Abstract
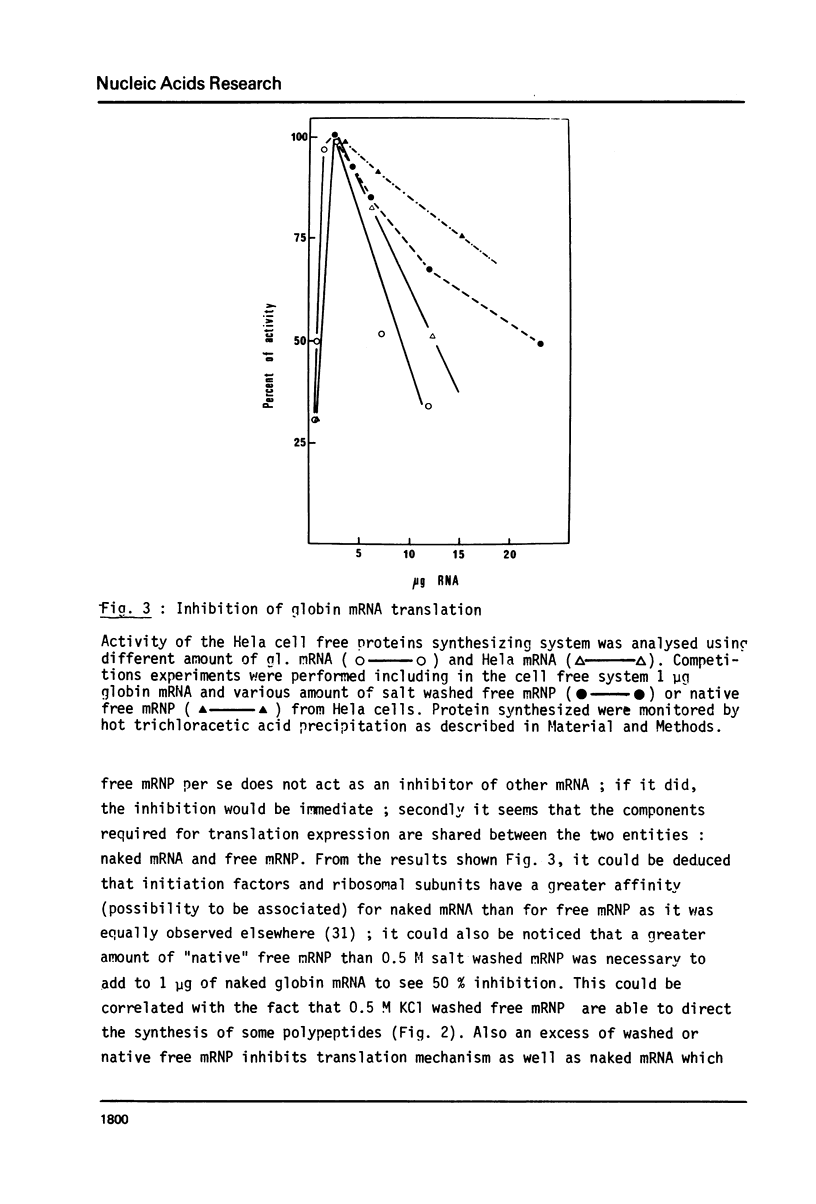
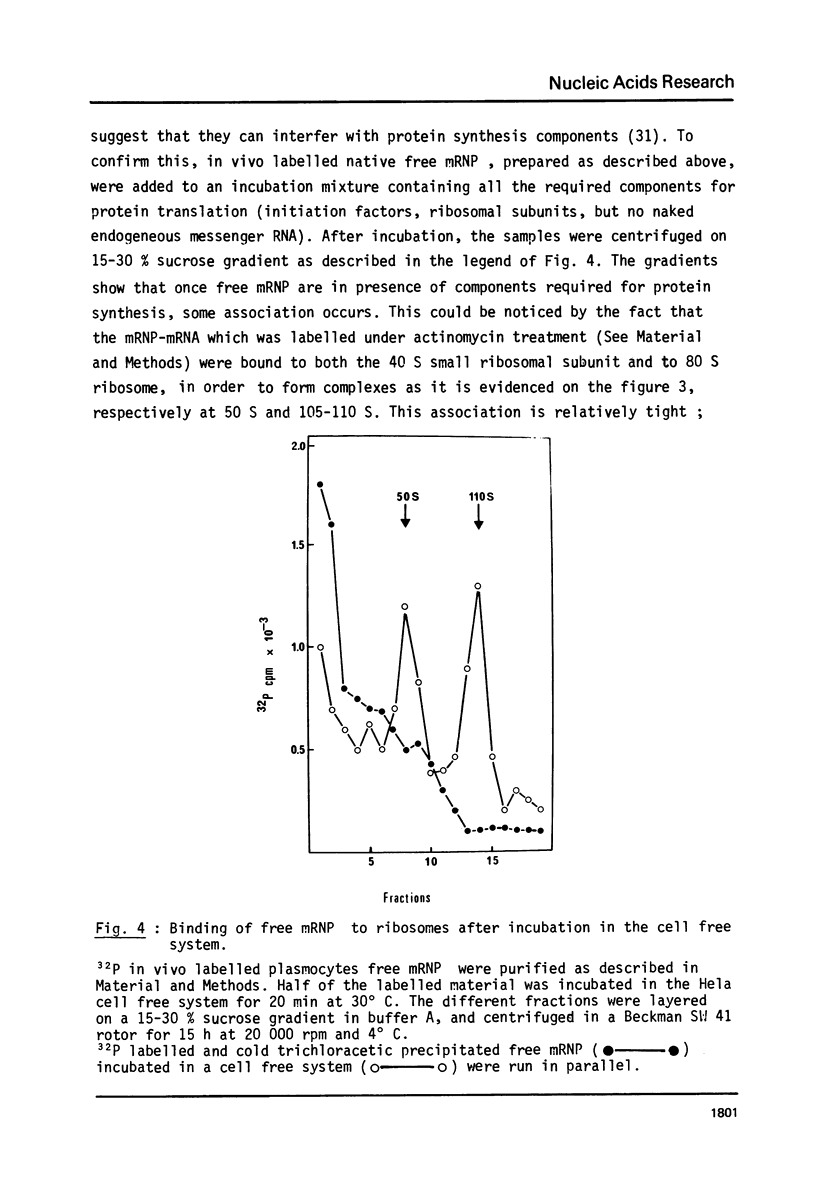
We analyzed the translational capacity of different kinds of free cytoplasmic messenger ribonucleoprotein complexes (free mRNP) in a Hela cell cell free system. Native free mRNP are not translated although free mRNP washed with 0.5 M KC1 can direct polypeptide synthesis. Furthermore, the 0.5 M KC1 wash possesses a factor which inhibits the translation of 0.5 M KC1 washed free mRNP as well as globin mRNA naked mRNA from plasmocytoma, or Hela cells. We also demonstrated that native free mRNP are able to form a complex with ribosomal subunits in the presence of initiation factors. This indicates that inhibition of translation by the 0.5 M KC1 wash occurs either at some point after initiation complex formation or at the elongation step.
