Summary
Recently, investigation of new neurons in memory formation has focused on a specific function – pattern separation. However, it has been difficult to reconcile the form of separation tested in behavioral tasks with how it is conceptualized according to computational and electrophysiology perspectives. Here, we propose a memory resolution hypothesis that considers the unique information contributions of broadly tuned young neurons and highly specific mature neurons and describe how the fidelity of memories can relate to spatial and contextual discrimination.
In the past decade, there has been considerable progress in describing what adult neurogenesis contributes to cognition. In addition to being thoroughly characterized at a cellular and circuit level(Zhao et al., 2008), neurogenesis has been a target of numerous computational and behavioral studies (Aimone and Gage, 2011; Deng et al., 2010; Inokuchi, 2011). Increasingly, the functional theories of neurogenesis have coalesced around several aspects of new neuron maturation (Aimone et al., 2010a). First, immature granule cells (GCs) show an increased intrinsic excitability and plasticity that distinguishes them from the less plastic and relatively silent older GC population (Esposito et al., 2005; Ge et al., 2007). Second, this immature state of GCs represents a critical developmental period in which they encode significant features of their environments (Kee et al., 2007; Tashiro et al., 2007). Finally, the process of neurogenesis is a key component of the pattern separation function of the dentate gyrus (DG) (Clelland et al., 2009; Sahay et al., 2011). Nevertheless, in many respects, this broader understanding of the DG’s function and how it relates to the hippocampus (Treves et al., 2008) has become the limiting factor to our understanding of the function of adult neurogenesis (Aimone et al., 2010b; Alme et al., 2010).
We believe that much of the uncertainty of how neurogenesis relates to DG function is in fact not due to a misunderstanding of the experimental and theoretical findings; rather, it is a challenge of description. Increasingly, descriptions of neurogenesis function have relied on the loaded term “pattern separation,” originally a computational concept that has taken on somewhat different meanings depending on its context. In this opinion piece, we hope to clarify our interpretation of the function of neurogenesis, and more generally the DG, by describing neurogenesis and DG function using a more consistent framework.
What do we mean by “pattern separation?”
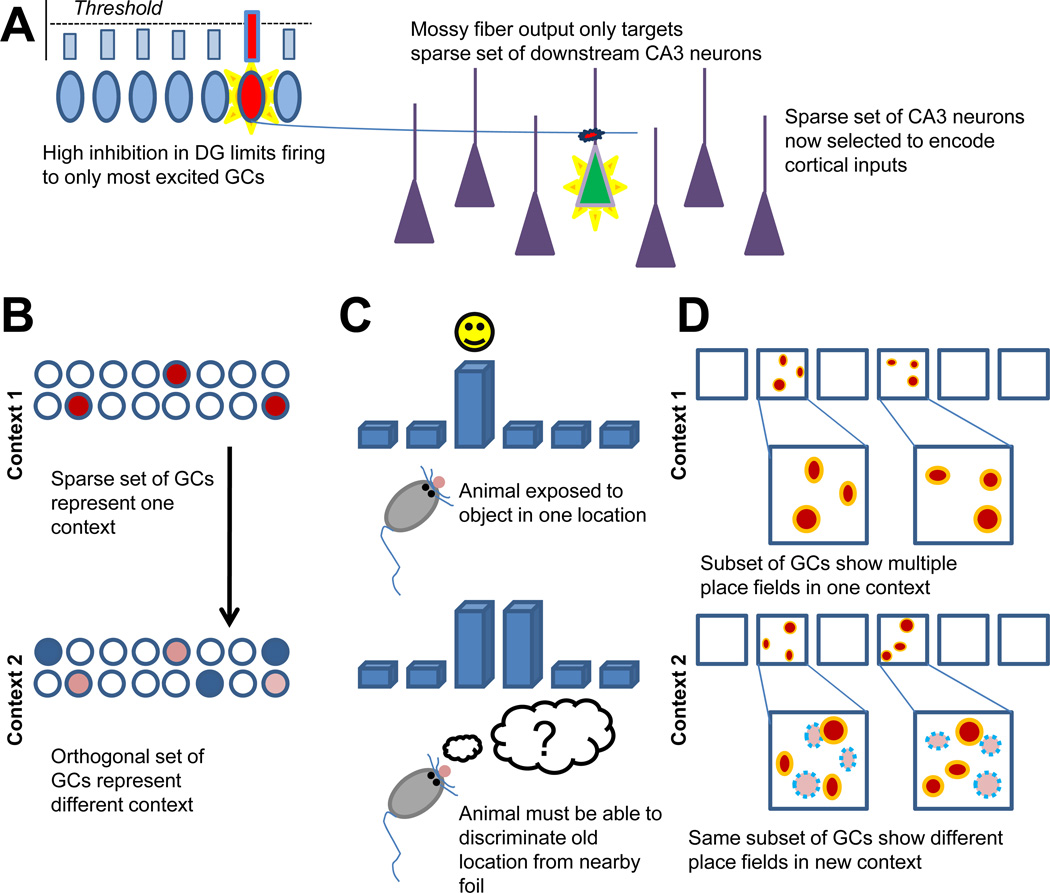
To understand the rationale for the predicted separation role for the DG, it is useful to briefly review the history of the pattern separation hypothesis (Figure 1). Although the early hippocampal modeling work of David Marr did not explicitly consider a separation role for the DG, he did predict that the recurrent axons within CA3 would be ideal for forming memory representations (Marr, 1971). Subsequent work on CA3-like recurrent networks demonstrated the value of uncorrelated inputs for attractor formation (Amit et al., 1987; Hopfield, 1982). This requirement for a separation device upstream of the CA3 was complemented by the anatomy of the DG and its unique mossy fiber projection to the CA3 (Amaral et al., 2007) (Figure 1A). Despite containing several times more neurons than either the CA3 or its entorhinal cortex (EC) inputs, the projection from DG to CA3 was extremely sparse by cortical standards, with each