Abstract
Chapter summary
Angiogenesis is a prominent feature of rheumatoid synovitis. Formation of new blood vessels permits a supply of nutrients and oxygen to the augmented inflammatory cell mass and so contributes to perpetuation of joint disease. Vascular endothelial growth factor (VEGF) is a potent endothelial cell-specific growth factor that is upregulated by proinflammatory cytokines and by hypoxia. Serum VEGF concentrations are elevated in rheumatoid arthritis (RA) and correlate with disease activity. Furthermore, serum VEGF measured at first presentation in RA is highly significantly correlated with radiographic progression of disease over the subsequent year. Power Doppler ultrasonography is a sensitive method for demonstrating the presence of blood flow in small vessels and there is a very close relation between the presence or absence of vascular flow signal on power Doppler imaging and the rate of early synovial enhancement on dynamic gadolinium-enhanced magnetic resonance imaging (MRI) of joints with RA. Images obtained by both dynamic enhanced MRI and power Doppler ultrasonography correlate with vascularity of synovial tissue as assessed histologically. In early RA, there is a striking association between joint erosions assessed on high-resolution ultrasonography and vascular signal in power Doppler mode. Collectively, these findings implicate vascular pannus in the erosive phase of disease and strongly suggest that proangiogenic molecules such as VEGF are targets for novel therapies in RA. Animal model data supports this concept. It seems likely that serological and imaging measures of vascularity in RA will become useful tools in the assessment of disease activity and response to therapy.
Keywords: angiogenesis, magnetic resonance imaging, power Doppler ultrasonography, rheumatoid arthritis, VEGF
Introduction
Rheumatoid arthritis (RA) is a common human disease, with a prevalence of about 1%. The clinical presentation is heterogeneous, with a wide spectrum of age of onset, degree of joint involvement, and severity. Up to 90% of patients with aggressive synovitis have radiologic evidence of bone erosion within 2 years of diagnosis, despite treatment [1]. However, at the onset of symptoms, it is difficult to predict which patients will follow a more severe disease course. It is now highly desirable to identify such patients at an early stage in their disease evolution, because of the advent of biologic agents that have the potential not only to significantly improve symptoms and signs of disease in a high proportion of patients [2], but also to arrest structural damage to joints [3]. At present, the economic burden of these new therapies is such that rationing of some sort is inevitable and it is therefore equally desirable to determine whether those patients receiving biologic therapies are responding adequately at an early stage of their treatment.
In health, angiogenesis – or growth of new blood vessels from pre-existing vasculature – occurs during growth and the female reproductive cycle. It is also a feature of tissue repair after injury and contributes to the pathogenesis of a number of disease states, including cancer, chronic gingivitis, diabetic retinopathy, and RA. Angiogenesis occurs as a coordinated process comprising proliferation and migration of endothelial cells, followed by the formation of capillary tubes, deposition of basement membrane, and proliferation and migration of pericytes and smooth muscle cells. Anastomoses are created and flow of blood is established. Vascular reorganisation follows, in a process requiring the regression of redundant vessels by apoptosis of endothelial cells [4]. In order to match function of the microvascular bed to local metabolic demand, the developing vessels begin to express vasoactive peptides and their receptors [5].
Angiogenesis in the synovial membrane of patients with RA is considered by many investigators to be an important early step in pathogenesis of RA and in the perpetuation of disease [5,6]. This chapter focuses on recent research findings arising from serologic and imaging measures of vascularity in RA. I discuss the role of vascular endothelial growth factor (VEGF), a marker of angiogenesis, in the pathophysiology of RA, and emerging evidence that serum VEGF concentrations correlate with disease activity and fall when synovitis is successfully suppressed by therapy. I will also discuss the potential of angiogenic markers, such as VEGF, to predict disease outcome. I review developments in imaging technologies that permit assessment of synovial vascularity and discuss their potential application in the evaluation of RA disease activity, prediction of disease progression, and monitoring of response to therapy. I examine the implications of these findings for our understanding of disease pathogenesis and consider the potential of VEGF and angiogenesis as a target for therapy.
Historical background: the role of VEGF in RA
Angiogenesis, regulated by a complex set of inducers and inhibitors, arises when hypoxic, diseased, or injured tissues secrete proangiogenic molecules. Many endothelial growth factors have been demonstrated in RA synovium [6-8] and tenosynovium [9]. Of these, VEGF is the most endothelial-cell-specific factor characterised to date [10-12]. It also induces vascular permeability [13]. In RA synovium, VEGF expression is upregulated in macrophages and fibroblasts [14,15] and we have demonstrated protein expression in synovial endothelial cells by immunohistochemistry [16] (Fig. 1). Co-culture of activated monocytes or neutrophils from RA synovial fluids with unstimulated, semiconfluent RA fibroblast-like synoviocytes results in synergistic increases in the expression and secretion of bioactive VEGF [17]. Furthermore, this induction of VEGF is significantly inhibited by anti-integrin antibodies, a finding implying that VEGF expression in RA synovitis is regulated not only by proinflammatory cytokines, but also by the physical interaction of activated leukocytes and fibroblast-like synoviocytes [17].
Figure 1.
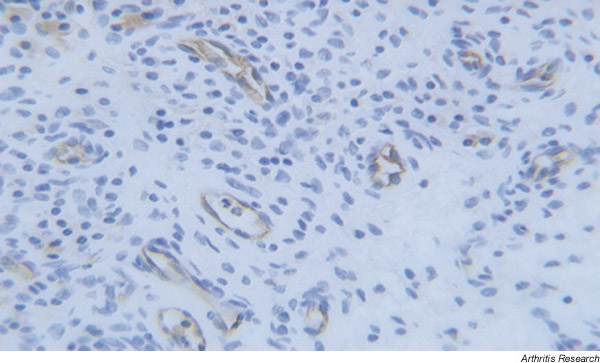
Staining of rheumatoid synovium for vascular endothelial growth factor, showing expression of the factor in endothelial cells.
VEGF exists as several isoforms, generated by alternative splicing of VEGF mRNA. RT-PCR analysis shows the VEGF(121) isoform to be expressed constitutively in RA synovial tissues, whereas the VEGF(165) isoform is expressed in less than half the tissues examined [18,19]. Microvascular density is significantly higher in RA synovial tissues expressing VEGF(165) [18]. The proportion of microvessels expressing CD31 that are activated, as assessed by a monoclonal antibody recognising the VEGF/KDR receptor complex, is significantly greater in RA than in osteoarthritis or normal synovial tissue [20]. VEGF receptor Flt-1 (VEGFR-1) protein is expressed on microvessels in close proximity to VEGF protein [19] and there is a close correlation between expression of isoform VEGF(165) and that of its receptors KDR (VEGFR-2) and neuropilin [18].