Full text
PDF


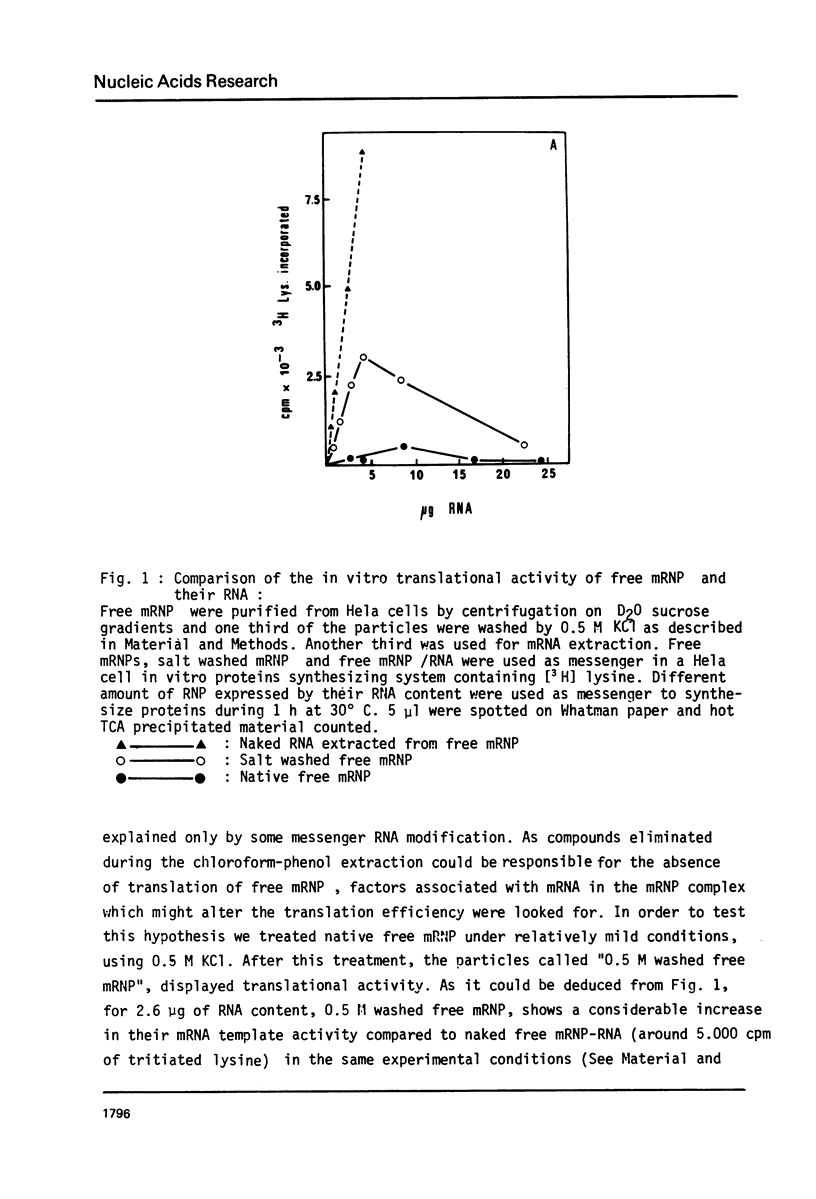
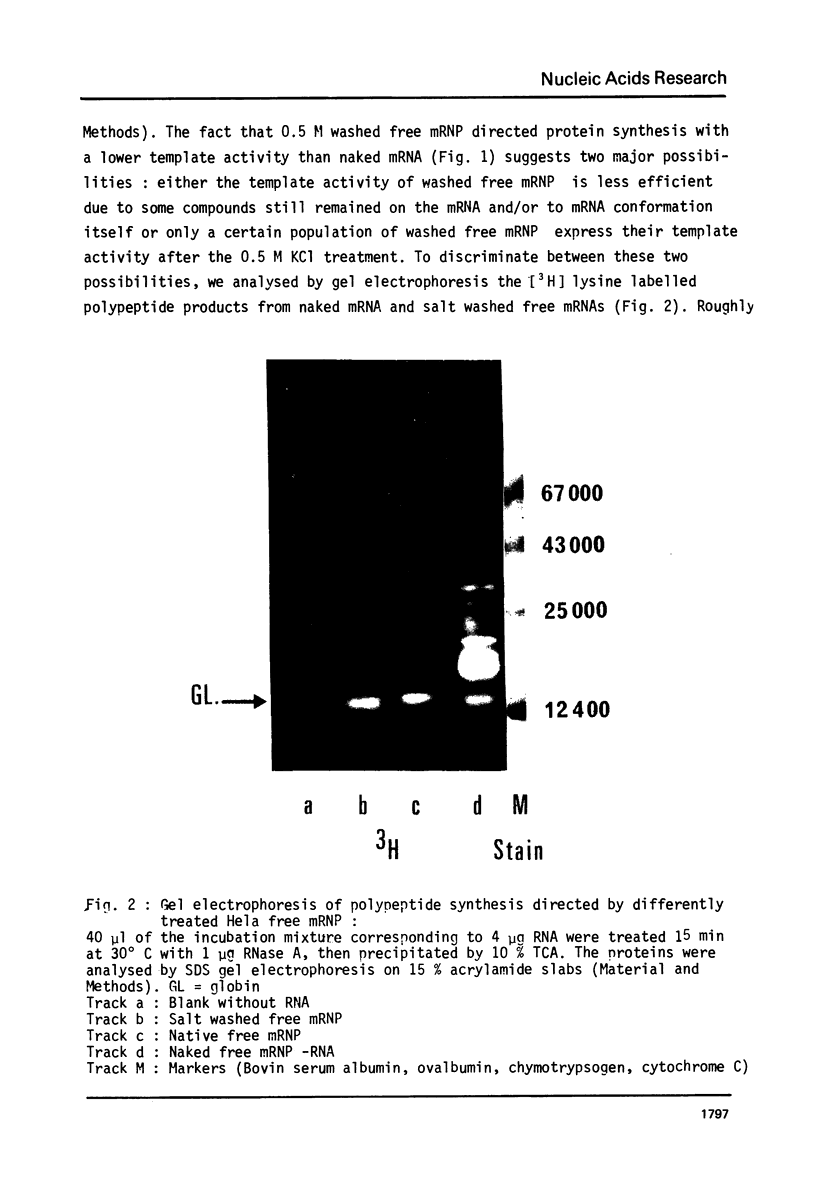
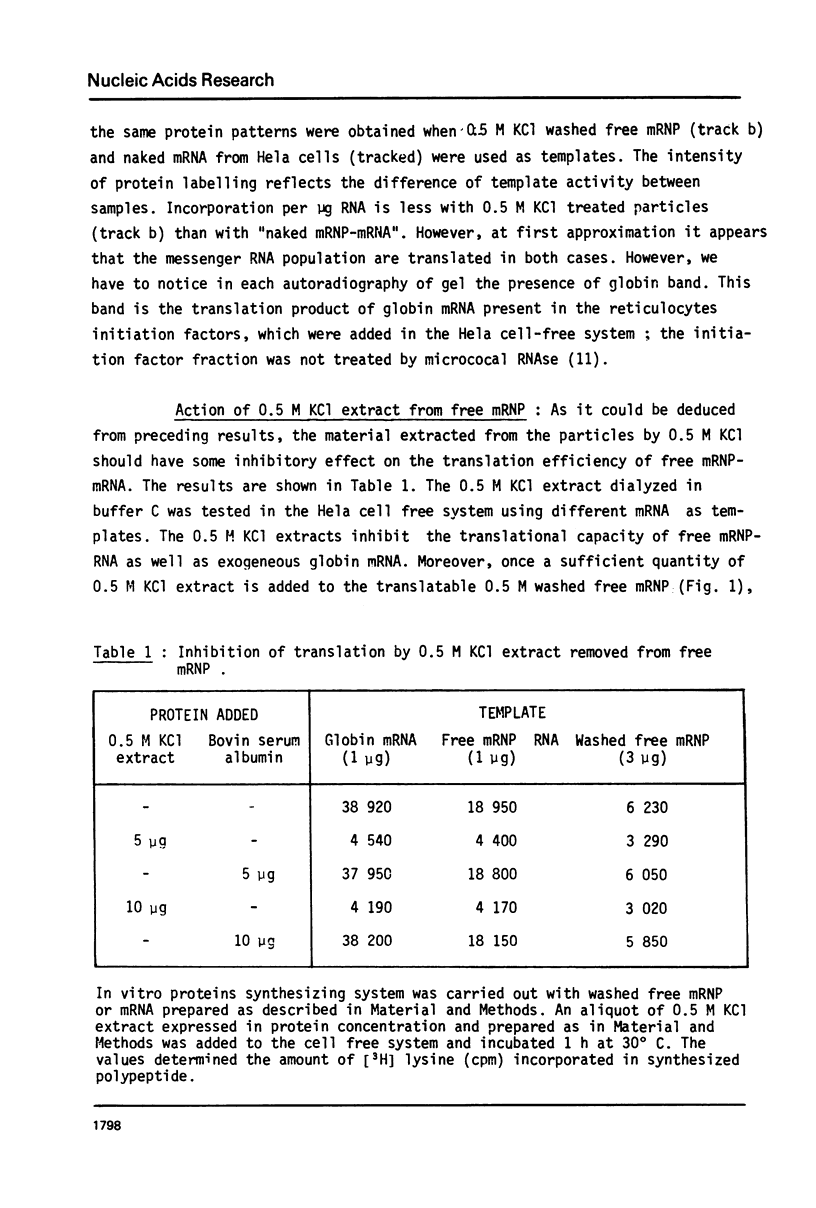




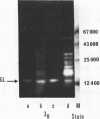
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bag J., Sarkar S. Studies on a nonpolysomal ribonucleoprotein coding for myosin heavy chains from chick embryonic muscles. J Biol Chem. 1976 Dec 10;251(23):7600–7609. [PubMed] [Google Scholar]
- Civelli O., Vincent A., Buri J. F., Scherrer K. Evidence for a translational inhibitor linked to globin mRNA in untranslated free cytoplasmic messenger ribonucleoprotein complexes. FEBS Lett. 1976 Dec 15;72(1):71–76. doi: 10.1016/0014-5793(76)80815-0. [DOI] [PubMed] [Google Scholar]
- Egly J. M., Schmitt M., Kempf J. Characterization of a protein kinase-phosphoprotein system in free cytoplasmic ribonucleoprotein particles of plasma cell tumours. Biochim Biophys Acta. 1976 Dec 13;454(3):549–557. doi: 10.1016/0005-2787(76)90280-x. [DOI] [PubMed] [Google Scholar]
- Geoghegan T., Cereghini S., Brawerman G. Inactive mRNA-protein complexes from mouse sarcoma-180 ascites cells. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5587–5591. doi: 10.1073/pnas.76.11.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M. Control of protein synthesis by hemin. Evidence that the hemin-controlled translational repressor inhibits formation of 80 S initiation complexes from 48 S intermediate initiation complexes. J Biol Chem. 1979 Apr 10;254(7):2370–2377. [PubMed] [Google Scholar]
