Abstract
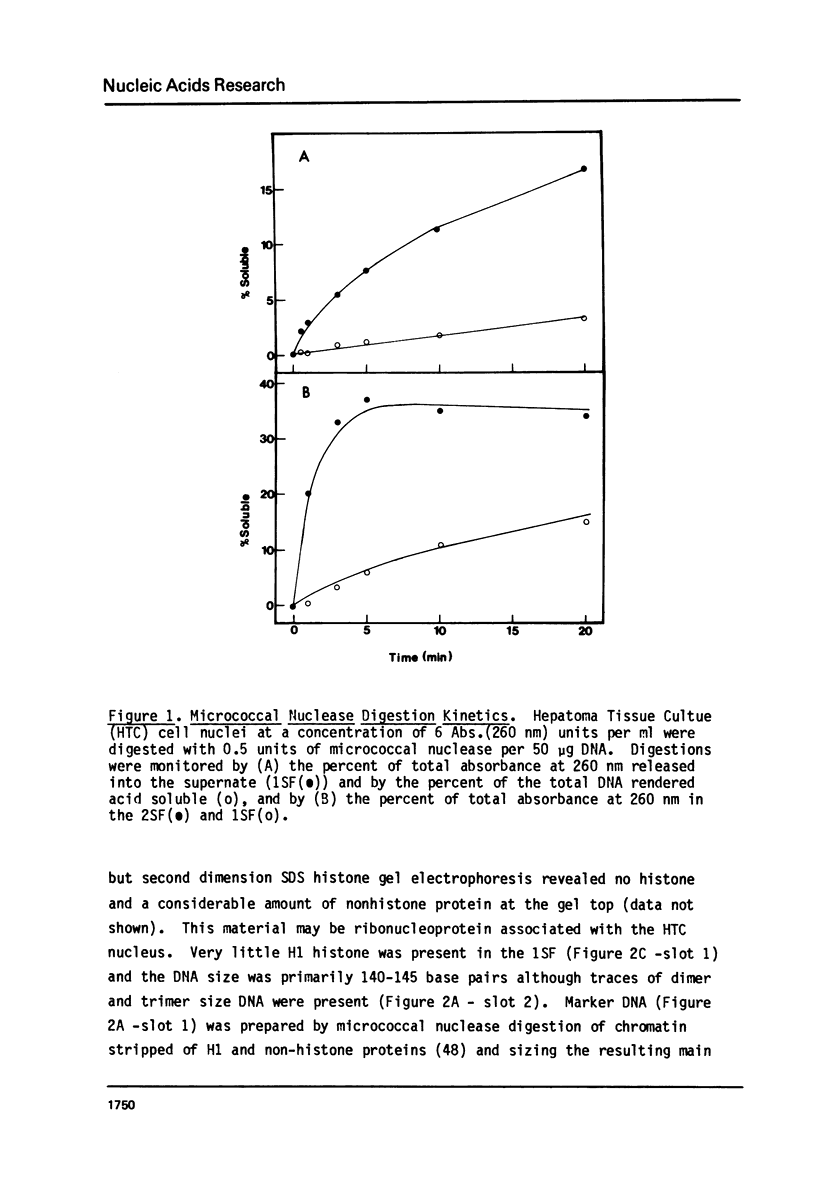
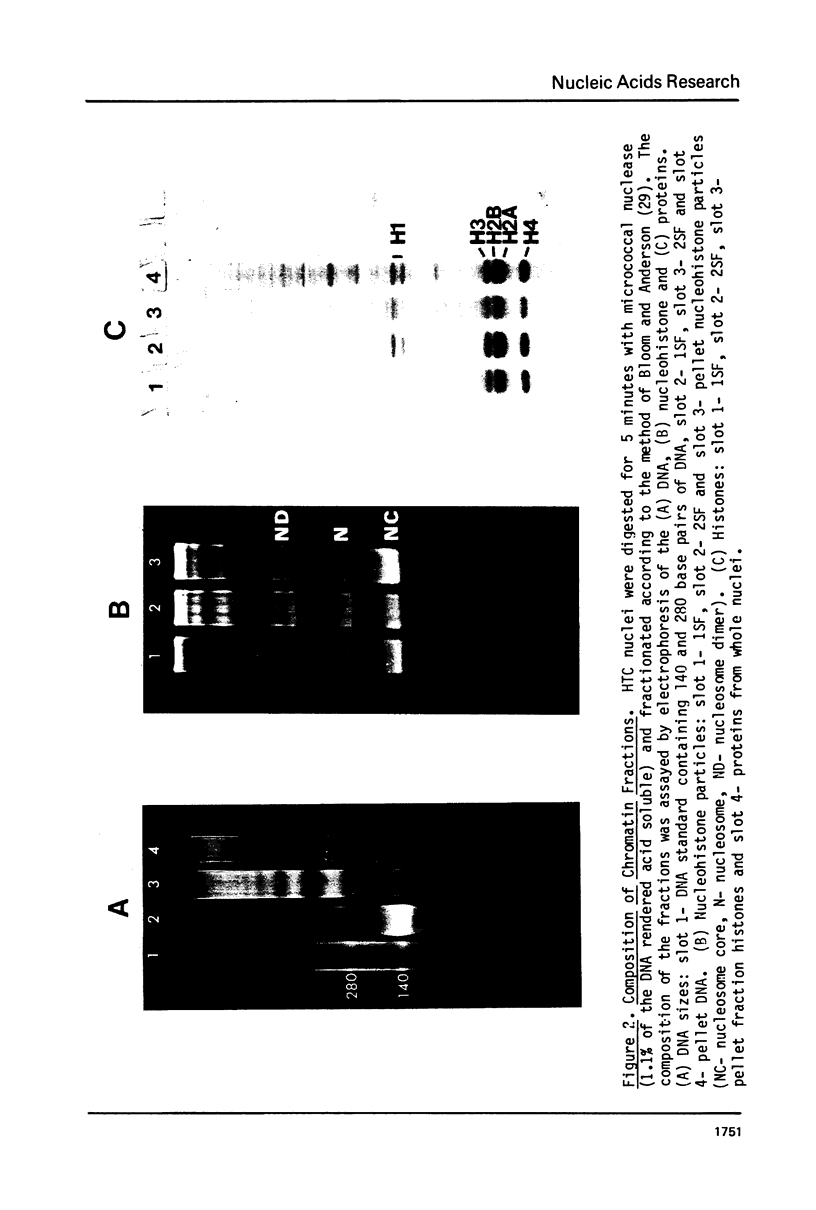
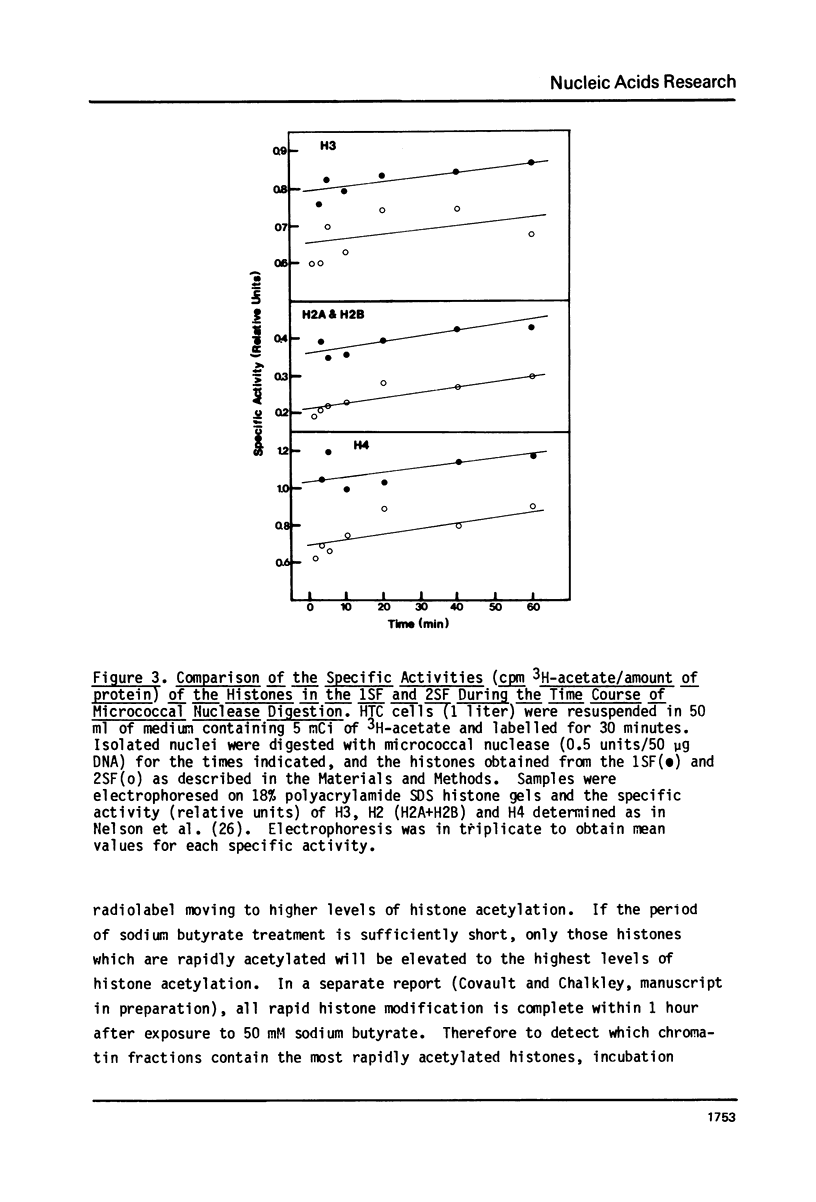
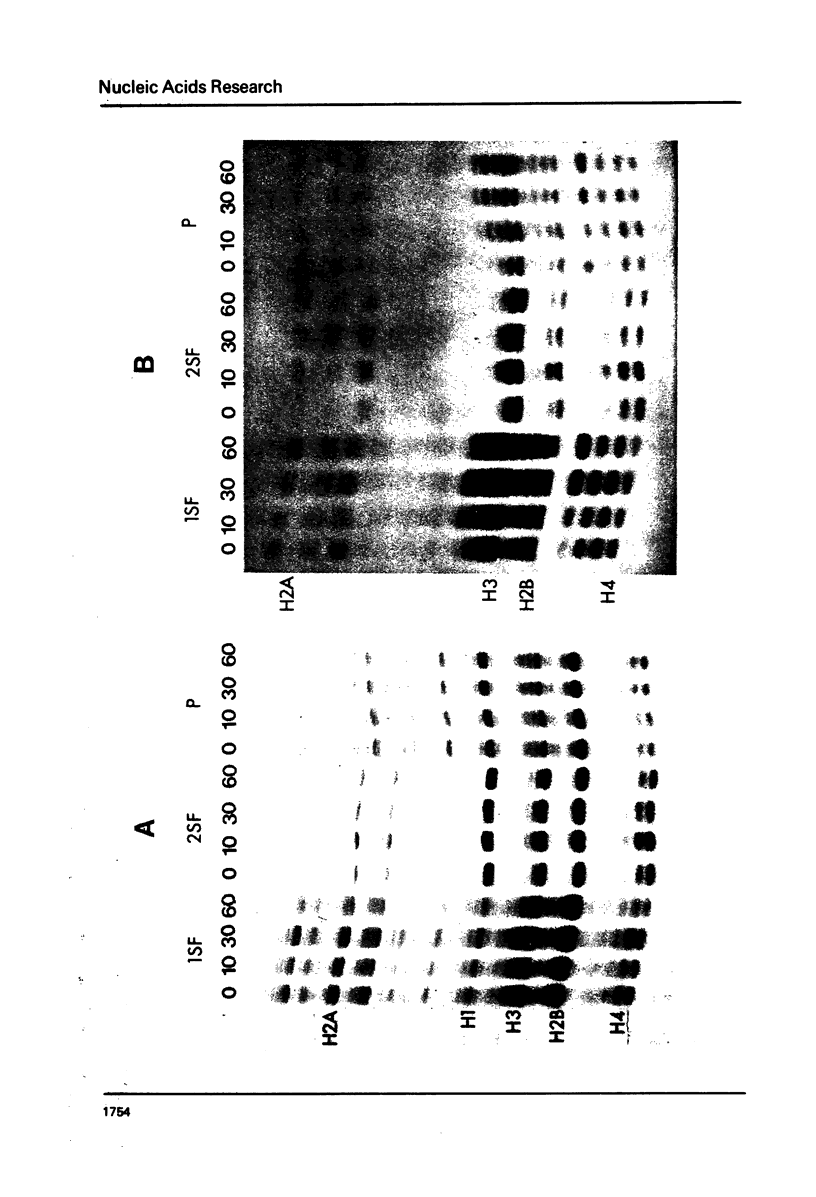
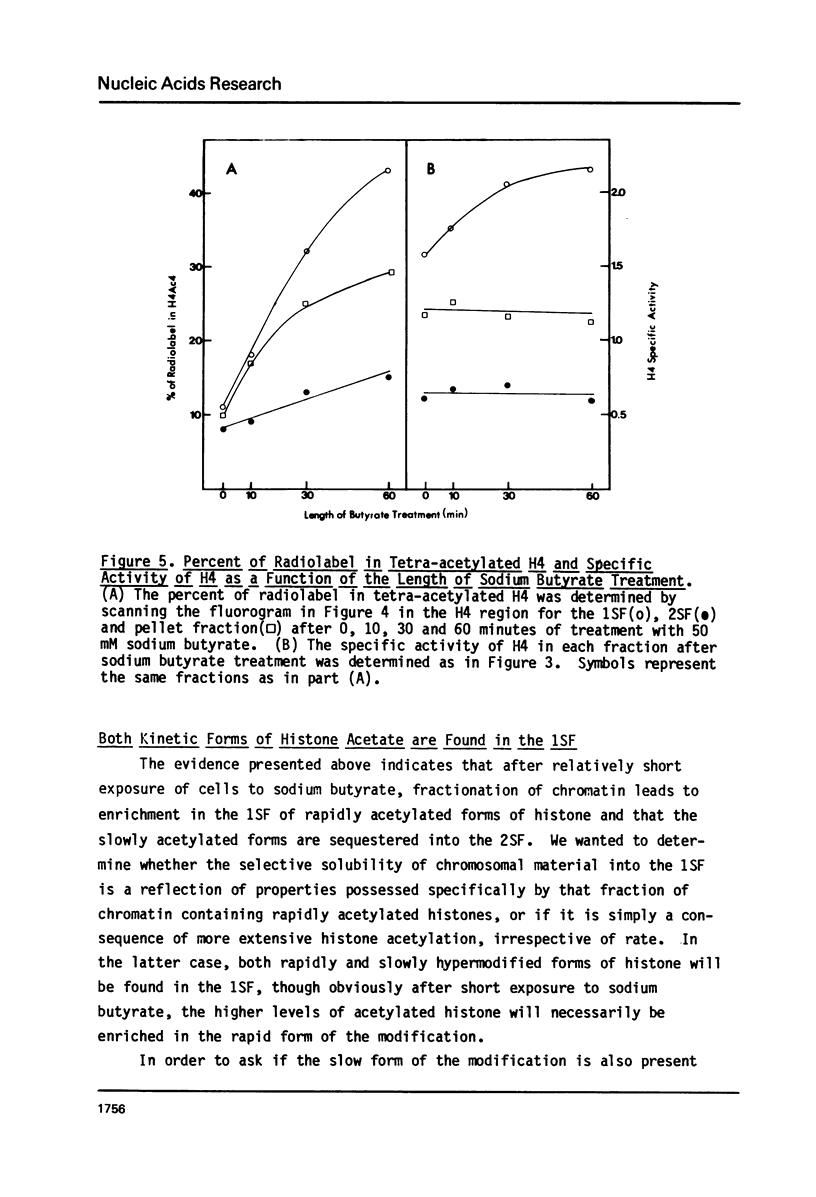
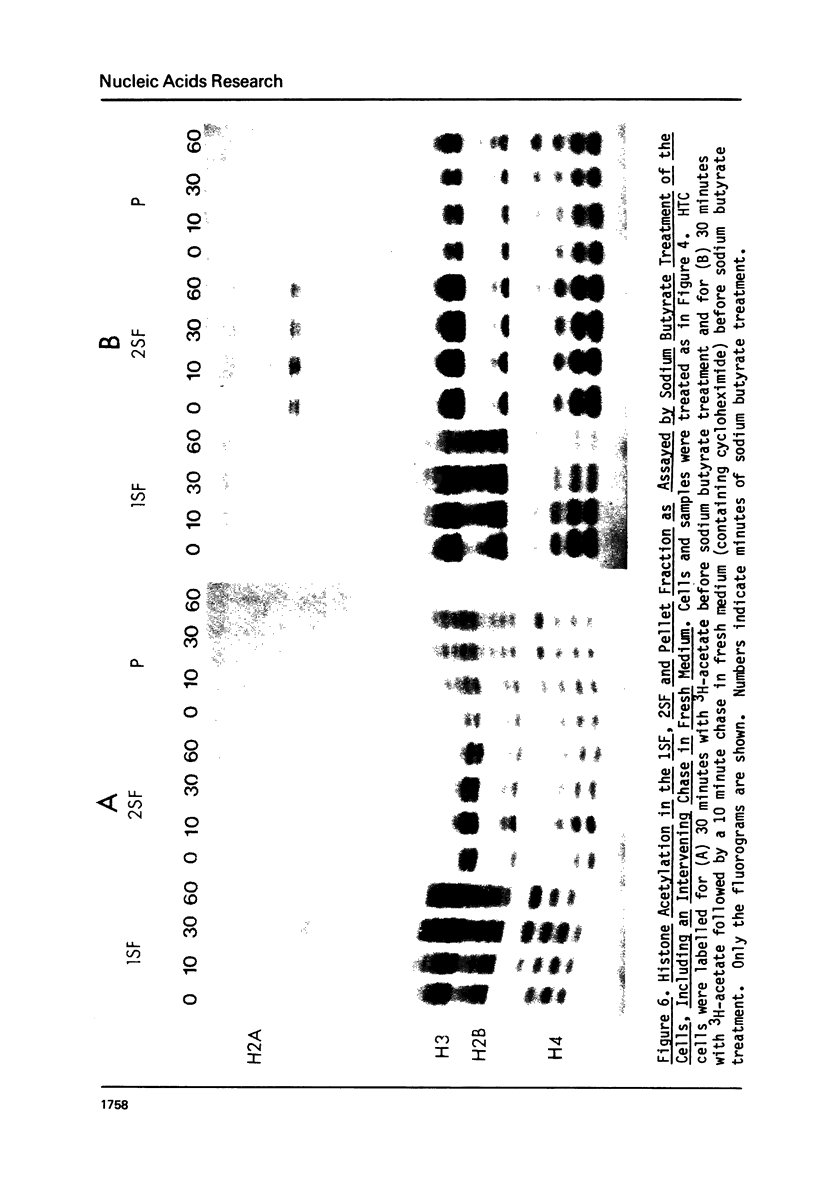
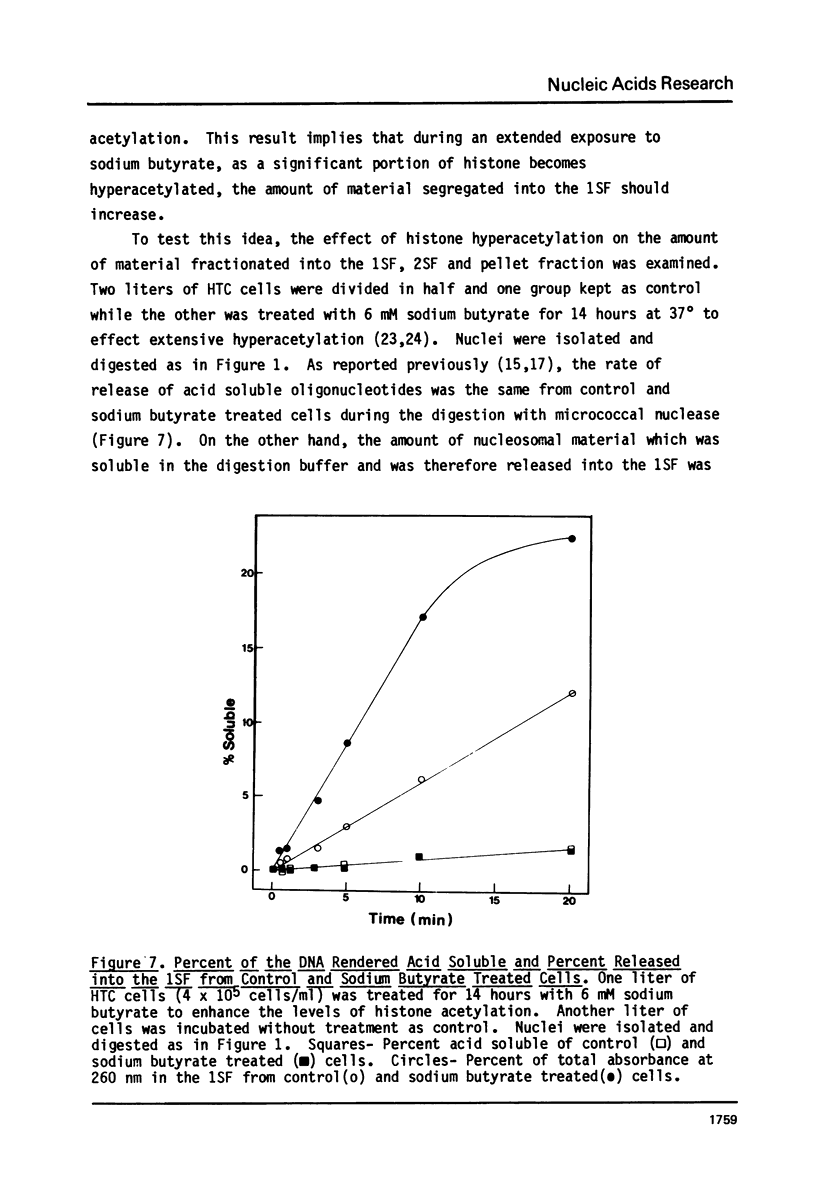
It has been previously shown that micrococcal nuclease digestion and subsequent fractionation of hen oviduct nuclei generates fractions enriched (first supernatant fraction - 1SF) and depleted (second supernatant fraction - 2SF) in ovalbumin genes, while a third fraction, the pellet fraction, contains about the same level of this gene as whole chromatin (Bloom and Anderson (1978) Cell 15, 141-150). We have utilized this fractionation method in an attempt to assess the extent and kinetics of histone acetylation associated with chromatin from the 1SF, 2SF, and pellet fraction. Hepatoma Tissue Culture (HTC) cells were labelled for 30 minutes in vivo with 3H-acetate, nuclei isolated and the chromatin fractionated. The specific activity of the histones in the 1SF was slightly greater than that of the 2SF (1.2 to 1.6 fold difference) independent of the length of nuclease digestion. If the labelling period is followed by short (10 to 60 minute) treatment of the cells with sodium butyrate, the more rapidly as well as more extensively acetylated histones are also preferentially found in the 1SF. This is in part the result of segregation of chromatin particles into the 1SF as the histones associated with these particles become hyperacetylated. That is, the extent of histone acetylation regulates the distribution of chromatin in the 1SF, 2SF and pellet fraction.