In the case of inflammatory tissue, several interdependent processes promote angiogenesis. Shear stress on the endothelial wall as a result of increased blood flow may enhance angiogenesis, as may extravasated plasma proteins such as fibrinogen products. Similarly, inflammatory cells – including macrophages, lymphocytes, mast cells, and fibroblasts – and their proangiogenic soluble products promote angiogenesis. Many cytokines promoting inflammation, such as tumour necrosis factor (TNF)-α, IL-1, and IL-8 also have proangiogenic activity. Hypoxia, which is often a feature of inflammation, is a potent inducer of VEGF. Furthermore, hypoxic culture conditions greatly augment the rate of VEGF secretion from cultured synovial fibroblasts stimulated by IL-1 and transforming growth factor (TGF)-β [21]. Direct measurements confirm that the RA joint is a hypoxic environment [22]. Contributory factors include the high metabolic demands of inflamed synovial tissue and the rapid rate of synovial proliferation, such that cells become more distant from the closest blood vessels, compounding the hypoxic state [23]. Tissue hypoxia in the rheumatoid joint results in increased VEGF mRNA stability [24] and enhances VEGF gene transcription through the binding of hypoxia-inducible transcription factors such as HIF-1 and HIF-2. Both of these factors are degraded within minutes of exposure to an oxygen tension >3–5% but are stabilised under conditions of hypoxia (<3% oxygen), then translocated to the nucleus, where they bind to hypoxia-responsive elements on hypoxia-inducible genes to upregulate their expression [25]. Thus, it is possible that the hypoxic environment in the rheumatoid joint, compounded by the high metabolic demands of synovial inflammation, promotes transcriptional changes permissive to perpetuation of synovitis.
Measuring VEGF in RA: relation to disease activity and response to therapy
VEGF is detectable in serum, synovial tissue, and fluids of patients with RA [14,15,26-30]. Human neutrophils secrete VEGF [31], and Kasama et al. report that concentrations of neutrophil-associated VEGF in RA synovial fluids correlate well with free VEGF in joint effusions and with the patient's disease activity [32]. Other investigators find no correlation between VEGF concentrations in serum and synovial fluids from the same RA patients [33]. Synovial fluid VEGF concentrations correlate with matrix metalloproteinase (MMP)-9 concentrations in the fluid in early inflammatory arthritis [34].
The source of VEGF in the serum is unclear. In vitro, human peripheral blood mononuclear cells have been shown to release VEGF in response to cytokines expressed in RA synovium, such as TNF-α [29]. Release of VEGF from platelets has also been reported [35]. Serum VEGF might therefore be derived from platelets, synovial-fluid neutrophils, inflamed synovial tissue, or other sources.
A number of groups have reported that VEGF concentrations are higher in the serum of RA patients than in healthy controls or patients with osteoarthritis [27,33,35-37]. Furthermore, serum VEGF concentrations correlate with individual and composite measures of RA disease activity, including acute phase markers and counts of swollen and tender joints [33,36,37]. In a study of patients attending my early clinics for inflammatory arthritis and established RA, serum VEGF concentrations were higher in patients with early RA than in patients with long-standing, treated RA [36]. This observation may represent a response to therapy, a view supported by studies in which serum VEGF concentrations have been measured before and after commencement of therapy [27,35,36]. In our series, a total of 27 early RA patients responsive to DMARD (disease-modifying antirheumatic drug) therapy exhibited a significant reduction in serum VEGF concentrations, in contrast to patients unresponsive to DMARD treatment, who showed no such significant change [36]. Treatment of RA patients with anti-TNF-α therapy results in marked reduction, but not normalisation, of serum VEGF concentrations [38]. The correlation of this reduction with changes in clinical and laboratory measures of disease activity suggests that the diminished vascular permeability consequent upon reduced VEGF concentrations contributes to the rapid decrease in counts of swollen joints observed after TNF blockade.
The significant reductions in serum VEGF concentrations following response to therapy in RA suggest that angiogenesis is an important process in perpetuating synovitis and raises the possibility that persistence of joint inflammation indicates an imbalance between inducers and inhibitors of angiogenesis. This concept is supported by the observation that concentrations of endostatin, an angiogenesis inhibitor, are not elevated in serum and synovial fluid samples from patients in whom serum VEGF is elevated [35]. Another naturally occurring inhibitor of angiogenesis is soluble Flt-1, a VEGF antagonist. Soluble Flt-1 (sFlt-1) is an alternatively spliced form of Flt-1, one of the tyrosine kinase receptors that mediate the action of VEGF [12,39,40]. We found that sFlt-1 is higher in groups with both early and long-standing RA than in controls, and that elevated sFlt-1 concentrations correlate with VEGF concentrations in serum of the same RA patients [36]. The raised concentrations of sFlt-1 observed in RA are presumably insufficient to inhibit VEGF activity. This finding is analogous to reports in RA of elevated concentrations of other proinflammatory cytokines and their naturally occurring inhibitors.
Can angiogenic markers predict disease outcome?
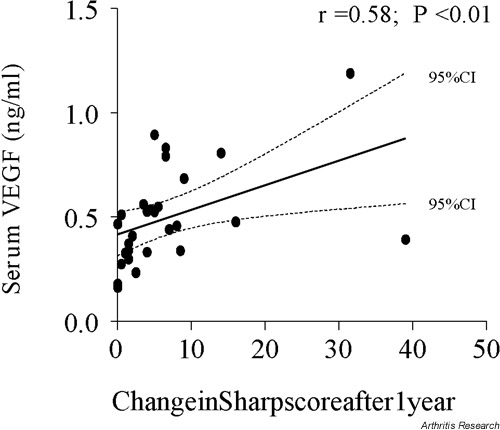
My colleagues and I have recently reported that serum VEGF concentrations at presentation with early RA correlate highly significantly with development of radiographic damage over the subsequent year [36] as assessed in radiographs of the hands and feet by the van der Heijde modification of Sharp's method [41] (Fig. 2). We have also observed that RA patients with persistent disease activity despite conventional therapy have relatively high serum VEGF concentrations at first presentation. If confirmed in larger series, these observations would support the case for early introduction of a more aggressive therapeutic regime in patients with highly elevated serum VEGF concentrations early in their disease course. It might also be speculated that, in the future, serial serum measurements of angiogenic markers may help to determine whether vascular pannus, with its potential to cause cartilage and bone destruction, is adequately suppressed at any given stage of disease evolution.
Figure 2.
Relation between serum VEGF concentrations, measured at first presentation in a cohort of patients with early RA, and joint damage over the following year, expressed as change in the van der Heijde modification of the Sharp score. Original data reported in [36]. VEGF, vascular endothelial growth factor.
