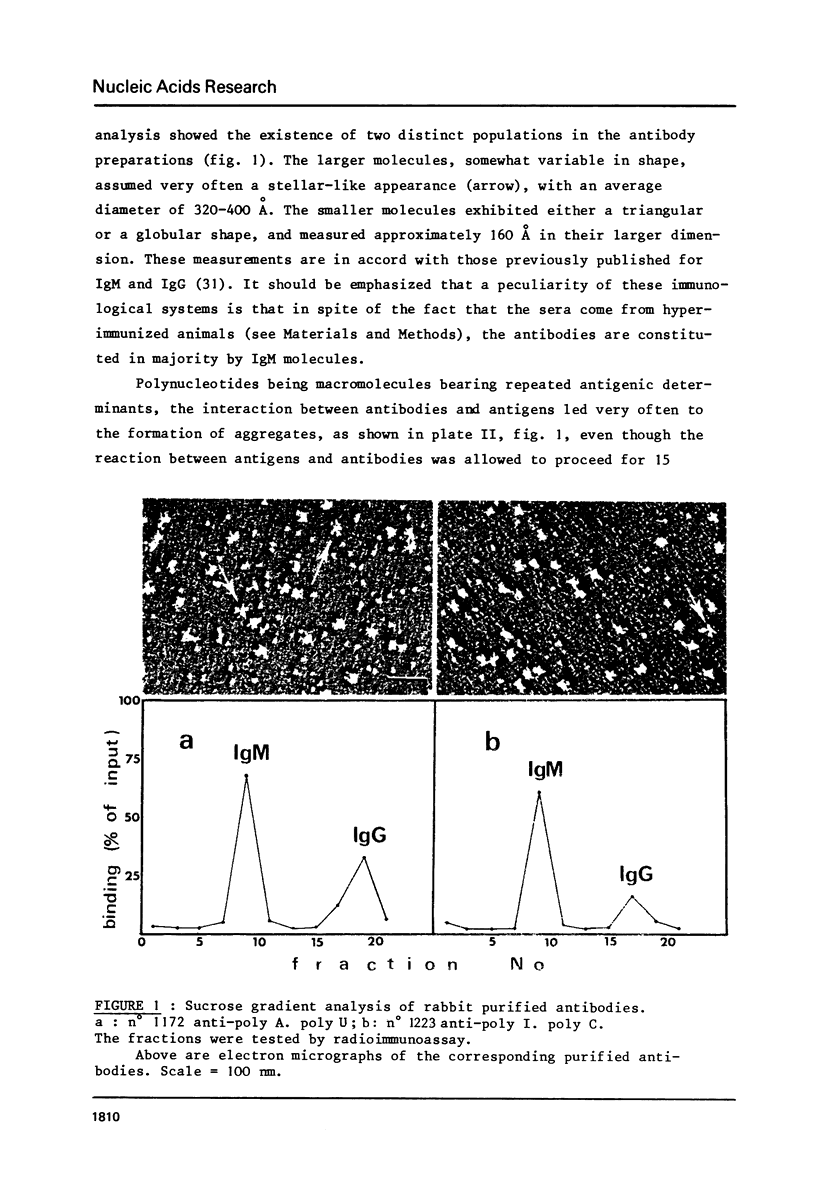
Abstract
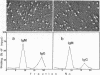
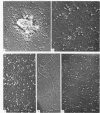
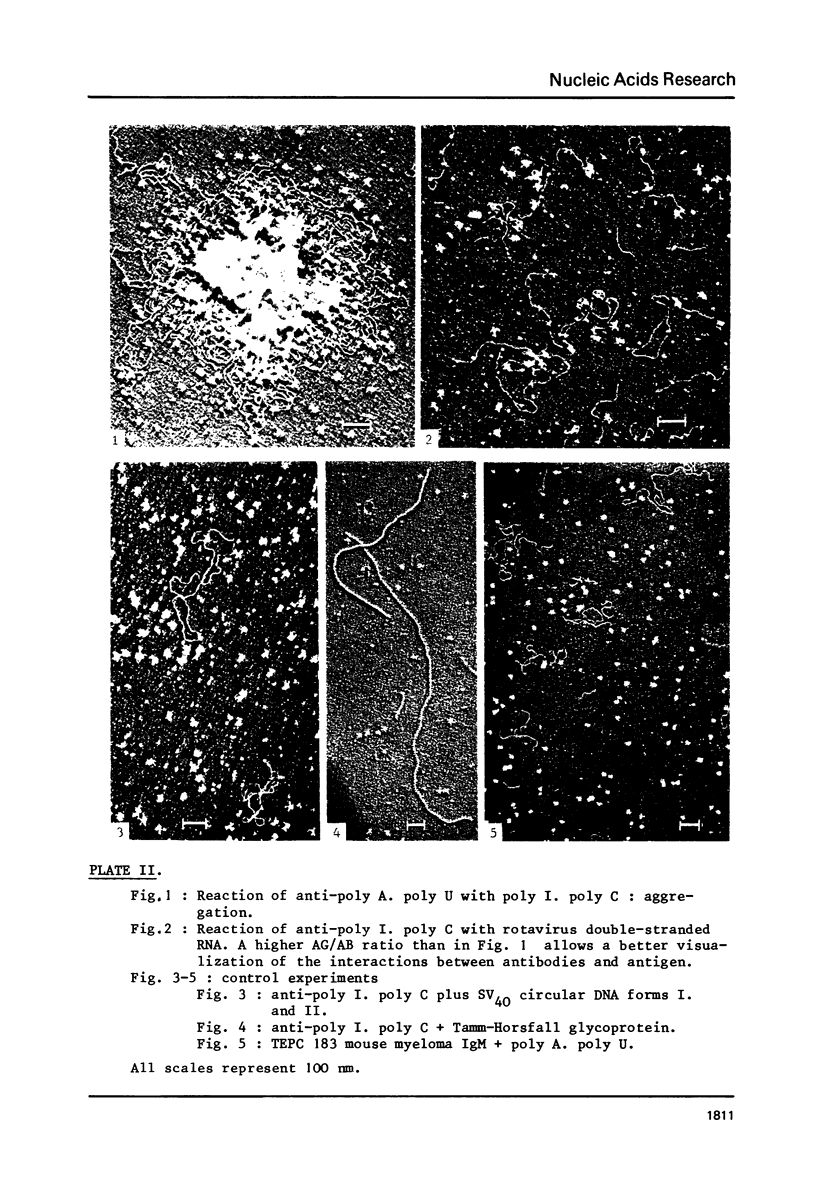
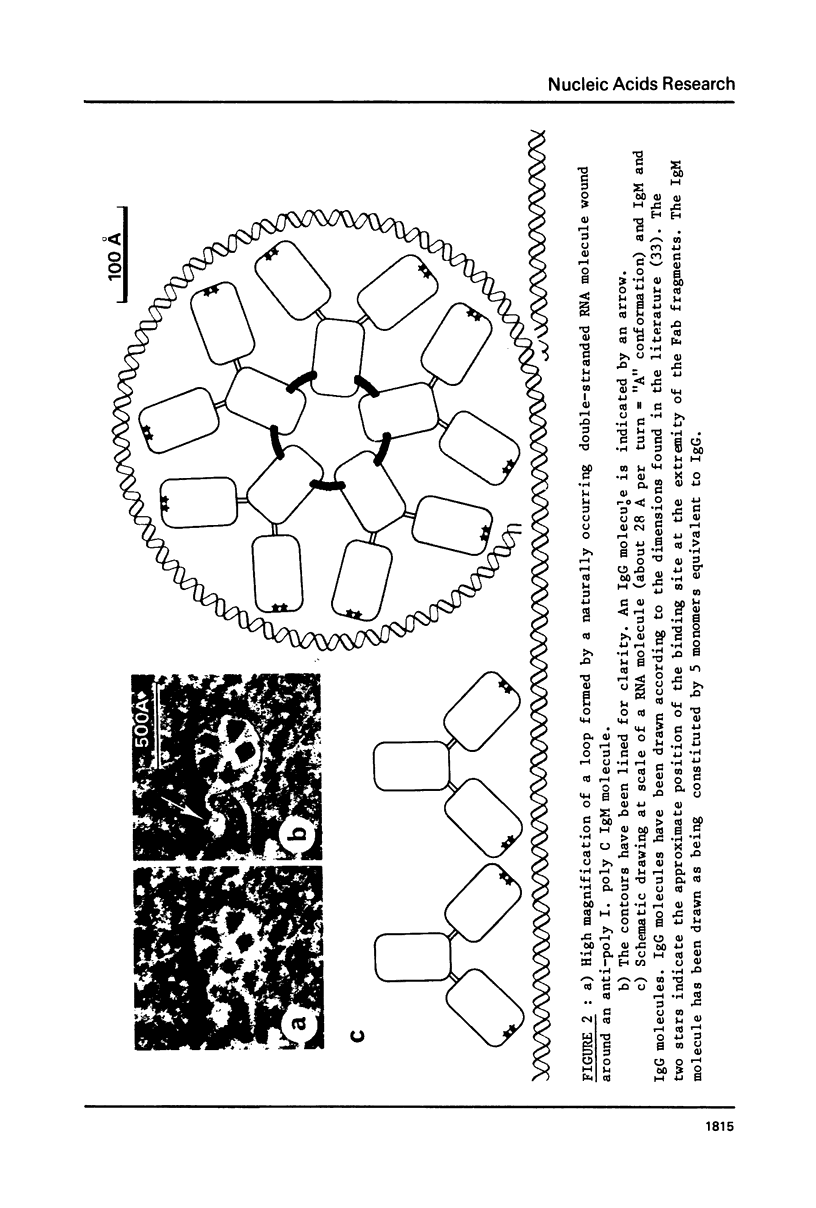
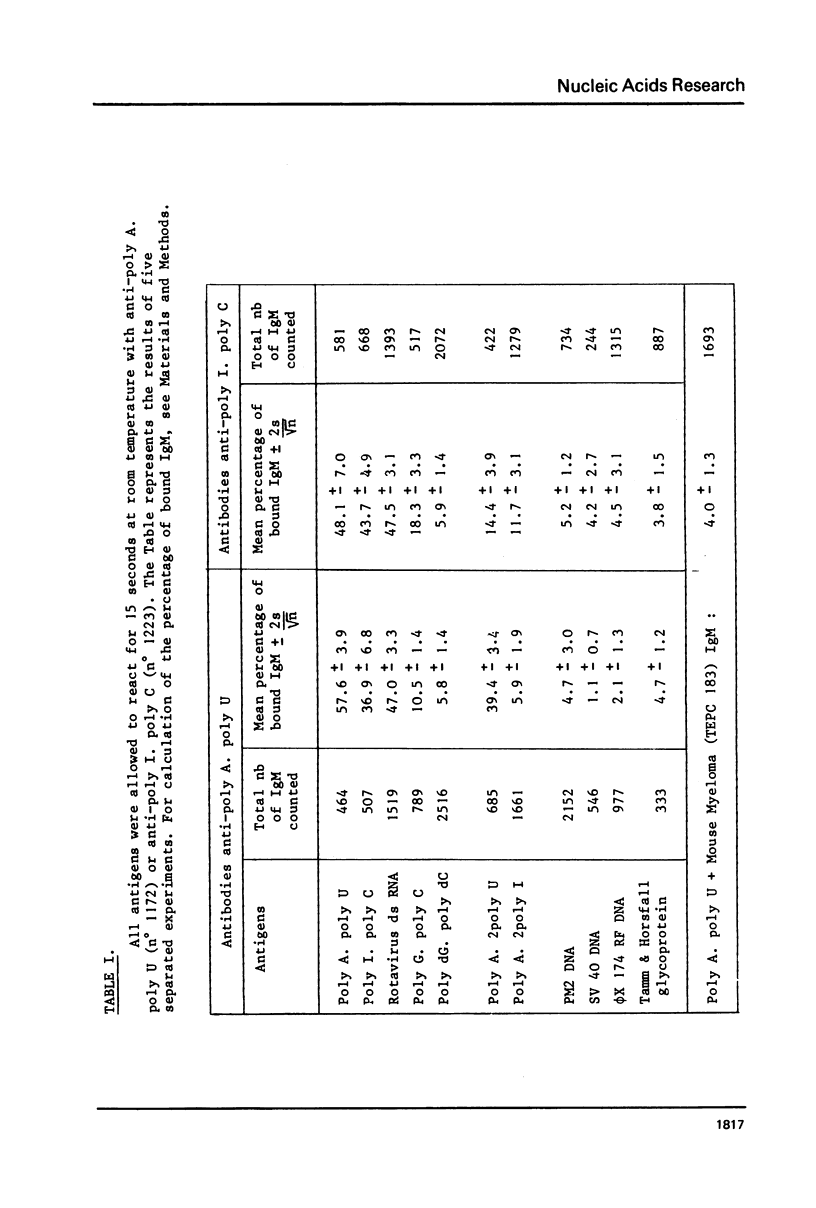
The reactions between purified anti-poly A. poly U and-poly I. poly C. antibodies (IgG and IgM), and synthetic and natural polynucleotides were visualized at the molecular level. This was achieved by the use of fine tungsten bidirectional shadowing of molecules adsorbed onto thin carbon films, combined with dark field electron microscopic observation. A progression was observed from monogamous multivalency (binding of a single multifunctional antigen molecule with several combining sites of the same antibody molecule simultaneously) (Crothers and Metzger, 1972, Immunochemistry, 9, 341-357), to aggregation. Different types of figures were observed, among which loops formed by the coiling of the antigen around a single IgM molecule were very frequently seen. The tendency of IgG antibodies to bind cooperatively to certain antigens was also noted. In contrast, cross-links were seldom encountered. The cross-reactivity of different polynucleotides was also assessed by a quantitative analysis. The length of antigen associated to an antibody molecule (either IgG or IgM) was also measured.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Lakmaker F. Immunology of DNA. V. Analysis of DNA/anti-DNA complexes. J Immunol Methods. 1976;13(3-4):241–252. doi: 10.1016/0022-1759(76)90071-5. [DOI] [PubMed] [Google Scholar]
- Arimura H. Correlation between molecular size and interferon- inducing activity of poly I:C. Acta Virol. 1975 Nov;19(6):457–466. [PubMed] [Google Scholar]