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright S. C., Nelson P. P., Garrard W. T. Histone molar ratios among different electrophoretic forms of mono- and dinucleosomes. J Biol Chem. 1979 Feb 25;254(4):1065–1073. [PubMed] [Google Scholar]
- Alfageme C. R., Zweidler A., Mahowald A., Cohen L. H. Histones of Drosophila embryos. Electrophoretic isolation and structural studies. J Biol Chem. 1974 Jun 25;249(12):3729–3736. [PubMed] [Google Scholar]
- Balhorn R., Tanphaichitr N., Chalkley R., Granner D. K. The effect of inhibition of deoxyribonucleic acid synthesis on histone phosphorylation. Biochemistry. 1973 Dec 4;12(25):5146–5150. doi: 10.1021/bi00749a019. [DOI] [PubMed] [Google Scholar]
- Bellard M., Gannon F., Chambon P. Nucleosome structure III: the structure and transcriptional activity of the chromatin containing the ovalbumin and globin genes in chick oviduct nuclei. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):779–791. doi: 10.1101/sqb.1978.042.01.078. [DOI] [PubMed] [Google Scholar]
- Bloom K. S., Anderson J. N. Conformation of ovalbumin and globin genes in chromatin during differential gene expression. J Biol Chem. 1979 Oct 25;254(20):10532–10539. [PubMed] [Google Scholar]
- Bloom K. S., Anderson J. N. Fractionation of hen oviduct chromatin into transcriptionally active and inactive regions after selective micrococcal nuclease digestion. Cell. 1978 Sep;15(1):141–150. doi: 10.1016/0092-8674(78)90090-9. [DOI] [PubMed] [Google Scholar]
