Abstract
Aims/hypothesis
Two US randomized trials found a lower incidence of type 2 diabetes among women treated by menopausal hormone therapy (MHT) with oral conjugated equine estrogen combined with medroxyprogesterone acetate. The purpose of this study was to evaluate the influence of various MHTs, according to their formulation and route of administration, on new-onset diabetes in a cohort of postmenopausal French women.
Methods
The association between MHT use and new-onset diabetes was investigated by Cox regression analysis in 63,624 postmenopausal women of the French E3N cohort. Cases of diabetes were identified through self-report or drug reimbursement record linkage, and further validated.
Results
1220 new-onset diabetes cases were validated. We observed a lower risk of new-onset diabetes among women having ever used MHT (Hazard ratio: HR=0.82 [0.72 – 0.93]), compared to MHT never users. Adjustment for BMI during follow-up rather than baseline BMI did not substantially modify this association. An oral route of estrogen administration was associated with a greater decrease in diabetes risk than a cutaneous route (HR=0.68 [0.55–0.85] vs 0.87 [0.75–1.00], P for homogeneity=0.028). When further taking into account the type of progestagen used in combined MHT, we were not able to show significant differences between progestagens.
Conclusion
MHT appeared to be associated with a lower risk of new-onset diabetes. This relation was not mediated by changes in BMI. Further studies are needed to confirm the stronger effect of oral administration of estrogen compared to cutaneous administration.
Keywords: Adult; Aged; Diabetes Mellitus; epidemiology; Estrogens, Conjugated (USP); therapeutic use; Female; Hormone Replacement Therapy; Humans; Medroxyprogesterone Acetate; therapeutic use; Middle Aged; Postmenopause; Proportional Hazards Models; Questionnaires; Randomized Controlled Trials as Topic
Keywords: adult diabetes, postmenopause, menopausal hormone therapy, cohort study
Introduction
Diabetes mellitus is one of the most common chronic diseases in the industrialized world [1]. In France, the prevalence of treated diabetes was estimated to be 3.6% in 2005 [2]. The World Health Organization predicts that the number of patients with diabetes worldwide will rise from 171 million in 2000 to more than 366 million in 2030 [3]. Diabetes prevalence is generally similar in men and women [3] and higher in overweight and obese subjects [4–6].
Steroid hormones may influence diabetes onset. Animal studies have indeed suggested that ovarian hormone deficiency is associated with increased insulin resistance [7, 8]. Impaired glucose tolerance and insulin resistance are known to increase with age [9] but it is still unclear if menopause per se modifies this increase [10]. Compared to premenopausal women, postmenopausal women have similar glucose and insulin levels and a relatively minor deterioration in glucose tolerance, but they have an increased insulin resistance, produce less insulin and clear it more slowly [11]. Moreover, menopausal hormone therapy (MHT) was shown to reverse the effects of menopause on insulin secretion and clearance [11].
Two randomized trials, the Heart and Estrogen/Progestin Replacement Study (HERS) and the Women’s Health Initiative (WHI) trial, examined the effects of 0.625 mg conjugated equine estrogen alone or combined with 2.5 mg medroxyprogesterone acetate (MPA) on diabetes incidence [12–14]. These trials found a significant reduction in diabetes incidence of 20% to 30% in users of combined MHT [12, 13], and of 12% in users of estrogen alone [14]. The WHI trial further observed a significant fall in insulin resistance during the first year of follow-up in women treated with combined MHT or estrogens alone [13, 14]. A recent meta-analysis on components of the metabolic syndrome, which included 107 randomized controlled trials of at least 8 weeks duration showed similarly that MHT reduced the risk of new-onset diabetes [15]. Results of observational studies are inconsistent [16–19].
Most trials have evaluated oral conjugated equine estrogens given alone or associated with either MPA or 19-nortestosterone derivatives, but not with other combined estrogen–progestagen therapies used in other parts of the world, nor with non-oral estrogens. So, it is still unclear whether some MHTs are more beneficial than others. In France, estrogen, mostly estradiol administered through the skin, is used alone or in combination with a variety of progestagens. The purpose of the present study was to evaluate the influence of MHTs, their type and route of administration, on the risk of new-onset diabetes in a cohort of postmenopausal French women.
Subjects and methods
Subjects
The E3N (Etude Epidemiologique de Femmes de la Mutuelle Générale de l’Education Nationale, MGEN) prospective cohort was initiated in France in 1990 to investigate factors associated with cancers in women [20]. The cohort included 98,995 women living in France, aged 40–65 years in 1990, who were covered by the national health insurance plan for teachers and coworkers. All women signed an informed consent, in compliance with the rules of the French National Commission for Computed Data and Individual Freedom (Commission National Informatique et Libertés) from which approval was obtained. In 1990 and at follow-up (1992, 1993, 1995, 1997, 2000, 2002 and 2005), women completed self-administered questionnaires with demographic and anthropometric characteristics, reproductive history, health status, parental diabetes, and smoking status.