- Heywood S. M., Kennedy D. S., Bester A. J. Separation of specific initiation factors involved in the translation of myosin and myoglobin messenger RNAs and the isolation of a new RNA involved in translation. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2428–2431. doi: 10.1073/pnas.71.6.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood S. M., Kennedy D. S. Purification of myosin translational control RNA and its interaction with myosin messenger RNA. Biochemistry. 1976 Jul 27;15(15):3314–3319. doi: 10.1021/bi00660a023. [DOI] [PubMed] [Google Scholar]
- Ilan J., Ilan J. Translation of maternal messenger ribonucleoprotein particles from sea urchin in a cell-free system from unfertilized eggs and product analysis. Dev Biol. 1978 Oct;66(2):375–385. doi: 10.1016/0012-1606(78)90246-4. [DOI] [PubMed] [Google Scholar]
- Jacobs-Lorena, Baglioni C. Synthesis of rabbit globin by reticulocyte postribosomal supernatant and heterologous ribosomes. Eur J Biochem. 1973 Jun 15;35(3):559–565. doi: 10.1111/j.1432-1033.1973.tb02873.x. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Kaumeyer J. F., Young E. M., Raff R. A. A test for masked message: the template activity of messenger ribonucleoprotein particles isolated from sea urchine eggs. Dev Biol. 1978 Apr;63(2):279–298. doi: 10.1016/0012-1606(78)90134-3. [DOI] [PubMed] [Google Scholar]