- Boffa L. C., Vidali G., Mann R. S., Allfrey V. G. Suppression of histone deacetylation in vivo and in vitro by sodium butyrate. J Biol Chem. 1978 May 25;253(10):3364–3366. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Pollard H. B. The presence of F3-F2a1 dimers and F1 oligomers in chromatin. Biochem Biophys Res Commun. 1975 May 5;64(1):282–288. doi: 10.1016/0006-291x(75)90250-8. [DOI] [PubMed] [Google Scholar]
- Bostock C. J., Christie S., Hatch F. T. Accessibility of DNA in condensed chromatin to nuclease digestion. Nature. 1976 Aug 5;262(5568):516–519. doi: 10.1038/262516a0. [DOI] [PubMed] [Google Scholar]
- Candido E. P., Reeves R., Davie J. R. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978 May;14(1):105–113. doi: 10.1016/0092-8674(78)90305-7. [DOI] [PubMed] [Google Scholar]
- Cousens L. S., Gallwitz D., Alberts B. M. Different accessibilities in chromatin to histone acetylase. J Biol Chem. 1979 Mar 10;254(5):1716–1723. [PubMed] [Google Scholar]
- Davie J. R., Candido E. P. Acetylated histone H4 is preferentially associated with template-active chromatin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3574–3577. doi: 10.1073/pnas.75.8.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie J. R., Candido P. M. Chromatin subunits contain normal levels of major acetylated histone species. J Biol Chem. 1977 Sep 10;252(17):5962–5966. [PubMed] [Google Scholar]