Definition and validation of an incident case of diabetes
A first set of potential cases of diabetes included women who had self-reported either diabetes, a diabetes diet, use of diabetic drugs, or a hospitalization for diabetes in at least one of the eight questionnaires sent up until July 2005. A total of 4289 self-reported potential cases were identified. Among them, 2315 cases were validated because women were identified from drug reimbursement file provided by the health insurance as having been reimbursed for a diabetic drug between January 1st, 2004 (date when the file became available) and June 30th, 2007 (date of endpoint in the present study). Among the 1974 women without diabetic drug reimbursement, women alive and with an accurate address (n=1735) were mailed a questionnaire specifically designed to validate diabetes. From the 1480 women who completed this questionnaire (response rate: 84 %), 342 potential cases were confirmed if glucose concentrations at diagnosis were reported to comply with WHO recommendations (fasting ≥7.0 mmol/l or random glucose ≥11.1 mmol/l) [21], or women reported taking diabetic drugs, and/or their last values of fasting glucose or HbA1c levels were reported to be ≥7.0 mmol/l and/or ≥7% respectively [22, 23]. A total of 2657 self-reported diabetes cases were thus validated.
A second set of potential cases of diabetes was identified exclusively from the drug reimbursement file (n=1216) without prior report of diabetes in any of the eight study questionnaires. We mailed the diabetes specific questionnaire to 1139 of these women and 734 completed it. We considered as non-cases, women who declared they were non diabetic and who had been reimbursed for diabetic drugs only once before June 30th, 2007 (n=233); as validated diabetic cases women who confirmed diabetes in the diabetes specific questionnaire (n=458) and those who did not answer the diabetes specific questionnaire but had diabetic drugs reimbursed at least twice (n=381). Other potential cases were considered as non-validated (n=144).
Altogether, a total of 3496 diabetes cases diagnosed until June 30th, 2007 were thus validated in the E3N cohort.
Although this procedure did not systematically allow differentiation between type 1 and type 2 diabetes mellitus, the age range of our population implies that incident cases considered in our analyses are mostly type 2 diabetes mellitus. Prevalent diabetes cases were excluded from analyses (see below).
Identification of MHT use
Information on lifetime use of hormonal treatments was recorded in the 1992 questionnaire and included the start date and duration of each episode of hormone use, together with the corresponding brand name. To help women remember what brand they had taken, they were given a booklet with color photographs of hormonal treatments marketed in France. The information was updated in each of the subsequent questionnaires.
Population for analysis and follow-up
The present analysis included only the women who responded to a dietary history questionnaire sent in 1993 (Figure 1), which formed the baseline of the current report in order to be able to adjust for energy and alcohol intake. After two reminders to non-respondents, 77,613 dietary questionnaires were returned (81.1% response rate). Of these questionnaires, 2104 were excluded because of miscoding and 985 because respondents did not give their consent to a follow-up by the health insurer (MGEN) in case of dropout. We also excluded 1490 questionnaires with an unreasonable report of total energy intake, as defined by the 1st and 99th percentile of the ratio of energy intake to basal metabolic rate computed on the basis of age, height, and weight at the time of the dietary survey [24] and 368 women who did not complete the part of this questionnaire that inquired about their health status.
Figure 1.
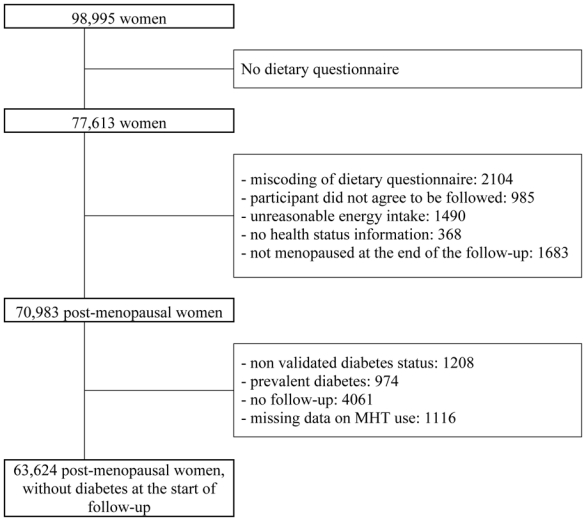
Exclusion criteria for the analysis of the influence of MHT on diabetes risk in the French E3N study.