Techniques for imaging synovial vasculature in RA
Blood flows at high velocity in large vessels and can be readily detected by conventional colour Doppler sonography, which encodes the mean Doppler frequency shift. However, at the microvascular level, which is of interest with respect to rheumatoid synovitis, blood flow is at lower velocities that are less readily detectable by conventional colour Doppler sonography. Power Doppler sonography, on the other hand, encodes the amplitude of the power spectral density of the Doppler signal and is a sensitive method for demonstrating the presence of blood flow in small vessels. Several recent studies have demonstrated that power Doppler ultrasound is capable of detecting inflammatory hyperaemia in RA synovitis [42-46]. Furthermore, the signal intensity on power Doppler imaging of synovitis in rheumatoid knee joints correlates well with the histologic assessment of synovial membrane microvascular density in tissue taken at arthroplasty from the previously imaged site [45]. Quantitative assessment of vascularised synovium in metacarpophalangeal joints of patients with RA, as demonstrated by power Doppler, has been reported to correlate with ESR [47]. However, in this cohort of RA patients in the established phase of disease (mean 7.6 years), there was no preponderance of blood flow near joint erosions. In contrast, in a cohort of 39 patients with early RA (disease duration <3 years), we have observed a striking association between the presence of bone erosion in metacarpophalangeal joints and increased intra-articular blood flow at the site of bone damage [48] (Fig. 3). This finding is consistent with the hypothesis that vascularity is associated with the active phase of erosive disease. Laser Doppler imaging of metacarpophalangeal joints in RA has also been reported to correlate with pain scores and synovitis detected on grey-scale high-resolution ultrasound [49]. As disease in many patients with RA continues to progress in spite of suppression of clinically evident synovitis, these noninvasive tools will enhance our ability to detect active vascularised synovium and may also therefore influence the use of DMARD and biologic therapies in such patients.
Figure 3.
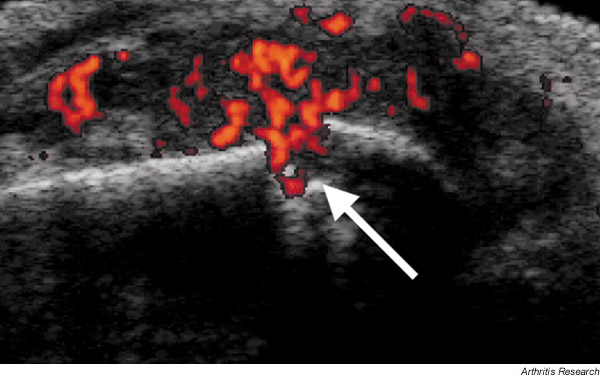
Combined high-resolution ultrasound and power Doppler imaging of a metacarpophalangeal joint in RA, seen in longitudinal section. The red colouring represents vascular signal and the arrow indicates a vascularised erosion.
We have investigated the capability of power Doppler and high-resolution ultrasound imaging to discriminate between patients receiving infliximab or placebo infusions added to pre-existing methotrexate treatment over the first 18 weeks of therapy in a clinical trial [50]. Median reduction in synovial thickness as assessed by high-resolution ultrasound was 50% in the infliximab group, versus an increase of 1.2% in the placebo group (P = 0.014). Similarly, median color Doppler area diminished by 98.4% in the infliximab group, versus a reduction of only 30.7% in the placebo group, a statistically significant difference (P = 0.017). The total number of vascularised erosions decreased by a median of 1.0 in the infliximab group, whereas there was no change from baseline in the placebo group (P = 0.001). In this study, ultrasonographic measures of joint vascularity were better able to discriminate between patients receiving infliximab and those receiving placebo infusions than were changes in DAS28, a composite measure of disease activity.
It has recently been shown that there is a very close relation between the presence or absence of vascular flow signal on power Doppler imaging and the rate of early synovial enhancement on dynamic gadolinium-enhanced magnetic resonance imaging (MRI) of RA metacarpophalangeal joints [51]. The ability to distinguish between RA joint effusion and synovial proliferation using MRI has been greatly improved by the introduction of paramagnetic contrast agents. The early, post-gadolinium synovial membrane enhancement in RA joints, determined by dynamic MRI, is considered to reflect synovial perfusion and permeability [52-54]. Histopathologic analyses of synovial tissue samples obtained from arthrotomy or arthroscopic or blind biopsies from knee joints after dynamic MRI imaging indicate that vascular density and blood-vessel fractional area correlate significantly with the early enhancement rate of synovial membrane [54,55]. Dynamic MRI with a gadolinium-based blood-pool agent has been employed as a technique for measurement of abnormal capillary permeability in synovial tissue of arthritic knees in rabbits [56]. MRI-derived microvascular characteristics comprising plasma volume, fractional leak rate, and permeability-surface area product correlated positively with histologic findings.
A novel approach to detecting angiogenesis in vivo using MRI has been described in a tumour model in rabbits. This technique, using a paramagnetic contrast agent targeted to endothelial αVβ3, an integrin expressed on new blood vessels, by linkage to a monoclonal antibody, successfully provided enhanced and detailed images of rabbit carcinomas and, furthermore, disclosed angiogenic 'hot spots' not visible by standard MRI [57].
Another approach to imaging RA synovial vasculature, which has the disadvantage, relative to the previously described methodologies, that it exposes patients to ionis-ing radiation, is the use of radiolabelled peptides that bind endothelium. For example, a radiolabelled F(ab)2 (antigen-binding fragment) of a monoclonal antibody that recognises an epitope of E-selectin has been successfully used to image RA synovium [58] and a radiolabelled E-selectin binding peptide has been used to image activated synovial endothelium in rat adjuvant arthritis [59]. This is a particularly attractive endothelial adhesion molecule to target, because it is not constitutively expressed but instead is synthesised de novo in response to proinflammatory cytokines and expressed on the luminal surface of the endothelium, where it is readily accessible to circulating radioligands [60]. Furthermore, the monoclonal antibody is internalised as a result of receptor recycling, with very little shedding into the general circulation. Nonetheless, positive images of activated endothelium obtained with specific proteins and peptides will inevitably include a component due to nonspecific uptake [61]. Neovascular antigens in inflammatory tissue are other potentially useful targets. Although neovascularisation may be nonspecifically imaged with technetium-labelled isonitriles [62], targeting epitopes associated with αV integrins, which are specific to new blood vessels [63,64], is a more attractive goal.