GC only terminating on roughly a dozen CA3 neurons. Furthermore, these synapses, known as mossy terminals, were noticeably larger and positioned very proximal to the soma of CA3 pyramidal neurons. The synapse characteristics suggested that the mossy terminals might be “detonators” for their CA3 targets and the sparse projection suggested that the DG might be responsible for establishing decorrelated patterns in the CA3 network (McNaughton and Morris, 1987; O'Reilly and McClelland, 1994; Treves and Rolls, 1992). The DG pattern separation theory was thus born (Figure 1B).
Figure 1. Summary of the basis for the pattern separation hypothesis.
A. Mechanism of DG pattern separation (sparse coding, high inhibition, strong sparse MF synapses) B. Cartoon of computational pattern separation. C. Example of behavioral pattern separation. D. Example of in vivo electrophysiology pattern separation
It was, however, a confluence of biological evidence for the pattern separation theory that solidified a general consensus in the community. The theory relied on several presuppositions that ultimately held up under experimental scrutiny. First, the mossy fibers should be very powerful, even detonator-like. In vivo patch clamp studies showed that they actually were, demonstrating that a single mossy fiber, when bursting, is capable of firing a downstream CA3 neuron (Henze et al., 2002). Second, the GC population should be essentially silent, with sparse overall activity. Early in vivo studies of the DG supported this prediction, and slice physiology demonstrated that GCs experienced a high level of tonic inhibition (Jung and McNaughton, 1993). Third, the DG should be particularly important for encoding, a function that was demonstrated by creative behavioral approaches (Kesner, 2007; Lee and Kesner, 2004).
Finally, in addition to the components of the proposed mechanism holding up under direct inspection, experiments that looked at behaviors that could be considered pattern separation have reliably supported a role for the DG (Figure 1C). Rats with lesions in their DG, but not CA1, showed a deficit on the spatial discrimination of objects that was dependent on their distance from each other on a cheeseboard (Gilbert et al., 2001). A mouse transgenic line with impaired plasticity localized to the DG showed an inability to distinguish between a shocked and non-shocked context over time (McHugh et al., 2007). Functional MRI showed that the presentation of objects that were highly similar, but not identical, to previously seen objects elicited increased blood flow in the human DG/CA3 region (Bakker et al., 2008). And, as mentioned above, a series of studies focusing on adult-born neurons suggested a pattern separation function (Clelland et al., 2009; Sahay et al., 2011). All of this evidence supported the idea that the DG is responsible for separating memories that are formed in the hippocampus.
Is “pattern separation” a proper assumption?
Nevertheless, although the proposed separation function for the DG has increasingly become accepted in the community, there are several problems with pattern separation as a function. For one, while the electrophysiology literature supports the prediction that few GCs are active at any given instant, the population behavior of GCs is not consistent with what the theoretical models predict, with the same subpopulation of neurons responding to multiple contexts, separating by “rate coding” instead of “population coding” (Alme et al., 2010; Leutgeb et al., 2007) (Figure 1D).