In the present analysis, only postmenopausal women were included. Women were considered postmenopausal if they had had 12 consecutive months without menstrual periods (unless due to hysterectomy), had undergone bilateral oophorectomy, had ever used MHT, or self-reported that they were postmenopausal. Age at menopause was defined as age at last menstrual period (if the last menstrual period occurred before MHT use, and if the cessation of menstruation was not due to hysterectomy), age at bilateral oophorectomy; or, in decreasing order of priority, self-reported age at menopause, age at start of MHT, age at start of menopausal symptoms; or, if no information was available, age 47 years if menopause was artificial, and age 51 otherwise, ages which corresponded to the median values for artificial and natural menopause in the cohort, respectively.
Among the women with a validated dietary questionnaire (n=72,666), 70,983 women were postmenopausal at the time of the 8th questionnaire; we excluded those with non validated diabetes or no date of diagnosis (n=1208), those who had been diagnosed with diabetes before the dietary questionnaire or first report of menopause (n=974), those with no follow-up (n=4061), and those who did not answer the questionnaire (sent in 1992) about lifetime use of MHT while being postmenopausal at that date (n=1116), leaving 63,624 women for analysis.
Follow-up started either at the date of return of the dietary questionnaire for the women who were already postmenopausal, otherwise at the date of the first report of menopause. Women contributed person-time until the date of diagnosis of diabetes, date of last completed questionnaire if the 2005 questionnaire was not completed, or June 30th 2007, whichever occurred first.
Statistical analysis
We used Cox proportional hazards regression models stratified by 5-year interval birth cohorts, with age as the timescale, to estimate the hazards ratios (HR) for diabetes and 95% confidence intervals (CI) [25]. We controlled for potential confounders by adjusting the model for educational level, physical activity in 1993 (energy expenditure calculated by multiplying the duration of daily physical activities by the estimated metabolic energy spent [26]), age at menarche, parity, breastfeeding, type of menopause, age of menopause, family history of diabetes, cholesterol level, hypertension, alcohol intake, energy intake, smoking, and body mass index (BMI). Data on smoking and hypertension were entered in the models as time-dependant variables. Cutoffs of covariables are indicated in the footnotes of table 2. We replaced missing values by the modal value (all were categorical variables) when in fewer than 5% of women, or else by a “missing” category.
Table 2.
Hazard ratios [95% CI] for new-onset diabetes according to duration and recency of use, route of estrogen administration, compared with MHT never-use, in the E3N-EPIC Study, France (1993–2007, n=63,624).
| Case / Total | Model 1a |
Model 2b |
|
|---|---|---|---|
| HR [95% CI] | HR [95% CI] | ||
| Never-use | 518 / 18230 | 1 [Reference] | 1 [Reference] |
| Ever-use | 702 / 45394 | 0.82 [0.72 – 0.93] | 0.75 [0.66 – 0.85] |
| Duration of MHT use in ever-users | |||
| 0–2 years | 144 / 7300 | 0.79 [0.64 – 0.96] | 0.75 [0.61 – 0.91] |
| 2–5 years | 202 / 11868 | 0.89 [0.74 – 1.06] | 0.84 [0.70 – 1.00] |
| >5 years | 294 / 23460 | 0.78 [0.66 – 0.91] | 0.70 [0.59 – 0.82] |
| Unknown duration | 62 / 2766 | 0.92 [0.70 – 1.21] | 0.75 [0.57 – 1.00] |
| P-value for homogeneity among duration of use | 0.41 | 0.32 | |
| Use of MHT in ever-users | |||
| Current use | 422 / 7657 | 0.83 [0.71 – 0.96] | 0.78 [0.68 – 0.89] |
| Past use (>1 year before) | 244 / 35384 | 0.78 [0.66 – 0.93] | 0.90 [0.76 – 1.07] |
| Unknown recency | 36 / 2353 | 1.01 [0.72 – 1.43] | 0.99 [0.70 – 1.39] |
| P-value for homogeneity among recency of use | 0.55 | 0.09 | |
| Route of estrogen administrationc | |||
| Oral | 125 / 11263 | 0.68 [0.55 – 0.85] | 0.61 [0.50 – 0.76] |
| Cutaneous | 425 / 25740 | 0.87 [0.75 – 1.00] | 0.78 [0.67 – 0.90] |
| Other routed | 49 / 2533 | 0.81 [0.59 – 1.11] | 0.76 [0.56 – 1.04] |
| Unknown route | 103 / 5858 | 0.84 [0.67 – 1.05] | 0.73 [0.59 – 0.92] |
| P-value for homogeneity among oral and cutaneous route | 0.028 | 0.031 | |
Model 1: Adjusted for age (time scale), age at menarche (<13 / ≥13 years), parity (nulliparous / parous), breastfeeding (no / <12 months / ≥12 months / unknown), age at menopause, type of menopause (artificial / natural / unknown), family history of diabetes (none / only one parent / both parents), physical activity in 1993 (<19.8 / 19.8–33.4 / 33.4–53.2 / ≥ 53.2 MET-h/week), alcohol intake (continuous), total energy intake exclusive of alcohol (continuous), educational level (≤ 9 / 10–11 / 12–14 / 15–16 / ≥ 17 y), baseline cholesterol level (≤ 2 / >2 g/l), hypertension (yes / no, time-dependant variable), smoking (never / former / current smoker, time-dependant variable) and baseline BMI (<22 / 22–25 / 25–27 / 27–30 / ≥30 kg/m2). Further stratified on year of birth (1925–1930 / 1930–1935 / 1935–1940 / 1940–1945 / 1945–1950);
Model 2: Model 1 with adjustment for BMI (<22 / 22–25 / 25–27 / 27–30 / ≥30 kg/m2) as a time-dependent variable;
Corresponding to the MHT used for the greatest length of time;
Vaginal, intramuscular, nasal.