- Flint S. J., Weintraub H. M. An altered subunit configuration associated with the actively transcribed DNA of integrated adenovirus genes. Cell. 1977 Nov;12(3):783–794. doi: 10.1016/0092-8674(77)90277-x. [DOI] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel A., Zolan M., Axel R. Genes transcribed at diverse rates have a similar conformation in chromatin. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4867–4871. doi: 10.1073/pnas.74.11.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldknopf I. L., French M. F., Musso R., Busch H. Presence of protein A24 in rat liver nucleosomes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5492–5495. doi: 10.1073/pnas.74.12.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld J. M., Garrard W. T., Bagi G., Wilson R. F., Bonner J. Partial purification of the template-active fraction of chromatin: a preliminary report. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2193–2197. doi: 10.1073/pnas.71.6.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grouse L., Chilton M. D., McCarthy B. J. Hybridization of ribonucleic acid with unique sequences of mouse deoxyribonucleic acid. Biochemistry. 1972 Feb 29;11(5):798–805. doi: 10.1021/bi00755a019. [DOI] [PubMed] [Google Scholar]
- Hagopian H. K., Riggs M. G., Swartz L. A., Ingram V. M. Effect of n-butyrate on DNA synthesis in chick fibroblasts and HeLa cells. Cell. 1977 Nov;12(3):855–860. doi: 10.1016/0092-8674(77)90284-7. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Mayfield J. E., Bonner J. Sequence composition of rat ascites chromosomal ribonucleic acid. Biochemistry. 1974 Feb 26;13(5):849–855. doi: 10.1021/bi00702a002. [DOI] [PubMed] [Google Scholar]