Along these lines, while much of the behavioral literature arguing for a pattern separation function is consistent, there are also alternative explanations. Instead of studying the ability of animals to distinguish different input patterns concurrently, the behavioral studies of the roles of the DG and neurogenesis in pattern separation have typically been designed to examine how animals’ responses to their present situation can be altered by their memories of the past input patterns (which are different from the current ones). Two types of strategies have been used in behavioral tasks for pattern separation. In some tasks, animals were trained to distinguish two input patterns, such as conditioned (CS+) and unconditioned (CS−) contexts. Specifically, initial training enabled the animals to generalize their conditioned responses to both CS+ and CS− contexts, and their ability to discriminate the CS+ and CS− contexts was subsequently tested through continuing reinforcement of the CS+ context but not the CS− context (McHugh et al., 2007; Sahay et al., 2011). It is possible that performance changes resulting from alterations in DG and/or neurogenesis may be due to defects in pattern separation, but it is also possible that other processes, such as inhibitory learning, may be involved. In other tasks, animals were trained to learn one pattern and were subsequently tested, using a working memory framework, for their ability to discriminate a learned pattern from another pattern (Clelland et al., 2009; Creer et al., 2010; Gilbert et al., 2001; Hunsaker and Kesner, 2008; Saxe et al., 2007). Paradigms using this type of task are also able to evaluate behavioral performance as a function of the extent of input pattern differences such as by varying the distance of spatial location systematically in the cheeseboard spatial discrimination task (Gilbert et al., 2001), further supporting a relationship between the pattern separation ability and behavioral outcome. However, it remains difficult to rule out in these tasks that animals may solve the task using different neural pathways according to the degree of dissimilarity between the input patterns. For example, in the cheeseboard spatial discrimination task, lesions of CA1 did not affect the performance at any of five tested pattern separation degrees, suggesting that the task could be solved independent of the trisynaptic pathway (Gilbert et al., 2001). On the other hand, lesions of CA3 affect working memory in general, making it difficult to test whether pattern separation relies on CA3 outputs other than Schaffer collaterals(Gilbert and Kesner, 2006).
Finally, there is a lack of a clear role for young neurons that would make them advantageous in the classic mechanism by which the DG provides separation. For instance, in our computational model, immature GCs are more active than the overall GC population, suggesting that young neurons actually decrease DG pattern separation in the classical sense when inputs are already highly dissimilar (Aimone et al., 2009). We interpreted this finding as a “pattern integration” effect, and hypothesized that this integration facilitated memory storage and discrimination in downstream regions. In contrast, we and others have also proposed that the plasticity of young neurons yields different functional populations at different times, potentially improving separation over time (Aimone et al., 2006; Becker and Wojtowicz, 2007). Nevertheless, it is still unclear how these proposed computational effects of immature neurons on pattern separation affect the discrimination tested in the behavioral tasks described above.
Notably, there is a potential for circularity in these interpretations (electrophysiological, behavioral, and computational) that suggest an involvement of the DG and neurogenesis in pattern separation. The initial hypothesis that the DG was responsible for pattern separation emerged from computational arguments based on basic observations of anatomy and physiology, as well as a theoretical consideration that a layer responsible for separation is beneficial to memory formation in a CA3-like network. Today, if one were presented with the full body of evidence concerning the DG, including adult neurogenesis and the physiology and behavioral results mentioned above, without any a priori assumptions, it is debatable whether “pattern separation” would even be suggested as a function.
Finally, it is worth noting that the idea that neural networks can encode two relatively similar inputs as distinct representations - and that such separation is beneficial for subsequent information processing and memory formation - is fairly fundamental to neural networks in general (O'Reilly and McClelland, 1994). Indeed, it is supposed that many brain regions have outputs that are less correlated than their inputs, and the computational act of remapping inputs to facilitate separation underlies several machine learning tools, such as support vector machines. As has been noted by others, pattern separation is a feature of most brain circuits; a role in pattern separation does not make the DG unique. In our opinion, the question is not “does the DG perform pattern separation?” but rather “what makes the separation in the DG unique?”
Do we mean “memory resolution?”
Rather than considering the function of the dentate gyrus as “pattern separation,” we propose that it may be better to refer to the DG’s function as controlling “memory resolution.” By memory resolution, we are referring to the extent of information encoded by the DG, and thus the downstream hippocampal regions, during memory formation. The encoding of more information yields memories that are robust enough to support finer discrimination in downstream regions. At one level, this difference in terminology is purely semantic –we are not proposing a radically different function for the DG than what is generally assumed. However, at a deeper level, a “memory resolution” lens is perhaps better suited for interpreting the results that the “pattern separation” lens has been increasingly unable to accommodate. Improving memory resolution can improve subsequent pattern separation at a behavioral level, even if the DG signal on its own does not “separate” in the manner originally predicted.