- Crothers D. M., Metzger H. The influence of polyvalency on the binding properties of antibodies. Immunochemistry. 1972 Mar;9(3):341–357. doi: 10.1016/0019-2791(72)90097-3. [DOI] [PubMed] [Google Scholar]
- Dubochet J., Ducommun M., Zollinger M., Kellenberger E. A new preparation method for dark-field electron microscopy of biomacromolecules. J Ultrastruct Res. 1971 Apr;35(1):147–167. doi: 10.1016/s0022-5320(71)80148-x. [DOI] [PubMed] [Google Scholar]
- Escaig J., Nicolas G. Cryo-fractures de matériel biologique réalisées á trés basses températures en ultra-vide. C R Acad Sci Hebd Seances Acad Sci D. 1976 Oct 27;283(10):1245–1248. [PubMed] [Google Scholar]
- FARR R. S. A quantitative immunochemical measure of the primary interaction between I BSA and antibody. J Infect Dis. 1958 Nov-Dec;103(3):239–262. doi: 10.1093/infdis/103.3.239. [DOI] [PubMed] [Google Scholar]
- Feinstein A., Munn E. A., Richardson N. E. The three-dimensional conformation of M and A globulin molecules. Ann N Y Acad Sci. 1971 Dec 31;190:104–121. doi: 10.1111/j.1749-6632.1971.tb13526.x. [DOI] [PubMed] [Google Scholar]
- Frink R. J., Eisenberg D., Glitz D. G. Localization of the site of adenylylation of glutamine synthetase by electron microscopy of an enzyme-antibody complex. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5778–5782. doi: 10.1073/pnas.75.12.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D. M. A circular dichroism study of poly dG, poly dC, and poly dG:dC. Biopolymers. 1974;13(10):2087–2102. doi: 10.1002/bip.1974.360131011. [DOI] [PubMed] [Google Scholar]
- Green N. M. Electron microscopy of the immunoglobulins. Adv Immunol. 1969;11:1–30. doi: 10.1016/s0065-2776(08)60476-9. [DOI] [PubMed] [Google Scholar]