- Jackson V., Shires A., Chalkley R., Granner D. K. Studies on highly metabolically active acetylation and phosphorylation of histones. J Biol Chem. 1975 Jul 10;250(13):4856–4863. [PubMed] [Google Scholar]
- Jackson V., Shires A., Tanphaichitr N., Chalkley R. Modifications to histones immediately after synthesis. J Mol Biol. 1976 Jun 25;104(2):471–483. doi: 10.1016/0022-2836(76)90282-5. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Allfrey V. G., Bradbury E. M., Matthews H. R. Altered nucleosome structure containing DNA sequences complementary to 19S and 26S ribosomal RNA in Physarum polycephalum. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1116–1120. doi: 10.1073/pnas.75.3.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Levy-Wilson B., Dixon G. H. Limited action of micrococcal nuclease on trout testis nuclei generates two mononucleosome subsets enriched in transcribed DNA sequences. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1682–1686. doi: 10.1073/pnas.76.4.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Wilson B., Watson D. C., Dixon G. H. Multiacetylated forms of H4 are found in a putative transcriptionally competent chromatin fraction from trout testis. Nucleic Acids Res. 1979 Jan;6(1):259–274. doi: 10.1093/nar/6.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B. W., Connor W., Dixon G. H. A subset of trout testis nucleosomes enriched in transcribed DNA sequences contains high mobility group proteins as major structural components. J Biol Chem. 1979 Feb 10;254(3):609–620. [PubMed] [Google Scholar]