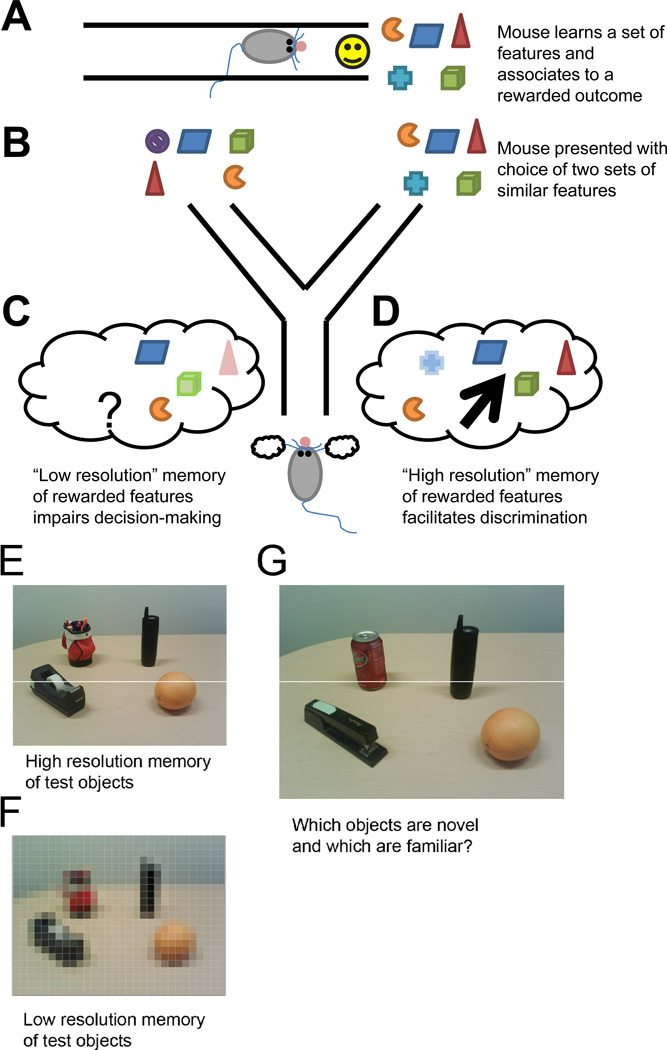
A simple example is shown in Figure 2. Suppose that an event (Figure 2A) is experienced and communicated to the hippocampus. The memory for this event is retrieved at some point in the future to make a decision (Figure 2B). Suppose the DG’s representation of this event consists of a very sparse representation and thus is at a low resolution. Some of the features that are encoded may be very precise, but the overall information stored in the memory is still sparse (Figure 2C). As a result, at a later time when the memory is compared to another experience, there is not sufficient information to determine whether the two experiences are the same or different. In this idealized example, the sparse code of the DG could actually impair later pattern separation by virtue of its weak memory encoding.
Figure 2. Cartoon demonstration of how memory resolution can lead to discrimination.
A. Cartoon example of “remembered” event. B. Task where A is being compared with a foil event. C. Low resolution “memory” of A impairs discrimination. D. High resolution “memory” of A enables discrimination. E. High resolution “memory” of real-world objects. F. Low resolution “memory” of objects. G. Discrimination is easier with high resolution memory
Now, suppose that the DG’s representation of the event utilizes more neurons and is thus at a higher resolution (Figure 2D). By the conventional pattern separation lens, this condition would actually hurt separation since the DG’s representation would be less sparse and thus less orthogonal to other memories. However, the information encoded in the memory is now sufficient for other brain regions to discriminate the memory from a current experience. Similarly, one can analogize the relative values of high and low resolution memories to that of a high resolution (Figure 2E) and a pixilated (Figure 2F) photograph. While the pixilated “memory” may contain information to make some distinctions, it is not nearly as informative as a high resolution memory (Figure 2G).
Memory resolution and neurogenesis
The examples in Figure 2 show how increased resolution can ultimately improve separation. But how does this proposed description account for adult neurogenesis, the process that we believe pattern separation struggles to explain? Does considering memory resolution provide any insight into the function of new neurons?
Several modeling studies, including our own, have noted that the presence of more active immature neurons in the DG would impair pattern separation in the classic sense since it would increase correlations across the GCs’ responses to inputs (Aimone et al., 2009; Weisz and Argibay, 2009). However, while the information encoded by immature neurons is lower and more redundant with other neurons, it is still possible that the young neurons could nonetheless add to the overall information content of the DG. This contribution could still be significant even if immature neurons only encode a fraction of the unique information that is contributed by mature GCs, since only a small number of mature GCs are active at any given instant. The immature neurons can therefore increase memory resolution, and thus an animal’s ability to discriminate at a behavioral level, even if their direct effect on DG orthogonalization is negative.
