Abstract
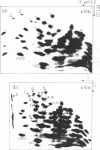
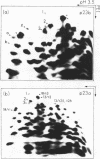
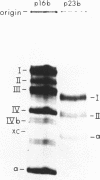
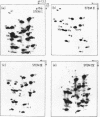
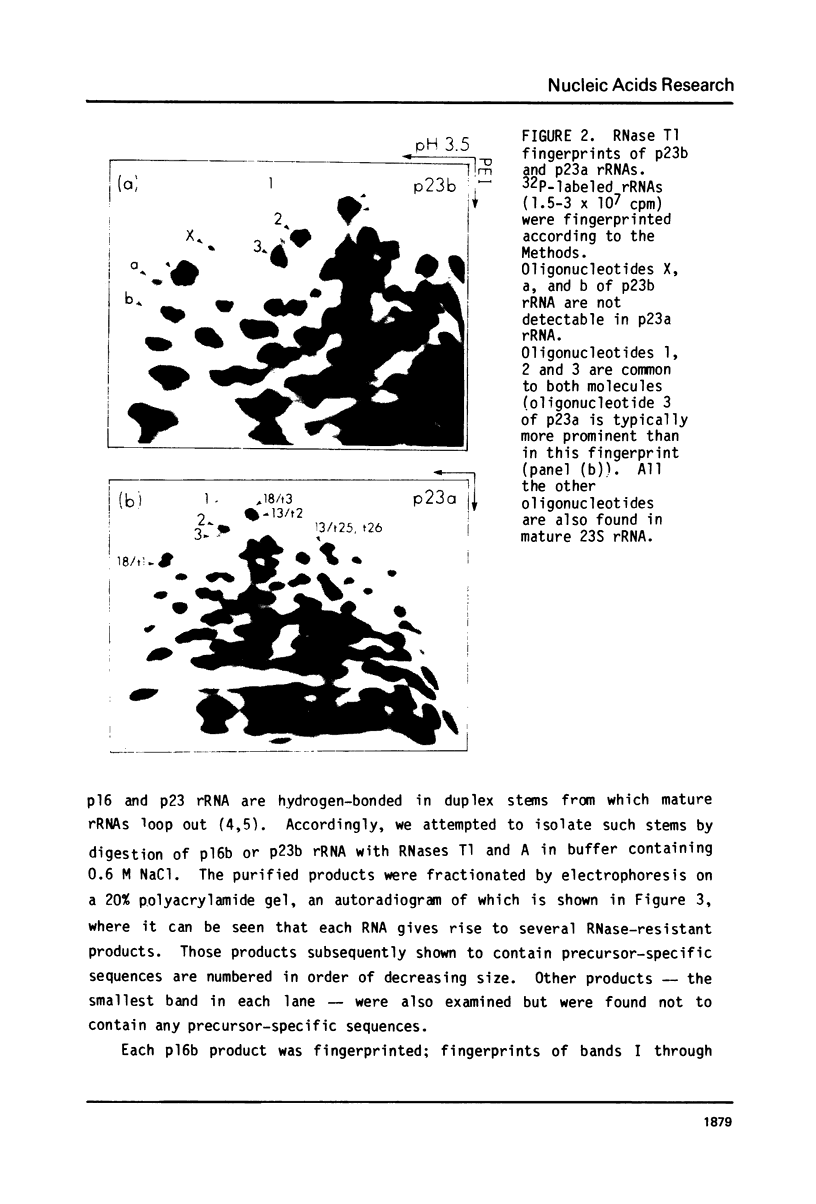
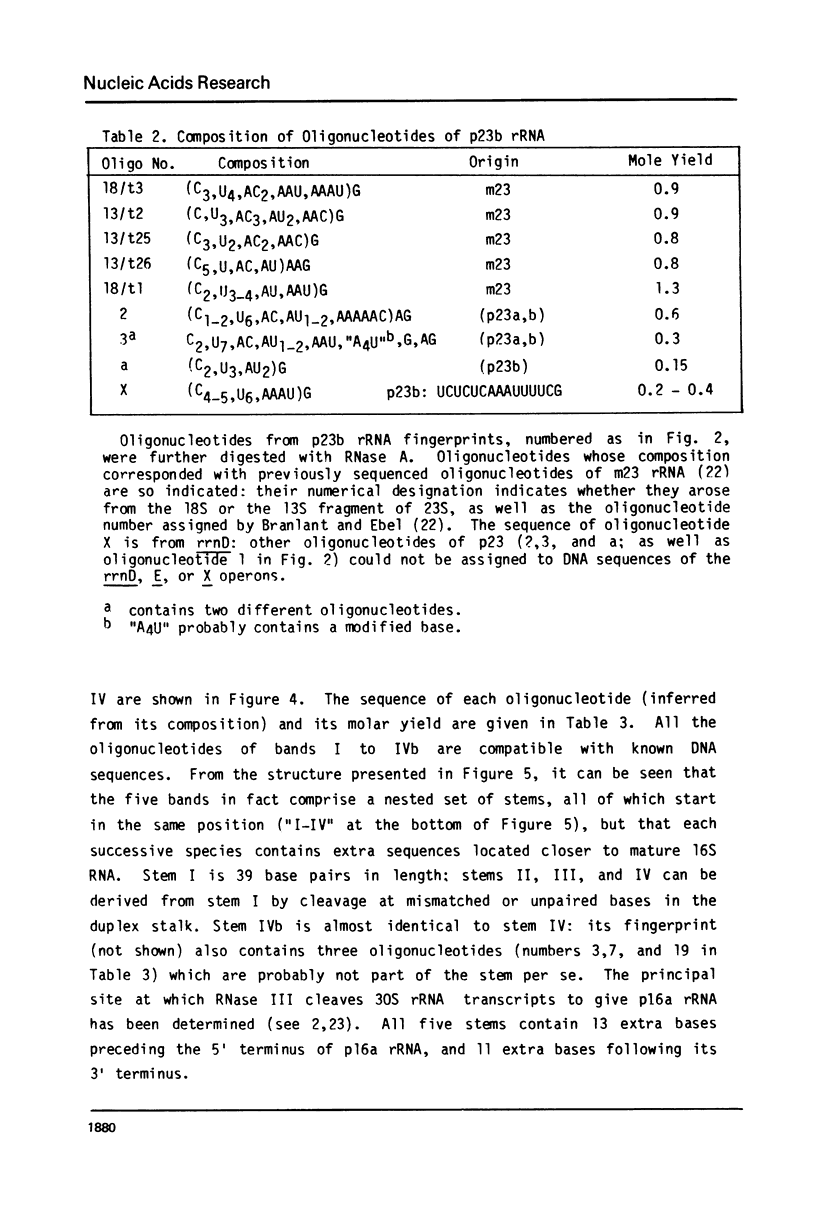
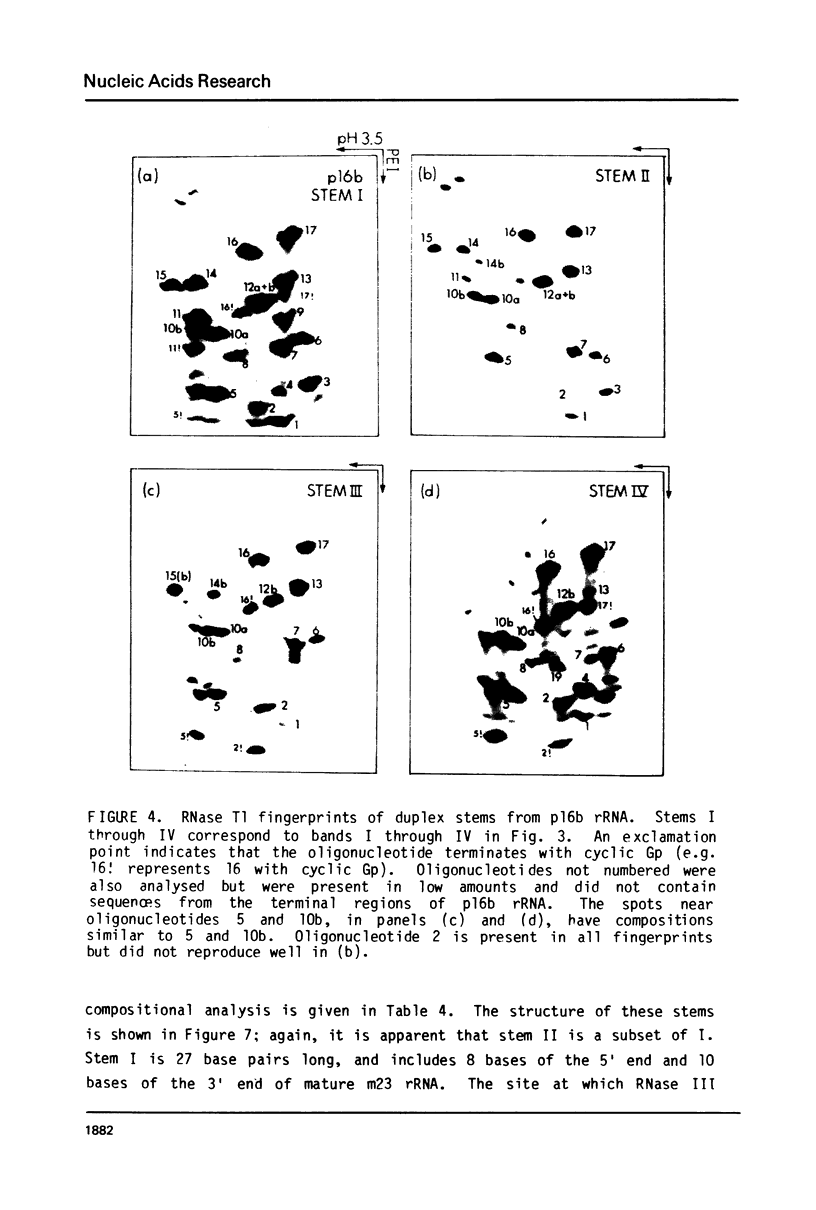
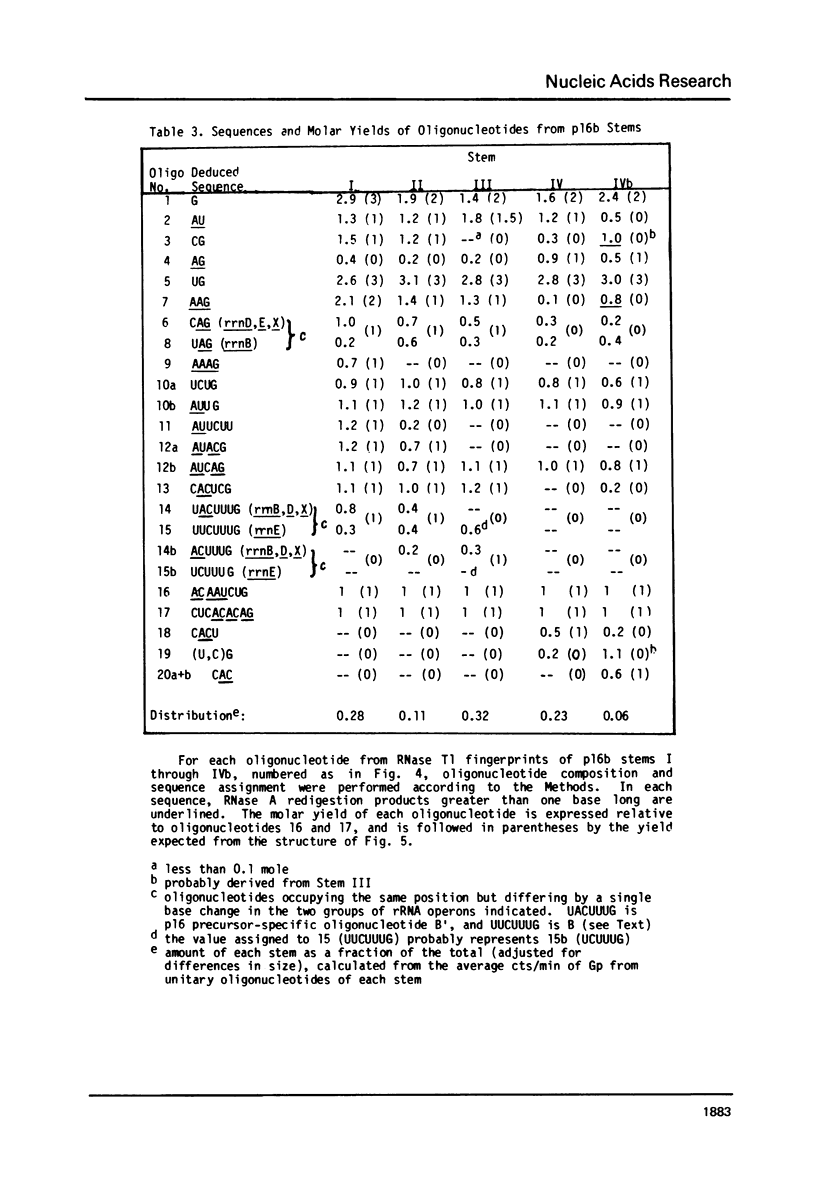
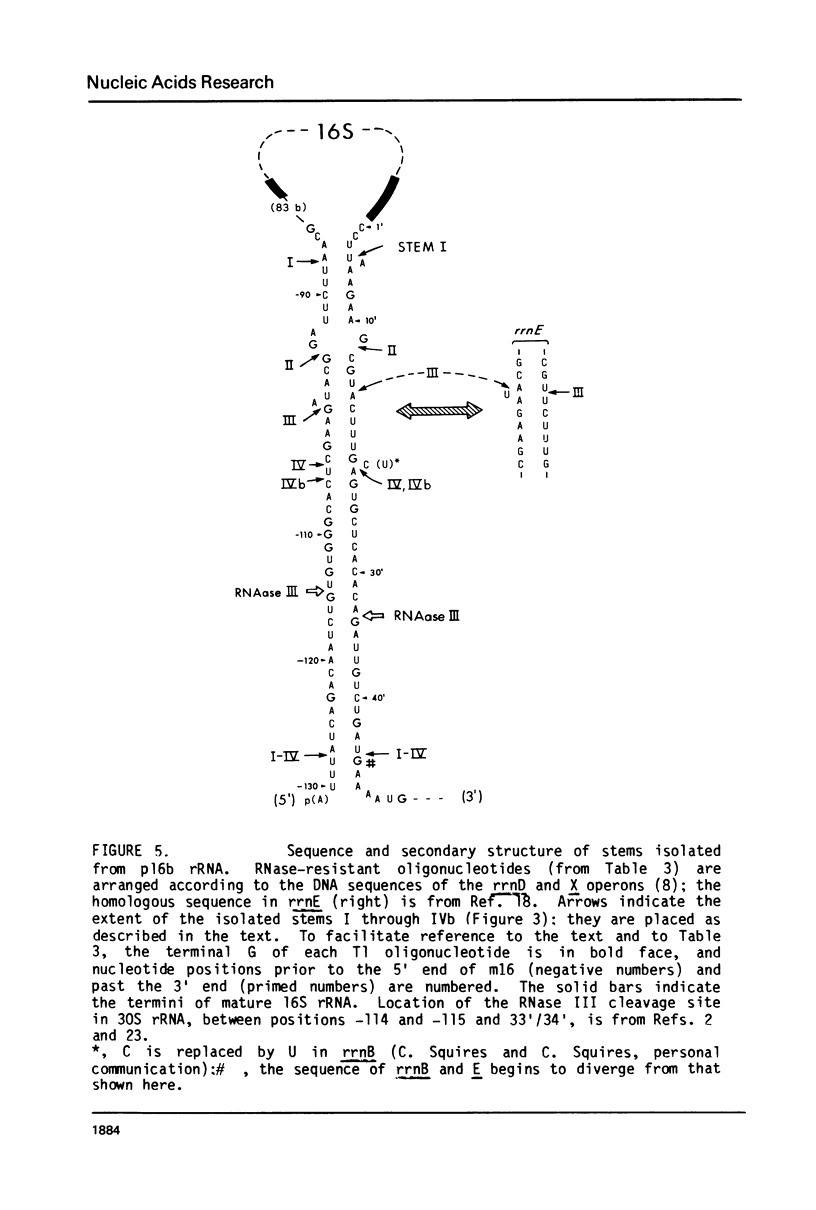
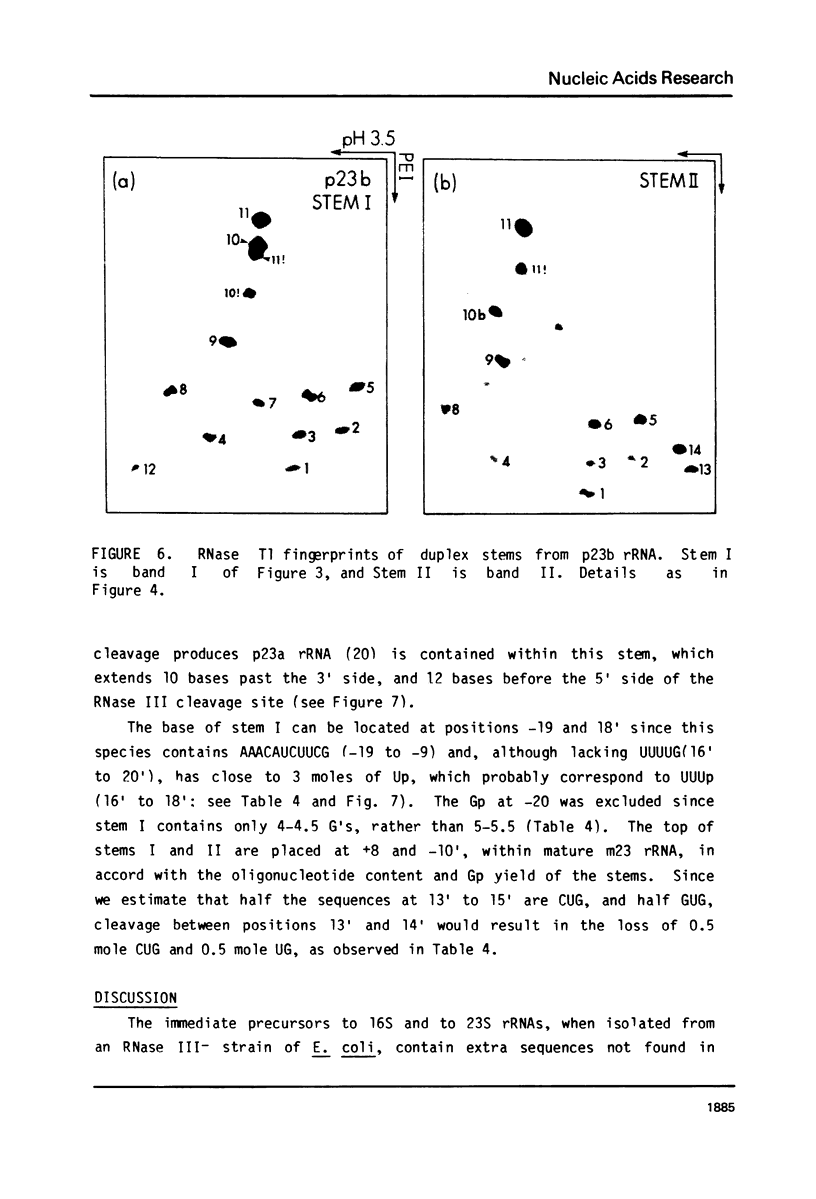
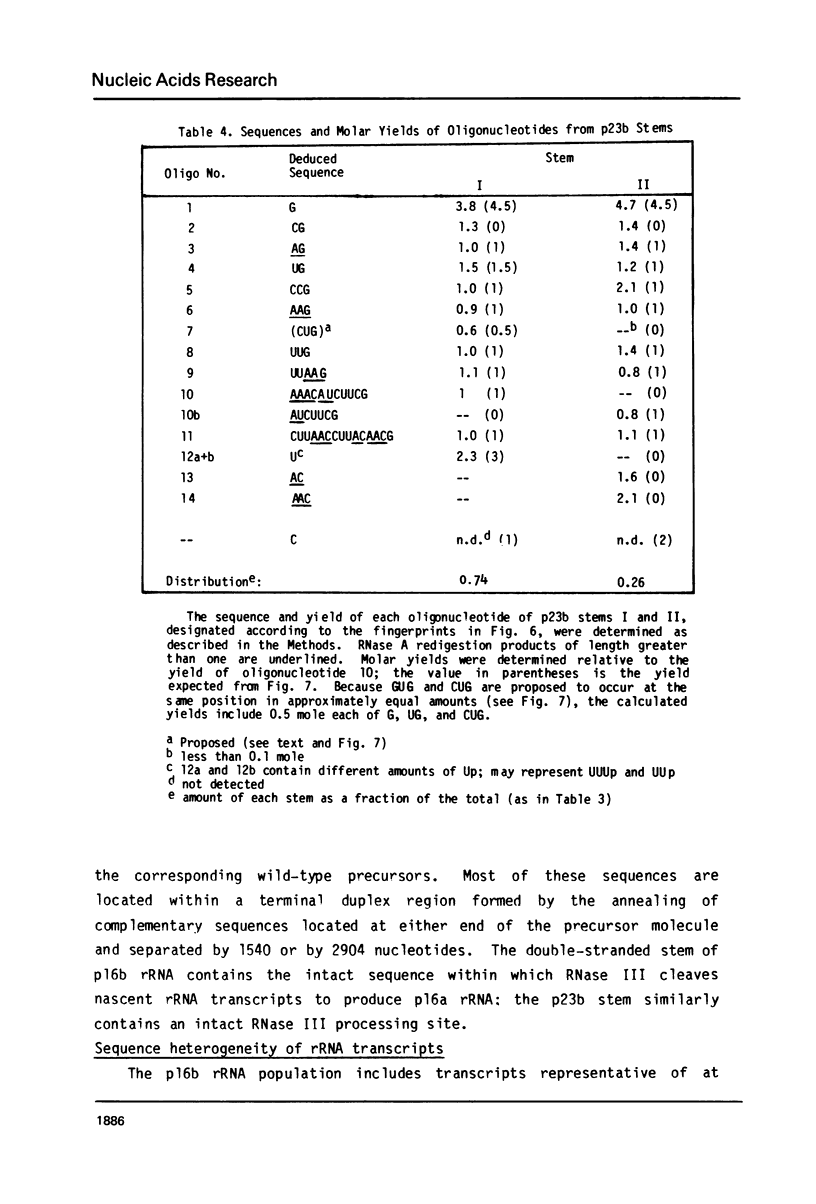
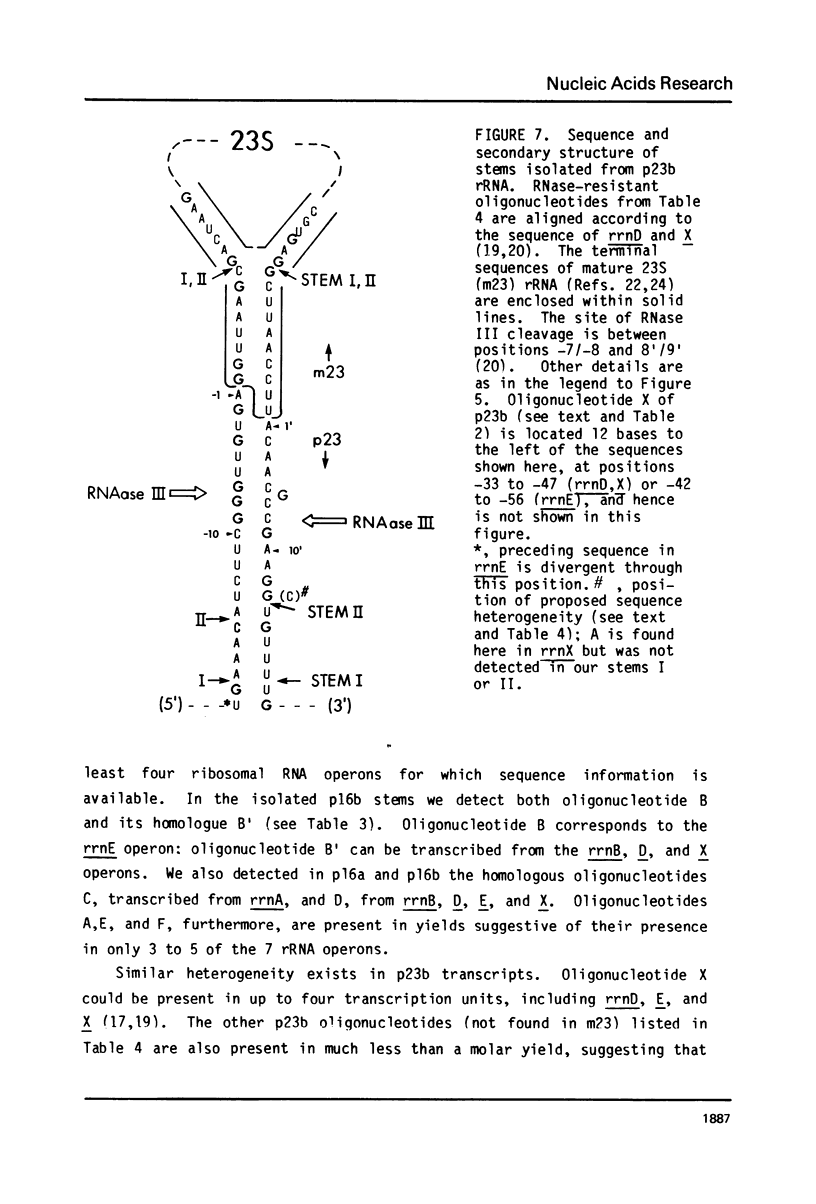
Escherichia coli cells lacking the ribosomal RNA processing enzyme RNase III do no excise the normal RNA precursors p16a (17S) and p23a from nascent rRNA transcripts. These cells produce, instead, slightly larger p16b and p23b precursors. Digestion of p16b or p23b rRNA with RNases A plus T1 yields double-stranded fragments composed