- Hornick C. L., Karuch F. Antibody affinity. 3. The role of multivalance. Immunochemistry. 1972 Mar;9(3):325–340. doi: 10.1016/0019-2791(72)90096-1. [DOI] [PubMed] [Google Scholar]
- Johnston M. I., Stollar B. D. Antigenic structure of double-stranded RNA analogues having varying activity in interferon induction. Biochemistry. 1978 May 16;17(10):1959–1964. doi: 10.1021/bi00603a024. [DOI] [PubMed] [Google Scholar]
- Koffler D., Carr R., Agnello V., Thoburn R., Kunkel H. G. Antibodies to polynucleotides in human sera: antigenic specificity and relation to disease. J Exp Med. 1971 Jul 1;134(1):294–312. doi: 10.1084/jem.134.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacour F., Nahon-Merlin E., Michelson M. Immunological recognition of polynucleotide structure. Curr Top Microbiol Immunol. 1973;62:1–39. doi: 10.1007/978-3-642-65772-6_1. [DOI] [PubMed] [Google Scholar]
- Lake J. A., Pendergast M., Kahan L., Nomura M. Localization of Escherichia coli ribosomal proteins S4 and S14 by electron microscopy of antibody-labeled subunits. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4688–4692. doi: 10.1073/pnas.71.12.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutsch G., Noll F., Theise H., Bielka H. Localization of ribosomal protein S2 in rat liver ribosomes by immune electron microscopy. Acta Biol Med Ger. 1977;36(2):287–289. [PubMed] [Google Scholar]
- Marck C., Thiele D. Poly(dG).poly(dC) at neutral and alkaline pH: the formation of triple stranded poly(dG).poly(dG).poly(dC). Nucleic Acids Res. 1978 Mar;5(3):1017–1028. doi: 10.1093/nar/5.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson A. M., Lacour F., Nahon-Merlin E. Propriétés immunochimiques du poly G-poly C et du poly G. C R Acad Sci Hebd Seances Acad Sci D. 1971 Jan 25;272(4):669–672. [PubMed] [Google Scholar]