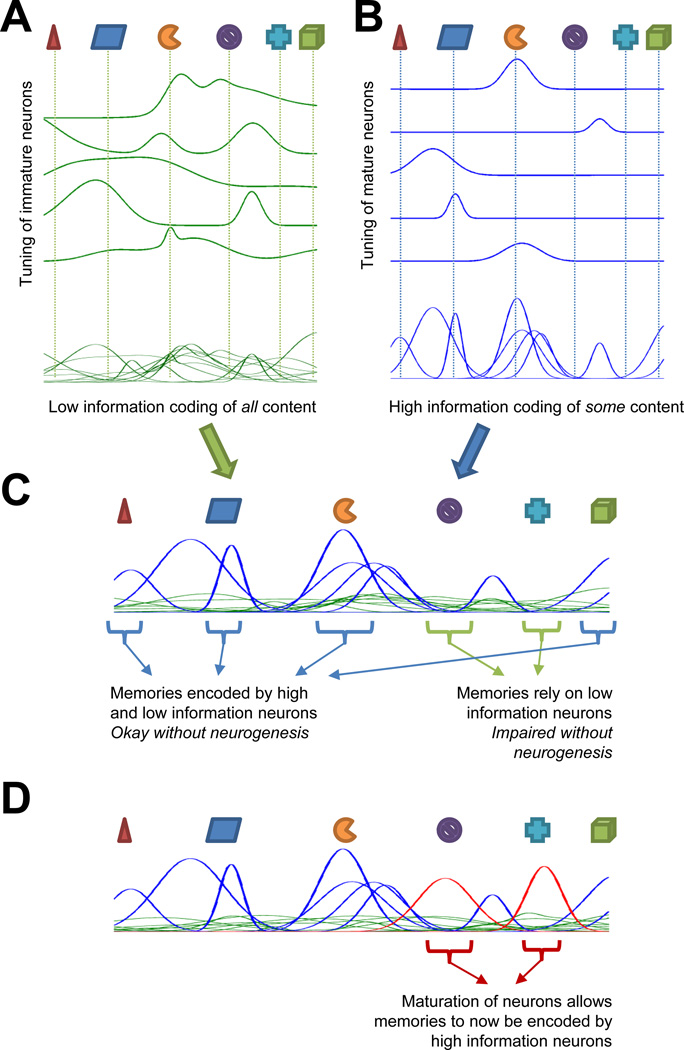
According to this framework, continuous neurogenesis results in a combination of signals from the DG to the CA3 that consists of two separate populations (Figure 3):
A population of broadly tuned GCs that weakly encode most of the features of the environment (Figure 3A)
A sparse population of sharply tuned GCs that strongly encode only those features that have been previously experienced (Figure 3B)
Figure 3. Cartoon depiction of how neurogenesis would affect memory resolution of novel and familiar features.
A. Immature GCs provide a complete yet low-information representation of experienced features. B. Mature GCs provide an incomplete yet high-information representation. C. Combination of mature and immature GCs provides a complete representation while preserving low correlations and overall information content. D. Maturation converts young GCs into high information neurons specific for experienced events
By itself, the latter population is most similar to the classic pattern-separating DG network; however, while its encoding may be nearly orthogonal, it may not relay enough information to allow subsequent discrimination (Figure 2). Likewise, on its own, the first population may contain information about the remembered event, but this information is in a sense “noisy” in that it lacks specificity. Combined, however, the two populations are capable of maximizing the information encoded while preserving the sparse coding of the overall active population (Figure 3C).
We propose that neurogenesis is actually capable of affecting this process in several ways. Clearly, the presence of “hyperexcitable” immature neurons provides a population of broadly tuned neurons, such as shown in Figure 3A. Due to their physiology and low connectivity, these immature neurons will be responsive to a wide range of inputs and overlap considerably with one another. While individually they are not as informative as mature cells (by virtue of their responding to many inputs), as a population they can still contain some specificity about their inputs. Importantly, because these neurons are responsive to a wide range of inputs, not as many young neurons are required to ensure that at least a few are responsive to any potential input to the DG. For this reason, the population of immature neurons does not need to be very large relative to the more sparsely active, sharply tuned mature neurons.
Less obvious, but equally as important, is the proposed role of neurogenesis in forming the sharply tuned GC population (Figure 3B). A sparse population is thought to be necessary for memory encoding in the hippocampus: attractor formation in the CA3 requires fairly separate inputs to adequately form memories that do not interfere with one another (Treves and Rolls, 1992). However, although the DG is large relative to other regions, there are not enough neurons available to ensure an ability to encode every possible input that may be experienced. For this reason, the experience-dependent specialization of maturing neurons to features of their environment is important to ensure that the mature GC population consists of neurons that are capable of responding to the key features of most environments. By directing maturing new neurons to preferentially respond to those inputs that they experienced when developing, the DG will be increasingly likely to have some neurons that will be capable of responding to any environment that the animal is likely to encounter (Figure 3D).
While the above description focuses on the DG, it is worth considering how this resolution may affect memory encoding within the CA3. It is important to note that the same CA3 neurons will receive inputs from a combination of both mature and immature neurons. While the potency of mossy fibers from immature GCs on CA3 is not fully understood (Toni et al., 2008), one possibility is that CA3 pyramidal neurons can only respond to single GCs if they are high information, and in contrast combinations of multiple active low information GCs may be required to induce CA3 activity. According to most classic hippocampal models, the active CA3 population, which only contains those neurons that receive inputs from informative mature GCs or groups of immature GCs, would then become bound to each other (through recurrent CA3 connections) and the direct EC inputs (Marr, 1971; Treves and Rolls, 1992). Thus when the “memory” is formed in CA3, rather than acting as an unsupervised training signal (i.e., random DG neurons active), the DG would provide a supervised cue based on the animal’s life experience up to that point.