- Levy B., Dixon G. H. Partial purification of transcriptionally active nucleosomes from trout testis cells. Nucleic Acids Res. 1978 Nov;5(11):4155–4163. doi: 10.1093/nar/5.11.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B., Dixon G. H. Renaturation kinetics of cDNA complementary to cytoplamic polyadenylated RNA from rainbow trout testis. Accessibility of transcribed genes to pancreatic DNase. Nucleic Acids Res. 1977 Apr;4(4):883–898. doi: 10.1093/nar/4.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M., Jackson V., Sealy L., Chalkley R. Comparative studies on highly metabolically active histone acetylation. Biochim Biophys Acta. 1979 Jan 26;561(1):248–260. doi: 10.1016/0005-2787(79)90508-2. [DOI] [PubMed] [Google Scholar]
- Nelson D. A., Perry M., Sealy L., Chalkley R. DNAse I preferentially digests chromatin containing hyperacetylated histones. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1346–1353. doi: 10.1016/0006-291x(78)90337-6. [DOI] [PubMed] [Google Scholar]
- Nelson D. A., Perry W. M., Chalkley R. Sensitivity of regions of chromatin containing hyperacetylated histones to DNAse I. Biochem Biophys Res Commun. 1978 May 15;82(1):365–363. doi: 10.1016/0006-291x(78)90617-4. [DOI] [PubMed] [Google Scholar]
