excel at its natural function of binding and presenting viral peptide epitopes to cytotoxic T cells. We have suggested that HLA-B27 may, however, act as a 'double-edged sword'. Thus, certain features of its peptide binding ability or cell biology (perhaps those favouring excellent antiviral responses) might also lead to autoimmunity. The recent demonstration that HLA-B27 can interact with a number of different immunoreceptors on different cell types has opened up promising new avenues of research into clarifying its role in the pathogenesis of spondyloarthropathy.
Glossary of terms
β2m = beta-2-microglobulin; HC10 = a monoclonal antibody with specificity for HLA class I heavy chains; HC-B27 = β2m-unassociated HLA-B27 heavy chain homodimer.
London, UK. 24-26 June 2002
Acknowledgement
This work was funded by the Medical Research Council and Arthritis Research Campaign.
References
- Brewerton DA, Caffrey M, Hart FD, James DCO, Nichols A, Sturrock RD. Ankylosing spondylitis and HL-A27. Lancet. 1973;i:904–907. doi: 10.1016/s0140-6736(73)91360-3. [DOI] [PubMed] [Google Scholar]
- Brown MA, Pile KD, Kennedy LG, Calin A, Darke C, Bell J, Wordsworth BP, Cornelis F. HLA class I associations of ankylosing spondylitis in the white population in the United Kingdom. Ann Rheum Dis. 1996;55:268–270. doi: 10.1136/ard.55.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewerton DA, Caffrey M, Hart FD, James DCO, Nichols A, Sturrock RD. Reiters disease and HL-A27. Lancet. 1974;ii:996–998. [Google Scholar]
- Orchard TR, Thiyagaraja S, Welsh KI, Wordsworth BP, Hill Gaston JS, Jewell DP. Clinical phenotype is related to HLA genotype in the peripheral arthropathies of inflammatory bowel disease. Gastroenterology. 2000;118:274–278. doi: 10.1016/s0016-5085(00)70209-5. [DOI] [PubMed] [Google Scholar]
- Allen RL, Bowness P, McMichael A. The role of HLA-B27 in spondyloarthritis. Immunogenetics. 1999;50:220–227. doi: 10.1007/s002510050596. [DOI] [PubMed] [Google Scholar]
- Hall F, Bowness P. In: HLA and MHC: Genes, Molecules and Function. Edited by Browning M, McMichael AJ, editor. Oxford: Bios Scientific;; 1996. HLA and disease: from molecular function to disease association. pp. 353–381. [Google Scholar]
- Townsend A, Rothbard J, Gotch F, Bahadur B, Wraith D, McMichael A. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986;44:959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Gotch F, Rothbard J, Howland K, Townsend A, McMichael A. Cytotoxic T lymphocytes recognise a fragment of influenza virus matrix protein in association with HLA-A2. Nature. 1987;326:881–882. doi: 10.1038/326881a0. [DOI] [PubMed] [Google Scholar]
- Benjamin R, Parham P. Guilt by association: HLA B27 and ankylosing spondylitis. Immunol Today. 1990;11:137–142. doi: 10.1016/0167-5699(90)90051-A. [DOI] [PubMed] [Google Scholar]
- Hermann E, Yu DT, Meyer zBK, Fleischer B. HLA-B27-restricted CD8 T cells derived from synovial fluids of patients with reactive arthritis and ankylosing spondylitis [see comments]. Lancet. 1993;342:646–650. doi: 10.1016/0140-6736(93)91760-J. [DOI] [PubMed] [Google Scholar]
- Hammer RE, Maika SD, Richardson JA, Tang J-P, Taurog JD. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human β2m: an animal model of HLA-B27-associated human disorders. Cell. 1990;63:1099–1112. doi: 10.1016/0092-8674(90)90512-d. [DOI] [PubMed] [Google Scholar]
- Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M, Fernandez SJL, Balish E, Hammer RE. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–2364. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breban M, Hammer RE, Ricardson JA, Taurog JD. Transfer of the inflammatory disease of HLA-B27 transgenic rats by bone marrow engraftment. J Exp Med. 1993;178:1607–1616. doi: 10.1084/jem.178.5.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare SD, Luthra HS, David CS. Spontaneous inflammatory arthritis in HLA-B27 transgenic mice lacking beta 2-microglobulin: a model of human spondyloarthropathies. J Exp Med. 1995;182:1153–1158. doi: 10.1084/jem.182.4.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare SD, Hansen J, Luthra HS, David CS. HLA-B27 heavy chains contribute to spontaneous inflammatory disease in B27/human beta2-microglobulin (beta2m) double transgenic mice with disrupted mouse beta2m. J Clin Invest. 1996;98:2746–2755. doi: 10.1172/JCI119100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DR, Gorga JC, Strominger JL, Wiley DC. The structure of HLA B27 reveals nonamer self-peptides bound in an extended conformation. Nature. 1991;353:321–325. doi: 10.1038/353321a0. [DOI] [PubMed] [Google Scholar]
- Jardetzky TS, Lane WS, Robinson RA, Madden DR, Wiley DC. Identification of self peptides bound to purified HLA-B27. Nature. 1991;353:326–329. doi: 10.1038/353326a0. [DOI] [PubMed] [Google Scholar]
- Rotzschke O, Falk F, Stevanovic S, Gnau V, Jung G, Rammensee H-G. Dominant aromatic/aliphatic C-terminal anchor in HLA-B*2702 and B*2705 peptide motifs. Immunogenetics. 1994;39:74–77. doi: 10.1007/BF00171803. [DOI] [PubMed] [Google Scholar]
- Bowness P, Allen RL, McMichael AJ. Identification of T cell receptor recognition residues for a viral peptide presented by HLA B27. Eur J Immunol. 1994;24:2357–2363. doi: 10.1002/eji.1830241015. [DOI] [PubMed] [Google Scholar]
- Colbert RA, Rowland-Jones SL, McMichael AJ, Frelinger JA. Allele-specific B pocket transplant in class 1 major histocompatibility complex protein changes requirement for anchor residue at P2 of peptide. Proc Natl Acad Sci USA. 1993;90:6879–6883. doi: 10.1073/pnas.90.14.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigaki N, Fruci D, Vigneti E, Starace G, Rovero P, Londei M, Butler RH, Tosi R. The peptide binding specificity of HLA-B27 subtypes. Immunogenetics. 1994;40:192–198. doi: 10.1007/BF00167079. [DOI] [PubMed] [Google Scholar]
- Colbert RA, Rowland-Jones SL, McMichael AJ, Frelinger JA. Differences in peptide presentation between B27 subtypes: the importance of the P1 side chain in maintaining high affinity peptide binding to B*2703. Immunity. 1994;1:121–130. doi: 10.1016/1074-7613(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Breur-Vriesendorp S, Dekker-Says A, Ivanyi P. Distribution of HLA-B27 subtypes in patients with ankylosing spondylitis: the disease is associated with a common determinant of the various B27 molecules. Ann Rheum Dis. 1987;46:353–356. doi: 10.1136/ard.46.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AVS, Allsopp CEM, Kwiatowski D, Anstey NM, Greenwood BM, McMichael AJ. HLA class I typing by PCR: HLA-B27 and an African subtype. Lancet. 1991;337:640–642. doi: 10.1016/0140-6736(91)92452-8. [DOI] [PubMed] [Google Scholar]
- Lopez-Larrea C, Sujirachato K, Mehr N, Chiewsilp P, Isarangkura D, Kanga U, Dominguez O, Coto E, Pena M, Setien F, Gonzalez-Roces S. HLA-B27 subtypes in Asian patients with ankylosing spondylitis. Evidence for new associations. Tissue Antigens. 1995;45:169–176. doi: 10.1111/j.1399-0039.1995.tb02436.x. [DOI] [PubMed] [Google Scholar]
- Khan MA. HLA-B27 polymorphism and association with disease [editorial] [see comments]. J Rheumatol. 2000;27:1110–1114. [PubMed] [Google Scholar]
- Nixon DF, Townsend ARM, Elvin JG, Rizza CR, Gallwey J, McMichael AJ. HIV-1 gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature. 1988;336:484–487. doi: 10.1038/336484a0. [DOI] [PubMed] [Google Scholar]