of sequences, located at both the 5' and the 3' end regions of the molecules. The terminal duplex, or stem, of p16b contains sequences surrounding the site of RNase III processing which is wild-type cells produces p16a rRNA: the p23b stem likewise contains an intact RNase III cleavage site. The results confirm our earlier prediction for the structure of rRNA transcripts, and also yield a definite secondary structure for the p16 stem, which was not uniquely determined by the corresponding DNA sequence. These experiments demonstrate the absence of significant RNase III processing activity in rnc-105 strains of E. coli, and implicate the participation of another endonuclease(s) in rRNA processing in mutant and wild-type cells.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apirion D., Watson N. Mapping and characterization of a mutation in Escherichia coli that reduces the level of ribonuclease III specific for double-stranded ribonucleic acid. J Bacteriol. 1975 Oct;124(1):317–324. doi: 10.1128/jb.124.1.317-324.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bram R. J., Young R. A., Steitz J. A. The ribonuclease III site flanking 23S sequences in the 30S ribosomal precursor RNA of E. coli. Cell. 1980 Feb;19(2):393–401. doi: 10.1016/0092-8674(80)90513-9. [DOI] [PubMed] [Google Scholar]
- Branlant C., Ebel J. P. Studies on the primary structure of Escherichia coli 23 SRNA. Nucleotide sequence of the ribonuclease T1 digestion products containing more than one uridine residue. J Mol Biol. 1977 Apr 15;111(3):215–256. doi: 10.1016/s0022-2836(77)80050-8. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Noller H. F. Complete nucleotide sequence of a 23S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):201–204. doi: 10.1073/pnas.77.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg A. E., Dahlberg J. E., Lund E., Tokimatsu H., Rabson A. B., Calvert P. C., Reynolds F., Zahalak M. Processing of the 5' end of Escherichia coli 16S ribosomal RNA. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3598–3602. doi: 10.1073/pnas.75.8.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegenheimer P., Apirion D. Escherichia coli ribosomal ribonucleic acids are not cut from an intact precursor molecule. J Biol Chem. 1975 Mar 25;250(6):2407–2409. [PubMed] [Google Scholar]
- Gegenheimer P., Apirion D. Processing of rRNA by RNAase P: spacer tRNAs are linked to 16S rRNA in an RNAase P RNAase III mutant strain of E. coli. Cell. 1978 Oct;15(2):527–539. doi: 10.1016/0092-8674(78)90021-1. [DOI] [PubMed] [Google Scholar]
- Gegenheimer P., Watson N., Apirion D. Multiple pathways for primary processing of ribosomal RNA in Escherichia coli. J Biol Chem. 1977 May 10;252(9):3064–3073. [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. Initiation of Escherichia coli ribosomal RNA synthesis in vivo. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5480–5484. doi: 10.1073/pnas.76.11.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelstaedt R., Oppermann H., Koch G. Poliovirus-induced infectious double-stranded RNA: Effect of RNA-degrading enzymes. Arch Virol. 1975;47(4):381–392. doi: 10.1007/BF01347980. [DOI] [PubMed] [Google Scholar]
- Nishimura S. Minor components in transfer RNA: their characterization, location, and function. Prog Nucleic Acid Res Mol Biol. 1972;12:49–85. [PubMed] [Google Scholar]