- Nelson D., Perry M. E., Chalkley R. A correlation between nucleosome spacer region susceptibility to DNase I and histone acetylation. Nucleic Acids Res. 1979 Feb;6(2):561–574. doi: 10.1093/nar/6.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D., Granner D., Chalkley R. Identification of a distinction between cytoplasmic histone synthesis and subsequent histone deposition within the nucleus. Biochemistry. 1974 Feb 12;13(4):746–749. doi: 10.1021/bi00701a017. [DOI] [PubMed] [Google Scholar]
- Once more into the breach. Nature. 1970 Jul 4;227(5253):1–1. doi: 10.1038/227001a0. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Perry M., Nelson D., Moore M., Chalkley R. Histone deacetylation in nuclei isolated from hepatoma tissue culture cells. Inhibition by sodium butyrate. Biochim Biophys Acta. 1979 Feb 27;561(2):517–525. doi: 10.1016/0005-2787(79)90159-x. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Allfrey V. G., Mirsky A. E. RNA synthesis and histone acetylation during the course of gene activation in lymphocytes. Proc Natl Acad Sci U S A. 1966 Apr;55(4):805–812. doi: 10.1073/pnas.55.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R., Candido E. P. Turnover of histone acetyl groups in cultured cells is inhibited by sodium butyrate. FEBS Lett. 1978 Jul 1;91(1):117–120. doi: 10.1016/0014-5793(78)80030-1. [DOI] [PubMed] [Google Scholar]
- Riggs M. G., Whittaker R. G., Neumann J. R., Ingram V. M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977 Aug 4;268(5619):462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Wangh L. J., Allfrey V. G. Processing of newly synthesized histone molecules. Science. 1975 Oct 10;190(4210):117–128. doi: 10.1126/science.1166303. [DOI] [PubMed] [Google Scholar]