- Nahon-Merlin E., Lacour F., Friend C., Revoltella R. Protective effect of immunization with nonviral antigens against Friend leukemia virus in mice. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2018–2021. doi: 10.1073/pnas.76.4.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahon-Merlin E., Michelson A. M., Verger C., Lacour F. Specificity of anti-poly G-poly C antibodies. J Immunol. 1971 Jul;107(1):222–226. [PubMed] [Google Scholar]
- Nahon E., Lacour F., Michelson M. Effet de la structure secondaire des polynucléotides sur leur réaction immunologique. C R Acad Sci Hebd Seances Acad Sci D. 1967 Jan 16;264(3):515–518. [PubMed] [Google Scholar]
- Nahon E., Michelson A. M., Lacour F. Antigénicité des complexes de polynucléotides. Biochim Biophys Acta. 1967 Nov 21;149(1):127–139. [PubMed] [Google Scholar]
- Politz S. M., Glitz D. G. Ribosome structure: localization of N6,N6-dimethyladenosine by electron microscopy of a ribosome-antibody complex. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1468–1472. doi: 10.1073/pnas.74.4.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Phizackerley R. P. Studies on the three-dimensional structure of immunoglobulins. Prog Biophys Mol Biol. 1976;31(1):67–93. doi: 10.1016/0079-6107(78)90005-6. [DOI] [PubMed] [Google Scholar]
- Potter M. Antigen-binding myeloma proteins of mice. Adv Immunol. 1977;25:141–211. [PubMed] [Google Scholar]
- Schur P. H., Monroe M. Antibodies to ribonucleic acid in systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1108–1112. doi: 10.1073/pnas.63.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollar B. D. The specificity and applications of antibodies to helical nucleic acids. CRC Crit Rev Biochem. 1975 May;3(1):45–69. doi: 10.3109/10409237509102552. [DOI] [PubMed] [Google Scholar]