In summary, the memory resolution hypothesis predicts that immature GCs provide a low-specificity yet densely sampled representation of cortical inputs, whereas mature GCs provide a highly specific yet sparse representation of an event. This combined representation maximizes the information encoded by hippocampal memories, thus increasing the memory’s resolution (behavioral discrimination), while keeping the memories formed distinct and minimizing interference in downstream attractor networks (computational pattern separation). Memories consisting of more familiar features would be expected to rely disproportionately on the mature population, and thus have a particularly high resolution and a relative insensitivity to the presence of young neurons. In contrast, adult neurogenesis is particularly important for the resolution of memories of particularly novel events since novel events would likely utilize fewer mature neurons. Notably, this mix between a mature neuron population optimally set up to respond to past experiences and a population of immature neurons with a capability to encode unforeseen events is reminiscent of the adaptive immune system where B and T cells are capable of responding to a novel infection by using naïve cells that must develop the ability to fight antigens, whereas memory B and memory T cells can facilitate a rapid immunological response to address re-exposure to a past infection.
Memory resolution and behavior
According to the memory resolution hypothesis, it is conceivable that damage to the DG or neurogenesis would affect the quality of the formed memory, which can only be detected when the behavioral task requires high memory precision. In agreement with this hypothesis, animals with problems in memory resolution will have difficulties in solving the “pattern separation” behavioral tasks when the similarity between patterns is high. For example, a lesion of the DG resulted in a deficit in distinguishing the spatial locations that were proximate but not distant from each other (Gilbert et al., 2001). Similarly, without enriched information mediated by plasticity of the DG, animals were delayed in discriminating different contexts (McHugh et al., 2007). In fact, the DG is involved in detecting fine spatial changes in the environment (Hunsaker et al., 2008), probably through adding more detailed information into memory to enhance its resolution.
Alterations in neurogenesis in the DG may also affect memory resolution. In particular, the immature neurons in the DG are mostly responsible for adding the broadly tuned but enriched information to the memory. As a result, an increase in the number of immature neurons improved animals’ performance on the tasks demanding high memory resolution (Creer et al., 2010; Sahay et al., 2011), whereas a decrease in neurogenesis resulted in deficits in solving these tasks (Clelland et al., 2009). Likewise, a reduction in memory resolution due to decreased neurogenesis could underlie an impaired performance on other hippocampus-mediated behavioral tasks. For example, high resolution memories could be predicted to be more robust and long lasting. Consistently, animals with reduced neurogenesis can perform well on short-term memory tests but not long-term memory tests in Morris water maze (Deng et al., 2009; Snyder et al., 2005). Similarly, variability in apparatus settings and testing paradigms may have different effects on memory resolution, which could help explain the detection of behavioral phenotypes in some cases but not others (Dupret et al., 2008; Garthe et al., 2009); reviewed by Deng et al., 2010).
What type of behavioral paradigms could be used to test memory resolution explicitly? While discrimination tasks are testing resolution, it is often unclear if deficits occurred during the initial encoding or at the time of retrieval. Since we predict the DG’s control of memory resolution is primarily associated with encoding, an ideal task would have distinct encoding and retrieval stages. While this is challenging in operant training tasks, it is potentially feasible using certain paradigms of context fear conditioning. In addition to discrimination tasks, it would also be interesting to consider tasks whose behavioral readout is parametric (e.g., probe trials in the Morris water maze and the Barnes maze); the proximity of a test behavior to a trained behavior could be a proxy for the resolution of the memory. We would further predict that the extent of neurogenesis dependence could be modulated by varying how familiar and novel features of the trained contexts are.
Memory resolution and physiology
Perhaps the greatest challenge to the pattern separation hypothesis has been the limited in vivo physiology data available in the DG. Specifically, when rats were exposed to two distinct environments, the same small population of DG neurons had multiple place fields within each environment, with almost no neurons being unique to one context or the other (Alme et al., 2010; Leutgeb et al., 2007). While the rate coding between contexts could be shown to be consistent with a pattern separation function, this observation was clearly at odds with the presumed population coding mechanism of the DG (Treves et al., 2008). One potential explanation suggested by the authors is that these broadly tuned DG neurons were in fact adult-born GCs, with older neurons having “retired” from the network (Alme et al., 2010).
The prediction that the broadly tuned DG neurons observed in vivo belong to an immature population of GCs is consistent with the role for immature neurons in memory resolution above. Nonetheless, the memory resolution hypothesis still predicts a population of GCs that are highly specific to a given context. Similarly, supporting evidence can be found in a mouse model where plasticity in the DG was impaired by a conditional knock-out of NMDA (McHugh et al., 2007). In these mice, in vivo recordings of CA1 neurons demonstrated that place fields were larger and that rate remapping between two environments was impaired in CA3. These observations are consistent with less information being communicated from the DG to these downstream regions in these mice.