- Pace N. R. Structure and synthesis of the ribosomal ribonucleic acid of prokaryotes. Bacteriol Rev. 1973 Dec;37(4):562–603. doi: 10.1128/br.37.4.562-603.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault J., Leavitt R. W. Inverted complementary terminal sequences in single-stranded RNAs and snap-back RNAs from vesicular stomatitis defective interfering particles. J Gen Virol. 1978 Jan;38(1):35–50. doi: 10.1099/0022-1317-38-1-35. [DOI] [PubMed] [Google Scholar]
- Sekiya T., Nishimura S. Sequence of the gene for isoleucine tRNA1 and the surrounding region in a ribosomal RNA operon of Escherichia coli. Nucleic Acids Res. 1979 Feb;6(2):575–592. doi: 10.1093/nar/6.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague K. U., Steitz J. A. The 3' terminal oligonucleotide of E. coli 16S ribosomal RNA: the sequence in both wild-type and RNase iii- cells is complementary to the polypurine tracts common to mRNA initiator regions. Nucleic Acids Res. 1975 Jun;2(6):787–798. doi: 10.1093/nar/2.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanami M. Analysis of the 5'-terminal nucleotide sequences of ribonucleic acids 1. the 5'-termini of Excherichia coli ribosomal RNA. J Mol Biol. 1967 Jan 28;23(2):135–148. doi: 10.1016/s0022-2836(67)80022-6. [DOI] [PubMed] [Google Scholar]
- Volckaert G., Fiers W. Micro thin-layer techniques for rapid sequence analysis of 32P-labeled RNA: double digestion and pancreatic ribonuclease analyses. Anal Biochem. 1977 Nov;83(1):228–239. doi: 10.1016/0003-2697(77)90531-0. [DOI] [PubMed] [Google Scholar]
- Volckaert G., Jou W. M., Fiers W. Analysis of 32P-labeled bacteriophage MS2 RNA by a mini-fingerprinting procedure. Anal Biochem. 1976 May 7;72:433–446. doi: 10.1016/0003-2697(76)90551-0. [DOI] [PubMed] [Google Scholar]
- Young R. A., Macklis R., Steitz J. A. Sequence of the 16 S-23 s spacer region in two ribosomal RNA operons of Escherichia coli. J Biol Chem. 1979 May 10;254(9):3264–3271. [PubMed] [Google Scholar]
- Young R. A., Steitz J. A. Complementary sequences 1700 nucleotides apart form a ribonuclease III cleavage site in Escherichia coli ribosomal precursor RNA. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3593–3597. doi: 10.1073/pnas.75.8.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer H. A., Gilbert S. F., Nomura M. DNA sequences of promoter regions for rRNA operons rrnE and rrnA in E. coli. Cell. 1979 May;17(1):201–209. doi: 10.1016/0092-8674(79)90308-8. [DOI] [PubMed] [Google Scholar]