- Sanders M. M. Fractionation of nucleosomes by salt elution from micrococcal nuclease-digested nuclei. J Cell Biol. 1978 Oct;79(1):97–109. doi: 10.1083/jcb.79.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealy L., Chalkley R. DNA associated with hyperacetylated histone is preferentially digested by DNase I. Nucleic Acids Res. 1978 Jun;5(6):1863–1876. doi: 10.1093/nar/5.6.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealy L., Chalkley R. The effect of sodium butyrate on histone modification. Cell. 1978 May;14(1):115–121. doi: 10.1016/0092-8674(78)90306-9. [DOI] [PubMed] [Google Scholar]
- Simpson R. T. Structure of chromatin containing extensively acetylated H3 and H4. Cell. 1978 Apr;13(4):691–699. doi: 10.1016/0092-8674(78)90219-2. [DOI] [PubMed] [Google Scholar]
- Stalder J., Seebeck T., Braun R. Accessibility of the ribosomal genes to micrococcal nuclease in Physarum polycephalum. Biochim Biophys Acta. 1979 Feb 27;561(2):452–463. doi: 10.1016/0005-2787(79)90153-9. [DOI] [PubMed] [Google Scholar]
- Vidali G., Boffa L. C., Bradbury E. M., Allfrey V. G. Butyrate suppression of histone deacetylation leads to accumulation of multiacetylated forms of histones H3 and H4 and increased DNase I sensitivity of the associated DNA sequences. Proc Natl Acad Sci U S A. 1978 May;75(5):2239–2243. doi: 10.1073/pnas.75.5.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]