Relevance to the understanding of pathogenesis
An inflamed synovium is central to the pathophysiology of RA. Histologically, RA synovitis is characterised by a mononuclear cell infiltrate and luxuriant vasculature [65]. Furthermore, the disease activity in a given joint is correlated with the synovial vascularisation [66,67]. Angiogenesis can be evident on microscopic examination of synovial biopsies from the earliest stages of disease evolution [68] and is observed as a fine network of vessels over the rheumatoid synovium at arthroscopic inspection of RA joints. Angiogenesis is integral to the development of inflammatory pannus, and without it, leukocyte ingress could not occur. Furthermore, formation of new blood vessels permits a supply of nutrients and oxygen to the augmented inflammatory cell mass and so contributes to the perpetuation of synovitis. Studies in experimental models of arthritis suggest that destruction of bone and cartilage may be more closely linked to angiogenesis than to pannus swelling [69,70]. In patients with early RA, my colleagues and I have observed a striking association between the presence of bone erosion on high-resolution ultrasound imaging of metacarpophalangeal joints and increased intra-articular blood flow in power Doppler mode imaging at the site of bone damage [48] These findings implicate vascular pannus in joint destruction in early RA and strongly support the contention that angiogenesis is a potential target for therapy in this disease.
VEGF and angiogenesis as a target for therapy
It is interesting to note that many of the anti-rheumatic drugs used in clinical practice have an effect on the vasculature. Anti-rheumatic drugs including gold, bucillamine, methotrexate, and corticosteroids inhibit production of VEGF by cultured synoviocytes [35]. Methotrexate, at concentrations equivalent to those attained in the serum of RA patients treated with this drug, inhibits basal and stimulated endothelial proliferation in vitro[71]. Thiol-containing and gold compounds may modulate neovascularisation indirectly by inhibiting production of monocyte/macrophage-derived angiogenic factors [72,73]. Cyclosporin A is known to inhibit activity of transcription factors of the nuclear factor of activated T-cells family. In addition, cyclosporin A has recently been shown to inhibit migration of primary endothelial cells and angiogenesis induced by VEGF, a novel mechanism that may account for some of the therapeutic activity of this drug in RA [74]. The effect appears to be mediated through inhibition of cyclooxygenase-2, the transcription of which is activated by VEGF in primary endothelial cells. Diminished vascular permeability consequent upon a reduction in VEGF is thought to be a factor contributing to the rapid reduction of joint swelling observed after anti-TNFα therapy [38]. We have shown that TNF blockade is also accompanied by reduced synovial angiogenesis as assessed by immunohistologic analysis of microvascular density and expression of αVβ3 integrin in synovial biopsy tissue taken before and 4 weeks after a single 10-mg/kg infliximab infusion [16].
Given the evidence discussed so far, there are strong grounds on which to suggest that targeting the newly formed vasculature of the RA pannus might modify disease progression. Identification of new molecular targets for immunotherapeutic intervention in RA ideally requires evidence of synovial tissue expression of the molecule at relevant sites, in vitro evaluation of its biologic function, and preclinical studies demonstrating clinical efficacy in experimental models [75]. I have already discussed the first two of these three requirements in considering angiogenesis, and molecules that promote it such as VEGF, as targets for therapy in RA. However, the third requirement, demonstration of clinical efficacy in experimental models, would perhaps represent the most convincing evidence in support of anti-angiogenic strategies in the treatment of human disease.
In collagen-induced arthritis in rats, administration of the anti-angiogenic agent AGM-1470 attenuates the clinical severity of established disease, with reduction in serum concentrations of VEGF [76] and histologic evidence of marked inhibition of pannus formation and neovascularisa-tion [77,78]. In murine collagen-induced arthritis, expression of biologically active VEGF was along a time course that paralleled the expression of VEGFR-1 and VEGFR-2 [79]. Furthermore, in this model, concentrations of VEGF expression correlated with clinical severity of disease and degree of neovascularisation. Administration of anti-VEGF antiserum before the onset of disease significantly delayed the development and severity of arthritis, whereas administration after disease onset had no effect on the progression or ultimate severity of the disease [79]. In another study employing the murine collagen-induced arthritis model, saline, normal rabbit immunoglobulin, or rabbit polyclonal anti-human VEGF(121) antibodies were administered to mice either before or after the onset of clinical disease [80]. Treatment with anti-VEGF antibodies before disease onset significantly delayed the development of arthritis and attenuated the severity of disease expression, although the frequency of the occurrence of disease did not differ from that in either control group. In this study, improvement in clinical and histological parameters of arthritis was observed even when anti-VEGF was administered after the onset of disease. Another approach to VEGF blockade in murine collagen-induced arthritis has been to administer exogenous sFlt-1 linked to polyethylene glycol (sFlt-PEG) to increase its in vivo half-life. In this model, daily intraperitoneal administration of this treatment from the first day of clinical arthritis was reported to ameliorate the severity of clinical disease expression and of bone and cartilage destruction on histopathological assessment [81].
Other anti-angiogenic agents successfully used in preclinical animal studies include the fungal derivative TNP-470 and specific antagonists of αVβ3. In a transgenic rodent model of inflammatory arthritis, administration of TNP-470 attenuated development of arthritis and alleviated clinical signs if administered at the early stages of clinical disease. Clinical improvement was associated with reduced cartilage and bone destruction [82]. There are preclinical in vivo data showing that selective targeting of new blood vessels may result in modification of disease. Intra-articular injections of specific antagonists of αVβ3 resulted in reduced synovial vascularity in rabbits after induction of arthritis with joint injections of a combination of ovalbumin and basic fibroblast growth factor. Diminished synovial vascularity was associated with a significant decrease in all arthritic parameters, including joint swelling and synovitis. Of note, the beneficial effects of angiogenesis inhibition were apparent in the established phase of disease [70]. Similarly, symptoms of adjuvant-induced arthritis in rats are significantly reduced by prophylactic or therapeutic administration of an orally active αVβ3 antagonist, with significant improvements in joint integrity seen on MRI [83].
What does the future hold in angiogenesis modulation and imaging of vasculature?
Given the central role played by angiogenesis and VEGF in arthritic disease, it might be predicted that suppression of angiogenesis and/or VEGF activity will be an effective treatment strategy in RA. However, it is possible that such an approach will be limited by toxicities related to inhibition of physiological processes involving VEGF-mediated angiogenesis. Examples include wound healing, the female reproductive cycle, and maintenance of cardiovascular health. Data arising from ongoing clinical trials of anti-angiogenic agents in the treatment of cancers should give valuable insight as to whether these theoretical concerns are well founded or not.
Studies evaluating new imaging technologies suggest that clinical joint examination and plain radiography are relatively insensitive [84] and may thus be insufficient measures for use in future clinical trials designed to assess reduction in symptoms and signs of rheumatoid disease as well as retardation of structural damage to joints. In a double-blind study in early RA in which patients received either infliximab or placebo infusions added to pre-existing, stable methotrexate therapy, my colleagues and I have recently shown that measurement of changes in serum VEGF concentration and ultrasonographic assessments of changes in synovial vascularity are able to discriminate between patients receiving either infliximab or placebo infusions [50]. We have also reported that the serum VEGF concentration at first presentation in early RA significantly correlates with radiological progression over the following year [36]. These findings predict that imaging technologies capable of evaluating synovial vascularity will have a practical value in determining prognosis and in assessment of response to therapy. Of the technologies discussed in this chapter, ultrasonographic imaging methods have the advantage that they are noninvasive, involve no ionising radiation, and are both more widely available and more economical than MRI. Furthermore, rheumatologists can be trained to undertake ultrasonographic imaging, thus obviating the delay incurred in referral to a radiologist.