- Kempf J., Egly J. M., Stricker C., Schmitt M., Mandel P. Isolation of cytoplasmic non-ribosomal ribonucleoprotein particles (informosomes). FEBS Lett. 1972 Oct 1;26(1):130–134. doi: 10.1016/0014-5793(72)80558-1. [DOI] [PubMed] [Google Scholar]
- Lee G. T., Engelhardt D. L. Peptide coding capacity of polysomal and non-polysomal messenger RNA during growth of animal cells. J Mol Biol. 1979 Apr 5;129(2):221–233. doi: 10.1016/0022-2836(79)90278-x. [DOI] [PubMed] [Google Scholar]
- Liautard J. P., Jeanteur P. Purification of histone messenger ribonucleoprotein particles from HeLa cell S-phase polysomes. Characterization of associated proteins. Nucleic Acids Res. 1979 Sep 11;7(1):135–150. doi: 10.1093/nar/7.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F. Model for the regulation of mRNA translation applied to haemoglobin synthesis. Nature. 1974 Oct 4;251(5474):385–388. doi: 10.1038/251385a0. [DOI] [PubMed] [Google Scholar]
- Nienhuis A. W., Falvey A. K., Anderson W. F. Preparation of globin messenger RNA. Methods Enzymol. 1974;30:621–630. doi: 10.1016/0076-6879(74)30060-2. [DOI] [PubMed] [Google Scholar]
- Perry R. P., La Torre J., Kelley D. E., Greenberg J. R. On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochim Biophys Acta. 1972 Mar 14;262(2):220–226. doi: 10.1016/0005-2787(72)90236-5. [DOI] [PubMed] [Google Scholar]