- Tischendorf G. W., Zeichhardt H., Stöffler G. Architecture of the Escherichia coli ribosome as determined by immune electron microscopy. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4820–4824. doi: 10.1073/pnas.72.12.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utter G., Biberfeld P., Norberg R., Thorstensson R., Fagraeus A. Ultrastructure of in vitro formed actin-anti-actin immune complexes. Exp Cell Res. 1978 Jun;114(1):127–133. doi: 10.1016/0014-4827(78)90044-7. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Larson J. E., Grant R. C., Shortle B. E., Cantor C. R. Physicochemical studies on polydeoxyribonucleotides containing defined repeating nucleotide sequences. J Mol Biol. 1970 Dec 28;54(3):465–497. doi: 10.1016/0022-2836(70)90121-x. [DOI] [PubMed] [Google Scholar]
- Wrigley N. G., Laver W. G., Downie J. C. Binding of antibodies to isolated haemagglutinin and neuraminidase molecules of influenza virus observed in the electron microscope. J Mol Biol. 1977 Jan 25;109(3):405–421. doi: 10.1016/s0022-2836(77)80020-x. [DOI] [PubMed] [Google Scholar]
- Younghusband H. B., Inman R. B. The electronmicroscopy of DNA. Annu Rev Biochem. 1974;43(0):605–619. doi: 10.1146/annurev.bi.43.070174.003133. [DOI] [PubMed] [Google Scholar]
- de Murcia G., Lang M. C., Freund A. M., Fuchs R. P., Duane M. P., Sage E., Leng M. Electron microscopic visualization of N-acetoxy-N-2-acetylaminofluorene binding sites in ColE1 DNA by means of specific antibodies. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6076–6080. doi: 10.1073/pnas.76.12.6076. [DOI] [PMC free article] [PubMed] [Google Scholar]