Concluding remarks
VEGF concentrations are elevated in the serum of patients with RA and correlate with individual and composite measures of the disease activity. Sensitive, noninvasive methods for visualising synovial vascularisation in RA, such as power Doppler sonography, are emerging as clinically important tools in the assessment of disease activity and hold promise as novel means of evaluating the response of patients to therapy. As arresting structural damage to joints becomes a realistic goal in the management of RA, vascular imaging and serologic markers may be more sensitive than disease activity scores in determining at an early stage of treatment which patients are responding satisfactorily.
Glossary of terms
Flt-1 = fms-like tyrosine kinase receptor-1, or VEGF receptor-1 (VEGFR-1); KDR = kinase-insert-domain-containing receptor, or VEGF receptor-2 (VEGFR-2); sFlt-1 = soluble Flt-1; VEGFR = vascular endothelial growth factor receptor.
London, UK. 24-26 June 2002
References
- Sharp JT, Wolfe F, Mitchell DM, Bloch DA. The progression of erosion and joint space narrowing scores in rheumatoid arthritis during the first twenty-five years of disease. Arthritis Rheum. 1991;34:660–668. doi: 10.1002/art.1780340606. [DOI] [PubMed] [Google Scholar]
- Maini RN, Taylor PC. Anti-cytokine therapy in rheumatoid arthritis. Ann Rev Med. 2000;51:207–229. doi: 10.1146/annurev.med.51.1.207. [DOI] [PubMed] [Google Scholar]
- Taylor PC. Anti-TNF therapies. Curr Opin Rheumatol. 2001;13:164–169. doi: 10.1097/00002281-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granuloma tissue and scar. Am J Pathol. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- Walsh DA. Angiogenesis and arthritis. Rheumatology (Oxford) 1999;38:103–112. doi: 10.1093/rheumatology/38.2.103. [DOI] [PubMed] [Google Scholar]
- Koch A. Angiogenesis: implications for rheumatoid arthritis. Arthritis Rheum. 1998;41:951–962. doi: 10.1002/1529-0131(199806)41:6<951::AID-ART2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Paleolog EM, Fava RA. Angiogenesis in rheumatoid arthritis: implications for future therapeutic strategies. Springer Semin Immunopathol. 1998;20:73–94. doi: 10.1007/BF00832000. [DOI] [PubMed] [Google Scholar]
- Paleolog EM, Miotla JM. Angiogenesis in arthritis: role in disease pathogenesis and as a potential therapeutic target. Angiogenesis. 1998;2:295–307. doi: 10.1023/A:1009229508096. [DOI] [PubMed] [Google Scholar]
- Jain A, Nanchahal J, Troeberg L, Green P, Brennan F. Production of cytokines, vascular endothelial growth factor, matrix metalloproteinases, and tissue inhibitor of metalloproteinases 1 by tenosynovium demonstrates its potential for tendon destruction in rheumatoid arthritis. Arthritis Rheum. 2001;44:1754–1760. doi: 10.1002/1529-0131(200108)44:8<1754::AID-ART310>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Brown LF, Detmar M, Claffey K, Nagy JA, Feng D, Dvorak AM, Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a multifunctional angiogenic cytokine. EXS. 1997;79:233–269. doi: 10.1007/978-3-0348-9006-9_10. [DOI] [PubMed] [Google Scholar]
- Achen MG, Stacker SA. The vascular endothelial growth factor family; proteins which guide the development of the vasculature. Int J Exp Pathol. 1998;79:255–265. doi: 10.1046/j.1365-2613.1998.700404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- Ferrara N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat. 1995;36:127–137. doi: 10.1007/BF00666035. [DOI] [PubMed] [Google Scholar]
- Fava RA, Olsen NJ, Spencer-Green G, Yeo KT, Yeo TK, Berse B, Jackman RW, Senger DR, Dvorak HF, Brown LF. Vascular permeability factor/endothelial growth factor (VPF/VEGF): accumulation and expression in human synovial fluids and rheumatoid synovial tissue. J Exp Med. 1994;180:341–346. doi: 10.1084/jem.180.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch AE, Harlow LA, Haines GK, Amento EP, Unemori EN, Wong WL, Pope RM, Ferrara N. Vascular endothelial growth factor. A cytokine modulating endothelial function in rheumatoid arthritis. J Immunol. 1994;152:4149–4156. [PubMed] [Google Scholar]
- Taylor PC, Patel S, Paleolog E, McCloskey RV, Feldmann M, Maini RN. Reduced synovial vascularity following TNFα blockade in rheumatoid arthritis [abstract]. Arthritis Rheum. 1998;41(suppl):S295. [Google Scholar]
- Kasama T, Shiozawa F, Kobayashi K, Yajima N, Hanyuda M, Takeuchi HT, Mori Y, Negishi M, Ide H, Adachi M. Vascular endothelial growth factor expression by activated synovial leukocytes in rheumatoid arthritis: critical involvement of the interaction with synovial fibroblasts. Arthritis Rheum. 2001;44:2512–2524. doi: 10.1002/1529-0131(200111)44:11<2512::AID-ART431>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Hosoda Y, Hirose S, Okada Y, Ikeda E. Expression of vascular endothelial growth factor isoforms and their receptors Flt-1, KDR, and neuropilin-1 in synovial tissues of rheumatoid arthritis. J Pathol. 2000;191:426–433. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH649>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Pufe T, Petersen W, Tillmann B, Mentlein R. Splice variants VEGF121 and VEGF165 of the angiogenic peptide vascular endothelial cell growth factor are expressed in the synovial tissue of patients with rheumatoid arthritis. J Rheumatol. 2001;28:1482–1485. [PubMed] [Google Scholar]
- Giatromanolaki A, Sivridis E, Athanassou N, Zois E, Thorpe PE, Brekken RA, Gatter KC, Harris AL, Koukourakis IM, Koukourakis MI. The angiogenic pathway "vascular endothelial growth factor/flk-1(KDR)-receptor" in rheumatoid arthritis and osteoarthritis. J Pathol. 2001;194:101–108. doi: 10.1002/path.842. [DOI] [PubMed] [Google Scholar]