- Robbins E., Borun T. W. The cytoplasmic synthesis of histones in hela cells and its temporal relationship to DNA replication. Proc Natl Acad Sci U S A. 1967 Feb;57(2):409–416. doi: 10.1073/pnas.57.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Spirin A. S. The second Sir Hans Krebs Lecture. Informosomes. Eur J Biochem. 1969 Aug;10(1):20–35. doi: 10.1111/j.1432-1033.1969.tb00651.x. [DOI] [PubMed] [Google Scholar]
- Spohr G., Granboulan N., Morel C., Scherrer K. Messenger RNA in HeLa cells: an investigation of free and polyribosome-bound cytoplasmic messenger ribonucleoprotein particles by kinetic labelling and electron microscopy. Eur J Biochem. 1970 Dec;17(2):296–318. doi: 10.1111/j.1432-1033.1970.tb01168.x. [DOI] [PubMed] [Google Scholar]
- Stulberg M. P., Isham K. R. Differential effects on RNA translation by a KC1 extract of reticulocyte ribosomes: characteristics of an inhibitory fraction. Biochem Biophys Res Commun. 1979 Oct 12;90(3):824–831. doi: 10.1016/0006-291x(79)91902-8. [DOI] [PubMed] [Google Scholar]
- Sundkvist I. C., Staehelin T. Structure and function of free 40 S ribosome subunits: Characterization of initiation factors. J Mol Biol. 1975 Dec 15;99(3):401–418. doi: 10.1016/s0022-2836(75)80135-5. [DOI] [PubMed] [Google Scholar]