Memory resolution and computational models
Finally, it is necessary to revisit the computational models of the hippocampus, DG, and adult neurogenesis. While some models have assumed the pattern separation function and have sought to reassess the mechanism by which the DG network decorrelates its inputs (Myers and Scharfman, 2009), there are other models that have explored other potential roles for the DG. Relevant to this discussion, there have been several models that discuss the DG’s contribution to hippocampal processing as being more sophisticated than simply separating inputs to the hippocampus, such as a proposal that the DG and hilus form a loop that acts as an error device to hetero-associations formed in CA3 (Lisman, 1999). Likewise, recent models that have explored the DG’s role in transforming the EC “grid cells” into the place cells common in the CA3 and CA1 may be better understood from a memory resolution view than from a pattern separation perspective (Renno-Costa et al., 2010).
The information content of the DG has been analyzed explicitly in several modeling studies. Indeed, it has been suggested that it is the high information content of a very few active GCs that is necessary for discrete attractor formation in CA3 (Treves and Rolls, 1992), and this analysis has been extended to show that the low firing rates and sparse connectivity of GCs, when their in vivo spatial behavior (Leutgeb et al., 2007) is considered, is important in determining the information content of place fields in CA3 (Cerasti and Treves, 2010). Although the contributions introduced by adult neurogenesis are not considered, this elegant information-based approach supports the idea that the properties of the DG contribute to the resolution of memories and that pattern separation can be considered a byproduct of this function.
Interestingly, other than a few exceptions, neurogenesis models have not directly discussed the role of new neurons in pattern separation; rather, they have emphasized two functions: a reduction of interference and an increase in hippocampal capacity. For instance, in models of the full hippocampal loop (EC→DG→CA3→CA1→EC), the presence of neurogenesis, either by replacement (Becker, 2005) or addition (Weisz and Argibay, 2009), has been shown to improve the whole network’s ability to store and recall information. While this avoidance of interference is similar to the classic pattern separation idea, the mechanism is again quite different from the classic proposal: neurogenesis is changing the neurons available to encode memories, so by definition the network encodes new information differently from old information. The interference reduction is thus increasing separation over time.
Although these neurogenesis models initialize new neurons differently, for a variety of reasons they reliably tend to be more plastic or trainable than “old” neurons. As a result, many of the neurogenesis models show a behavior consistent with the memory resolution mechanism shown in Figure 3: old neurons are responsible for encoding features similar to familiar memories and new neurons tend to be better suited for encoding novel features that are poorly encoded by the older neurons in the network (Aimone and Gage, 2011). The observation that the dichotomy of new and old neurons is preserved across a wide spectrum of models suggests that it may be a fairly robust prediction.
Summary
Although “pattern separation” as a concept evokes a strong intuitive understanding among hippocampal researchers, the term suffers from being both too general and too narrow at the same time. It is too general in that almost any behavior or physiology result can be considered a separation effect. As a result, it is very difficult to reconcile the “separation” behaviors that have been identified in the DG computationally, behaviorally, and physiologically (Figure 1). At the same time, despite being the site of adult neurogenesis, a unique and highly complex form of plasticity, the classic DG pattern separation theory has long constrained the DG into a relatively simple orthogonalization function.
The memory resolution concept suggested here seeks to alleviate the confusion associated with “pattern separation” by focusing on what information the DG contributes to hippocampal memories. Resolution is directly related to the amount of information incorporated into memories. Memories incorporating more information ultimately will facilitate discrimination in cognitive regions of the brain; likewise low-resolution memories will be difficult to separate (Figure 2). However, resolution also refers to the nature of how this information is encoded. Memory formation in hippocampus-like networks benefits from sparse coding and low correlations between memories. Highly specific and narrowly tuned, mature GCs carry considerable information individually, and in response to appropriate inputs are capable of generating a highly specific sparse code. However, in the absence of familiar inputs to drive mature GCs, the presence of broadly tuned young GCs will contribute to the encoding of memories while at the same time learn to become specialized, high-information neurons in the future. As a result, neurogenesis allows the resolution of novel and familiar memories to be appropriately tailored to balance the immediate (low correlation) and long-term (high information) requirements of memory encoding (Figure 3).
In conclusion, we are presenting memory resolution not as a novel function of new neurons but rather as a new perspective with which to view the range of proposed functions for neurogenesis and the DG. Indeed, we do not believe that a memory resolution view conflicts with other functions proposed for adult neurogenesis, such as a role in encoding temporal context or memory consolidation (Aimone et al., 2006; Becker and Wojtowicz, 2007; Kitamura et al., 2009); rather, we suspect that new neurons potentially affect multiple aspects of memory formation. Such proposed functions may indeed better fit into a memory resolution framework than into the classic pattern separation one. It is our hope that considering the DG in terms of memory resolution may diffuse the confusion due to the conflicting definitions associated with the “pattern separation” hypothesis and improve our understanding of how neurogenesis affects the DG and memory in the process.