- Berse B, Hunt JA, Diegel RJ, Morganelli P, Yeo K, Brown F, Fava RA. Hypoxia augments cytokine (transforming growth factor-beta (TGF-beta) and IL-1-induced vascular endothelial growth factor secretion by human synovial fibroblasts. Clin Exp Immunol. 1999;115:176–182. doi: 10.1046/j.1365-2249.1999.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PC, Miotla JM, Etherington P, Winlove P, Young S, Paleolog E, Maini RN. VEGF release is associated with hypoxia in inflammatory arthritis [abstract]. Rheumatology (Oxford) 2000;40(suppl):S7. [PubMed] [Google Scholar]
- Stevens CR, Blake DR, Merry P, Revell PA, Levick JR. A comparative study by morphometry of the microvasculature in normal and rheumatoid synovium. Arthritis Rheum. 1991;34:1508–1513. doi: 10.1002/art.1780341206. [DOI] [PubMed] [Google Scholar]
- Richard DE, Berra E, Pouyssegur J. Angiogenesis: how a tumor adapts to hypoxia. Biochem Biophys Res Commun. 1999;266:718–722. doi: 10.1006/bbrc.1999.1889. [DOI] [PubMed] [Google Scholar]
- Wiesener MS, Turley H, Allen WE, Willam C, Eckardt KU, Talks KL, Wood SM, Gatter KC, Harris AL, Pugh CW, Ratcliffe PJ, Maxwell PH. Induction of endothelial PAS domain protein-1 by hypoxia: characterisation and comparison with hypoxia-inducible factor-1 alpha. Blood. 1998;92:2260–2268. [PubMed] [Google Scholar]
- Jackson JR, Minton JA, Ho ML, Wei N, Winkler JD. Expression of vascular endothelial growth factor in synovial fibroblasts is induced by hypoxia and and interleukin 1beta. J Rheumatol. 1997;24:1253–1259. [PubMed] [Google Scholar]
- Harada M, Mitsuyama K, Yoshida H, Sakisaka S, Taniguchi E, Kawaguchi T, Ariyoshi M, Saiki T, Sakamoto M, Nagata K, Sata M, Matsuo K, Tanikawa K. Vascular endothelial growth factor in patients with rheumatoid arthritis. Scand J Rheumatol. 1998;27:377–380. doi: 10.1080/03009749850154429. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Kubo M, Kadono T, Yazawa N, Ihn H, Tamaki K. Serum concentrations of vascular endothelial growth factor in collagen diseases. Br J Dermatol. 1998;139:1049–1051. doi: 10.1046/j.1365-2133.1998.02563.x. [DOI] [PubMed] [Google Scholar]
- Bottomley MJ, Webb NJ, Watson CJ, Holt PJ, Freemont AJ, Brenchley PE. Peripheral blood mononuclear cells from patients with rheumatoid arthritis spontaneously secrete vascular endothelial growth factor (VEGF): specific up-regulation by tumour necrosis factor-alpha (TNF-alpha) in synovial fluid. Clin Exp Immunol. 1999;117:171–176. doi: 10.1046/j.1365-2249.1999.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima M, Yoshino S, Ishiwata T, Asano G. Role of vascular endothelial growth factor in angiogenesis of rheumatoid arthritis. J Rheumatol. 1995;22:1624–1630. [PubMed] [Google Scholar]
- Taichman N, Young S, Cruchley A, Taylor PC, Paleolog E. Human neutrophils secrete vascular endothelial growth factor. J Leukocyte Biol. 1997;62:397–400. doi: 10.1002/jlb.62.3.397. [DOI] [PubMed] [Google Scholar]
- Kasama T, Kobayashi K, Yajima N, Shiozawa F, Yoda Y, Takeuchi HT, Mori Y, Negishi M, Ide H, Adachi M. Expression of vascular endothelial growth factor by synovial fluid neutrophils in rheumatoid arthritis (RA). Clin Exp Immunol. 2000;121:533–538. doi: 10.1046/j.1365-2249.2000.01272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Joo YS, Kim WU, Min DJ, Min JK, Park SH, Cho CS, Kim HY. Vascular endothelial growth factor levels in the serum and synovial fluid of patients with rheumatoid arthritis. Clin Exp Rheumatol. 2001;19:321–324. [PubMed] [Google Scholar]
- Fraser A, Fearon U, Reece R, Emery P, Veale DJ. Matrix metalloproteinase 9, apoptosis, and vascular morphology in early arthritis. Arthritis Rheum. 2001;44:2024–2028. doi: 10.1002/1529-0131(200109)44:9<2024::AID-ART351>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Nagashima M, Wauke K, Hirano D, Ishigami S, Aono H, Takai M, Sasano M, Yoshino S. Effects of combinations of anti-rheumatic drugs on the production of vascular endothelial growth factor and basic fibroblast growth factor in cultured synoviocytes and patients with rheumatoid arthritis. Rheuma-tology (Oxford) 2000;39:1255–1262. doi: 10.1093/rheumatology/39.11.1255. [DOI] [PubMed] [Google Scholar]
- Ballara S, Taylor PC, Reusch P, Marme D, Maini RN, Paleolog E. Serum vascular endothelial growth factor (VEGF) and soluble VEGF receptor in inflammatory arthritis. Arthritis Rheum. 2001;44:2055–2064. doi: 10.1002/1529-0131(200109)44:9<2055::AID-ART355>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Sone H, Sakauchi M, Takahashi A, Suzuki H, Inoue N, Iida K, Shimano H, Toyoshima H, Kawakami Y, Okuda Y, Matsuo K, Yamada N. Elevated levels of vascular endothelial growth factor in the sera of patients with rheumatoid arthritis correlation with disease activity. Life Sci. 2001;69:1861–1869. doi: 10.1016/S0024-3205(01)01264-4. [DOI] [PubMed] [Google Scholar]
- Paleolog EM, Young S, Stark AC, McCloskey RV, Feldmann M, Maini RN. Modulation of angiogenic vascular endothelial growth factor (VEGF) by TNFα and IL-1 in rheumatoid arthritis. Arthritis Rheum. 1998;41:1258–1265. doi: 10.1002/1529-0131(199807)41:7<1258::AID-ART17>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Kendall RL, Wang G, Thomas KA. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem Biophys Res Commun. 1996;226:324–328. doi: 10.1006/bbrc.1996.1355. [DOI] [PubMed] [Google Scholar]
- Hornig C, Weich HA. Soluble VEGF receptors. Recombinant and naturally occurring forms involved in the regulation of angiogenesis. Angiogenesis. 1999;3:33–39. doi: 10.1023/A:1009033017809. [DOI] [PubMed] [Google Scholar]
- van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol. 2000;27:261–263. [PubMed] [Google Scholar]
- Newman JS, Laing TJ, McCarthy CJ, Adler RS. Power Doppler sonography of synovitis: assessment of therapeutic response – preliminary observations. Radiology. 1996;198:582–584. doi: 10.1148/radiology.198.2.8596870. [DOI] [PubMed] [Google Scholar]