- Huet S, Nixon DF, Rothbard J, Townsend ARM, Ellis SA, McMichael AJ. Structural homologies between two HLA B27 restricted peptides suggest residues important for interaction with HLA B27. Int Immunol. 1990;2:311–316. doi: 10.1093/intimm/2.4.311. [DOI] [PubMed] [Google Scholar]
- Goulder PJ, Phillips RE, Colbert RA, McAdam S, Ogg G, Nowak MA, Giangrande P, Luzzi G, Morgan B, Edwards A, McMichael AJ, Rowland JS. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- Bowness P, Moss PAH, Rowland-Jones SL, Bell JI, McMichael AJ. Conservation of T-cell receptor usage by HLA B27-restricted influenza-specific cytotoxic T-lymphocytes suggests a general pattern for antigen-specific major histocompatibility complex class-i-restricted responses. Eur J Immunol. 1993;23:1417–1421. doi: 10.1002/eji.1830230702. [DOI] [PubMed] [Google Scholar]
- Allen RL, Gillespie GM, Hall F, Edmonds S, Hall MA, Wordsworth BP, McMichael AJ, Bowness P. Multiple T cell expansions are found in the blood and synovial fluid of patients with reactive arthritis. J Rheumatol. 1997;24:1750–1757. [PubMed] [Google Scholar]
- Altman JD, Moss P, Goulder P, Barouch DH, McHeyzer WM, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- Sun MY, Bowness P. MHC class I multimers. Arthritis Res. 2001;3:265–269. doi: 10.1186/ar315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowness P, Allen RL, Barclay DN, Jones EY, McMichael AJ. Importance of a conserved TCR J alpha-encoded tyrosine for T cell recognition of an HLA B27/peptide complex. Eur J Immunol. 1998;28:2704–2713. doi: 10.1002/(SICI)1521-4141(199809)28:09<2704::AID-IMMU2704>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Kuon W, Holzhutter HG, Appel H, Grolms M, Kollnberger S, Traeder A, Henklein P, Weiss E, Thiel A, Lauster R, Bowness P, Radbruch A, Kloetzel PM, Sieper J. Identification of HLA-B27-restricted peptides from the Chlamydia trachomatis proteome with possible relevance to HLA-B27-associated diseases. J Immunol. 2001;167:4738–4746. doi: 10.4049/jimmunol.167.8.4738. [DOI] [PubMed] [Google Scholar]
- Benjamin RJ, Madrigal JA, Parham P. Peptide binding to empty HLA-B27 molecules of viable human cells. Nature. 1991;351:74–77. doi: 10.1038/351074a0. [DOI] [PubMed] [Google Scholar]
- Urban RG, Chicz RM, Lane WS, Strominger JL, Rehm A, Kenter MJH, Uytdehaag FGCM, Ploegh H, Uchanska ZB, Ziegler A. A subset of HLA-B27 molecules contains peptides much longer than nonamers. Proc Natl Acad Sci USA. 1994;91:1534–1538. doi: 10.1073/pnas.91.4.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RL, O'Callaghan CA, McMichael AJ, Bowness P. Cutting edge: HLA-B27 can form a novel beta 2-microglobulin-free heavy chain homodimer structure. J Immunol. 1999;162:5045–5048. [PubMed] [Google Scholar]
- Mear JP, Schreiber KL, Munz C, Zhu X, Stevanovic S, Rammensee HG, Rowland-Jones SL, Colbert RA. Misfolding of HLA-B27 as a result of its B pocket suggests a novel mechanism for its role in susceptibility to spondyloarthropathies. J Immunol. 1999;163:6665–6670. [PubMed] [Google Scholar]
- Young NT, Uhrberg M, Phillips JH, Lanier LL, Parham P. Differential expression of leukocyte receptor complex-encoded Ig-like receptors correlates with the transition from effector to memory CTL. J Immunol. 2001;166:3933–3941. doi: 10.4049/jimmunol.166.6.3933. [DOI] [PubMed] [Google Scholar]
- Boyle LH, Goodall JC, Opat SS, Gaston JS. The recognition of HLA-B27 by human CD4(+) T lymphocytes. J Immunol. 2001;167:2619–2624. doi: 10.4049/jimmunol.167.5.2619. [DOI] [PubMed] [Google Scholar]