Acknowledgments
We thank Mary Lynn Gage for editorial comments and Jamie Simon for assistance with illustrations. This work was funded in part by the James S. McDonnell Foundation and National Institutes of Health (R01-MH090258).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aimone JB, Deng W, Gage FH. Adult neurogenesis: integrating theories and separating functions. Trends Cogn Sci. 2010a;14:325–337. doi: 10.1016/j.tics.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Deng W, Gage FH. Put them out to pasture? What are old granule cells good for, anyway…? Hippocampus. 2010b;20:1124–1125. doi: 10.1002/hipo.20867. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Gage FH. Modeling new neuron function: a history of using computational neuroscience to study adult neurogenesis. Eur J Neurosci. 2011;33:1160–1169. doi: 10.1111/j.1460-9568.2011.07615.x. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alme CB, Buzzetti RA, Marrone DF, Leutgeb JK, Chawla MK, Schaner MJ, Bohanick JD, Khoboko T, Leutgeb S, Moser EI, et al. Hippocampal granule cells opt for early retirement. Hippocampus. 2010;20:1109–1123. doi: 10.1002/hipo.20810. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Scharfman HE, Lavenex P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies) Prog Brain Res. 2007;163:3–22. doi: 10.1016/S0079-6123(07)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit DJ, Gutfreund H, Sompolinsky H. Statistical Mechanics of Neural Networks near Saturation. Annals of Physics. 1987;173:30–67. [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S. A computational principle for hippocampal learning and neurogenesis. Hippocampus. 2005;15:722–738. doi: 10.1002/hipo.20095. [DOI] [PubMed] [Google Scholar]
- Becker S, Wojtowicz JM. A model of hippocampal neurogenesis in memory and mood disorders. Trends Cogn Sci. 2007;11:70–76. doi: 10.1016/j.tics.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Cerasti E, Treves A. How informative are spatial CA3 representations established by the dentate gyrus? PLoS Comput Biol. 2010;6:e1000759. doi: 10.1371/journal.pcbi.1000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One. 2009;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. The role of the dorsal CA3 hippocampal subregion in spatial working memory and pattern separation. Behav Brain Res. 2006;169:142–149. doi: 10.1016/j.bbr.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Henze DA, Wittner L, Buzsaki G. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat Neurosci. 2002;5:790–795. doi: 10.1038/nn887. [DOI] [PubMed] [Google Scholar]
- Hopfield JJ. Neural networks and physical systems with emergent collective computational abilities. Proc Natl Acad Sci U S A. 1982;79:2554–2558. doi: 10.1073/pnas.79.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. Evaluating the differential roles of the dorsal dentate gyrus, dorsal CA3, and dorsal CA1 during a temporal ordering for spatial locations task. Hippocampus. 2008;18:955–964. doi: 10.1002/hipo.20455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Rosenberg JS, Kesner RP. The role of the dentate gyrus, CA3a,b, and CA3c for detecting spatial and environmental novelty. Hippocampus. 2008;18:1064–1073. doi: 10.1002/hipo.20464. [DOI] [PubMed] [Google Scholar]
- Inokuchi K. Adult neurogenesis and modulation of neural circuit function. Curr Opin Neurobiol. 2011 doi: 10.1016/j.conb.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3:165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nature Neuroscience. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kesner RP. A behavioral analysis of dentate gyrus function. Prog Brain Res. 2007;163:567–576. doi: 10.1016/S0079-6123(07)63030-1. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H, Inokuchi K. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139:814–827. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Encoding versus retrieval of spatial memory: double dissociation between the dentate gyrus and the perforant path inputs into CA3 in the dorsal hippocampus. Hippocampus. 2004;14:66–76. doi: 10.1002/hipo.10167. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron. 1999;22:233–242. doi: 10.1016/s0896-6273(00)81085-5. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Morris RGM. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends in Neurosciences. 1987;10:408–415. [Google Scholar]
- Myers CE, Scharfman HE. A role for hilar cells in pattern separation in the dentate gyrus: a computational approach. Hippocampus. 2009;19:321–337. doi: 10.1002/hipo.20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- Renno-Costa C, Lisman JE, Verschure PF. The mechanism of rate remapping in the dentate gyrus. Neuron. 2010;68:1051–1058. doi: 10.1016/j.neuron.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011 doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew MV, Kandel ER, Hen R. Paradoxical Influence of hippocampal neurogenesis on working memory. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4642–4646. doi: 10.1073/pnas.0611718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves A, Rolls ET. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus. 1992;2:189–199. doi: 10.1002/hipo.450020209. [DOI] [PubMed] [Google Scholar]
- Treves A, Tashiro A, Witter ME, Moser EI. What is the mammalian dentate gyrus good for? Neuroscience. 2008;154:1155–1172. doi: 10.1016/j.neuroscience.2008.04.073. [DOI] [PubMed] [Google Scholar]
- Weisz VI, Argibay PF. A putative role for neurogenesis in neuro-computational terms: inferences from a hippocampal model. Cognition. 2009;112:229–240. doi: 10.1016/j.cognition.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]