- Hau M, Schultz H, Tony HP, Keberle M, Jahns R, Haerten R, Jenett M. Evaluation of pannus and vascularization of the metacarpophalangeal and proximal interphalangeal joints in rheumatoid arthritis by high-resolution ultrasound (multidimensional linear array). Arthritis Rheum. 1999;42:2303–2308. doi: 10.1002/1529-0131(199911)42:11<2303::AID-ANR7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Schmidt WA, Volker L, Zacher J, Schlafke M, Ruhnke M, Gromnica-Ihle E. Colour Doppler ultrasonography to detect pannus in knee joint synovitis. Clin Exp Rheum. 2000;18:439–444. [PubMed] [Google Scholar]
- Walther M, Harms H, Krenn V, Radke S, Faehndrich TP, Gohlke F. Correlation of power Doppler sonography with vascularity of the synovial tissue of the knee joint in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2001;44:331–338. doi: 10.1002/1529-0131(200102)44:2<331::AID-ANR50>3.3.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Giovagnorio F, Martinoli C, Coari G. Power Doppler sonography in knee arthritis–a pilot study. Rheumatol Int. 2001;20:101–104. doi: 10.1007/s002960000082. [DOI] [PubMed] [Google Scholar]
- Qvistgaard E, Rogind H, Torp-Pedersen S, Terslev L, Danneskiold-Samsoe B, Bliddal H. Quantitative ultrasonography in rheumatoid arthritis: evaluation of inflammation by Doppler technique. Ann Rheum Dis. 2001;60:690–693. doi: 10.1136/ard.60.7.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuer A, Blomley M, Cosgrove D, Maini RN, Taylor PC. Combined power colour doppler and greyscale ultrasound demonstrate an association between vascularity and erosion in rheumatoid arthritis [abstract]. Arthritis Rheum. 2000;43(suppl):S292. [Google Scholar]
- Ferrel WR, Balint PV, Egan CG, Lockhart JC, Sturrock RD. Metacarpophalangeal joints in rheumatoid arthritis: laser Doppler imaging – initial experience. Radiology. 2001;220:257–262. doi: 10.1148/radiology.220.1.r01jl26257. [DOI] [PubMed] [Google Scholar]
- Taylor PC, Steuer A, Charles P, Gruber J, Cosgrove D, Blomley M, Wagner C, Marsters P, DeWoody K, Maini RN. Early RA patients on infliximab therapy show significant changes in sonographic measures of joint vascularity and serum VEGF by 18 weeks [abstract]. Arthritis Rheum. 2001;44(suppl):S152. [Google Scholar]
- Szkudlarek M, Court-Payen M, Strandberg C, Klarlund M, Klausen T, Ostergaard M. Power Doppler ultrasonongraphy for assessment of synovitis in the metacarpophalangeal joints of patients with rheumatoid arthritis; a comparison with dynamic magnetic resonance imaging. Arthritis Rheum. 2001;44:2018–2023. doi: 10.1002/1529-0131(200109)44:9<2018::AID-ART350>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Konig H, Sieper J, Wolf KJ. Rheumatoid arthritis: evaluation of hypervascular and fibrous pannus with dynamic MR imaging enhanced with Gd-DTPA. Radiology. 1990;176:473–477. doi: 10.1148/radiology.176.2.2367663. [DOI] [PubMed] [Google Scholar]
- Gaffney K, Cookson J, Blake D, Coumbe A, Blades S. Quantification of rheumatoid synovitis by magnetic resonance imaging. Arthritis Rheum. 1995;38:1610–1617. doi: 10.1002/art.1780381113. [DOI] [PubMed] [Google Scholar]
- Gaffney K, Cookson J, Blades S, Coumbe A, Blake D. Quantitative assessment of the rheumatoid synovial microvascular bed by gadolinium-DTPA enhanced magnetic resonance imaging. Ann Rheum Dis. 1998;57:152–157. doi: 10.1136/ard.57.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard M, Stoltenberg M, Lovgreen-Nielsen P, Volck B, Sonne-Holm S, Lorenzen I. Quantification of synovitis by MRI: correlation between dynamic and static gadolinium-enhanced magnetic resonance imaging and microscopic and macroscopic signs of synovial inflammation. Magn Reson Imaging. 1998;16:743–754. doi: 10.1016/S0730-725X(98)00008-3. [DOI] [PubMed] [Google Scholar]
- van Dijke CF, Peterfy CG, Brasch RC, Lang P, Roberts TP, Shames D, Kneeland JB, Lu Y, Mann JS, Kapila SD, Genant HK. MR imaging of the arthritic rabbit knee joint using albumin-(Gd-DTPA)30 with correlation to histopathology. Magn Reson Imaging. 1999;17:237–245. doi: 10.1016/S0730-725X(98)00167-2. [DOI] [PubMed] [Google Scholar]
- Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KC. Detection of tumor angiogenesis in vivo by alphaVbeta3-targeted magnetic resonance imaging. Nat Med. 1998;4:623–626. doi: 10.1038/nm0598-623. [DOI] [PubMed] [Google Scholar]
- Chapman PT, Jamar F, Keelan ETM, Peters AM, Haskard DO. Use of a radiolabeled monoclonal antibody against E-selectin for imaging of endothelial activation in rheumatoid arthritis. Arthritis Rheum. 1996;39:1371–1375. doi: 10.1002/art.1780390815. [DOI] [PubMed] [Google Scholar]
- Zinn KR, Chaudhuri TR, Smyth CA, Wu Q, Liu HG, Fleck M, Mountz JD, Mountz JM. Specific targeting of activated endothelium in rat adjuvant arthritis with a 99mTc-radiolabeled E-selectin-binding peptide. Arthritis Rheum. 1999;42:641–649. doi: 10.1002/1529-0131(199904)42:4<641::AID-ANR6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Keelan ET, Licence ST, Peters AM, Binns RM, Haskard DO. Characterization of E-selectin expression in vivo with use of a radiolabeled monoclonal antibody. Am J Physiol. 1994;266:H278–290. doi: 10.1152/ajpheart.1994.266.1.H279. [DOI] [PubMed] [Google Scholar]
- Jamar F, Chapman PT, Manicourt DH, Glass DM, Haskard DO, Peters AM. A comparison between 111In-anti-E-selectin mAb and 99Tcm-labelled human non-specific immunoglobulin in radionuclide imaging of rheumatoid arthritis. Br J Radiol. 1997;70:473–481. doi: 10.1259/bjr.70.833.9227228. [DOI] [PubMed] [Google Scholar]
- Scopinaro F, Schillaci O, Scarpini M, Mingazzini PL, Di Macio L, Banci M, Danieli R, Zerilli M, Limiti MR, Centi Colella A. Technetium-99m sestamibi: an indicator of breast cancer invasiveness. Eur J Nucl Med. 1994;21:984–987. doi: 10.1007/BF00238124. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Montgomery AMP, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin αVβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct αV integrins. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- FitzGerald O, Bresnihan B. Synovial membrane cellularity and vascularity. Ann Rheum Dis. 1995;54:511–515. doi: 10.1136/ard.54.6.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblad S, Hedfors E. Intraarticular variation in synovitis. Local macroscopic and microscopic signs of inflammatory activity are significantly correlated. Arthritis Rheum. 1985;28:977–986. doi: 10.1002/art.1780280904. [DOI] [PubMed] [Google Scholar]
- Ostergaard M, Hansen M, Stoltenberg M, Gideon P, Klarland M, Jensen KE, Lorenzen I. Magnetic resonance imaging-determined synovial membrane volume as a marker of disease activity and a predictor of progressive joint destruction in the wrists of patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:918–929. doi: 10.1002/1529-0131(199905)42:5<918::AID-ANR10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Hirohata S, Sakakibara J. Angioneogenesis as a possible elusive trigger factor in rheumatoid arthritis [letter]. Lancet. 1999;353:1331. doi: 10.1016/S0140-6736(98)05912-1. [DOI] [PubMed] [Google Scholar]
- Firestein GS. Starving the synovium: angiogenesis and inflammation in rheumatoid arthritis. J Clin Invest. 1999;103:3–4. doi: 10.1172/JCI5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storgard CM, Stupack DG, Jonczyk A, Goodman SL, Fox RI, Cheresh D. Decreased angiogenesis and arthritic disease in rabbits treated with alphavbeta3 antagonist. J Clin Invest. 1999;103:47–54. doi: 10.1172/JCI3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata S, Matsubara T, Saura R, Tateishi H, Hirohata K. Inhibition of in vitro vascular endothelial cell proliferation and in vivo neovascularization by low-dose methotrexate. Arthritis Rheum. 1989;32:1065–1073. doi: 10.1002/anr.1780320903. [DOI] [PubMed] [Google Scholar]
- Koch AE, Cho M, Burrows J, Leibovich SJ, Polverini PJ. Inhibition of production of macrophage-derived angiogenic activity by the anti-rheumatic agents gold sodium thiomalate and auranofin. Biochem Biophys Res Commun. 1988;154:205–212. doi: 10.1016/0006-291x(88)90671-7. [DOI] [PubMed] [Google Scholar]
- Koch AE, Burrows JC, Polverini PJ, Cho M, Leibovich SJ. Thiol-containing compounds inhibit the production of monocyte/macrophage-derived angiogenic activity. Agents Actions. 1991;34:350–357. doi: 10.1007/BF01988728. [DOI] [PubMed] [Google Scholar]
- Hernandez GL, Volpert OV, Iniguez MA, Lorenzo E, Martinez-Martinez S, Grau R, Fresno M, Redondo JM. Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2. J Exp Med. 2001;193:607–620. doi: 10.1084/jem.193.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PC, Williams RO, Maini RN. Immunotherapy for rheumatoid arthritis. Curr Opin Immunol. 2001;13:611–616. doi: 10.1016/S0952-7915(00)00269-7. [DOI] [PubMed] [Google Scholar]
- Oliver SJ, Cheng TP, Banquerigo ML, Brahn E. Suppression of collagen-induced arthritis by an angiogenesis inhibitor, AGM-1470 in combination with cyclosporin: reduction of vascular endothelial growth factor (VEGF). Cell Immunol. 1995;166:196–206. doi: 10.1006/cimm.1995.9978. [DOI] [PubMed] [Google Scholar]
- Peacock DJ, Banquerigo ML, Brahn E. Angiogenesis inhibition suppresses collagen arthritis. J Exp Med. 1992;175:1135–1138. doi: 10.1084/jem.175.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver SJ, Banquerigo ML, Brahn E. Suppression of collagen-induced arthritis using an angiogenesis inhibitor, AGM-1470 and a microtubule stabilizer, taxol. Cell Immunol. 1994;157:291–299. doi: 10.1006/cimm.1994.1223. [DOI] [PubMed] [Google Scholar]
- Lu J, Kasama T, Kobayashi K, Yoda Y, Shiozawa F, Hanyuda M, Negishi M, Ide H, Adachi M. Vascular endothelial growth factor expression and regulation of murine collagen-induced arthritis. J Immunol. 2000;164:5922–5927. doi: 10.4049/jimmunol.164.11.5922. [DOI] [PubMed] [Google Scholar]
- Sone H, Kawakami Y, Sakauchi M, Nakamura Y, Takahashi A, Shimano H, Okuda Y, Segawa T, Suzuki H, Yamada N. Neutralization of vascular endothelial growth factor prevents collagen-induced arthritis and ameliorates established disease in mice. Biochem Biophys Res Commun. 2001;281:562–568. doi: 10.1006/bbrc.2001.4395. [DOI] [PubMed] [Google Scholar]
- Miotla JM, Maciewicz R, Kendrew J, Feldmann M, Paleolog E. Treatment with soluble VEGF receptor reduces disease severity in murine collagen-induced arthritis. Lab Invest. 2000;80:1195–1205. doi: 10.1038/labinvest.3780127. [DOI] [PubMed] [Google Scholar]
- de Bandt M, Grossin M, Weber AJ, Chopin M, Elbim C, Pla M, Gougerot-Pocidalo MA, Gaudry M. Suppression of arthritis and protection from bone destruction by treatment with TNP-470/AGM-1470 in a transgenic mouse model of rheumatoid arthritis. Arthritis Rheum. 2000;43:2056–2063. doi: 10.1002/1529-0131(200009)43:9<2056::AID-ANR17>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Badger AM, Blake S, Kapadia R, Sarkar S, Levin J, Swift BA, Hoffman SJ, Stroup GB, Miller WH, Gowen M, Lark MW. Disease-modifying activity of SB 27 an orally active, non-peptide alphavbeta3 (vitronectin receptor) antagonist, in rat adjuvant-induced arthritis. Arthritis Rheum. 3005;44:128–137. doi: 10.1002/1529-0131(200101)44:1<128::AID-ANR17>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Wakefield RJ, Gibbon WW, Conaghan PG, O'Connor P, McGonagle D, Pease C, Green MJ, Veale DJ, Isaacs JD, Emery P. The value of sonography in the detection of bone erosions in patients with rheumatoid arthritis. Arthritis Rheum. 2000;43:2762–2770. doi: 10.1002/1529-0131(200012)43:12<2762::AID-ANR16>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]