As overweight is a major risk factor for diabetes [27] and MHT use is associated with a lower increase in fat mass [15, 28–31], BMI may be an intermediate factor in the relation between MHT exposure and diabetes risk. We therefore decided to present two different models. The first was adjusted for potential confounders and baseline BMI; the second was adjusted for potential confounders and BMI during follow-up as a time-dependant variable.
Regarding MHT exposure status, the information reported in questionnaires “n” and earlier was used to prospectively categorize participants for the period between completion of questionnaire “n” and completion of questionnaire “n+1” (or end of follow-up). For women who did not answer questionnaire “n”, MHT exposure status was classified as missing for the period between the date at which questionnaire n was sent to the participants and the date of completion of the subsequent questionnaire (or end of follow-up). In estimating the HRs associated with different types of MHT, estimates were computed for the MHT used for the longest duration in the case of treatment change during follow-up.
All tests of statistical significance were two-sided, and the significance was set at the .05 level. The P-values for assessing possible heterogeneity in effect estimates were computed from likelihood ratio tests. We performed all analyses using SAS software, version 9.1 (SAS Institute Inc, Cary, NC).
Results
A total of 1220 new-onset diabetes cases were validated during 663,087 person-years of follow-up (mean duration: 10.4 years; SD: 3.6). Table 1 shows selected characteristics of women according to their use of MHT. Women having ever used MHT appeared more educated, had less often a family history of diabetes, were less often overweight, were more often parous, had an earlier menopause and had a higher cholesterol level than women who had never used MHT. Women who had ever used MHT also had a higher yearly increase in BMI during follow-up than never-users (0.07 ± 0.27 vs 0.06 ± 0.35 kg/m²/year) and this difference was significant even after adjustment for age and BMI at the start of follow-up (P < 0.001). Differences in women’s characteristics at the start of follow-up were also found between oral and transdermal routes of estrogen administration, pointing out towards a better cardio-metabolic profile of oral estrogen users, and between estrogen alone and estrogen plus progestagen combinations.
Table 1.
Selected characteristics (mean (SD), n (%)) of participants at the start of follow-up according to 1/ use of menopausal hormone therapy (MHT) use 2/ route of estrogen administration 3/ association of progestagen, as recorded at the end of the follow-up. E3N-EPIC study, France (1993–2007, n=63,624)
| 1- MHT use
|
2- Route of estrogen administration
|
3- Type of MHT
|
||||||
|---|---|---|---|---|---|---|---|---|
| Never | Ever | Oral | Transdermal/ Cutaneous | Other/ unknown | Estrogen alone | Estrogen + progestagen | Other / unknown | |
| N | 18230 | 45394 | 11263 | 25740 | 8391 | 4656 | 30905 | 9833 |
| Age at start of follow-up, y | 57.1 (5.5) | 54.8 (4.7)a | 53.6 (4.1) | 54.5 (4.3) b | 57.1 (5.4) | 54.8 (5.1) | 54 (4.1) c | 56.9 (5.4) |
| Age at menopause, y | 50.7 (3.9) | 50.1 (3.7) a | 50.2 (3.6) | 50.2 (3.5) | 49.7 (4.4) | 49.4 (4.4) | 50.3 (3.3) c | 49.8 (4.4) |
| University degree (%) | 6000 (32.9%) | 16465 (36.3%) a | 4427 (39.3%) | 9182 (35.7%) b | 2856 (34.0%) | 1440 (30.9%) | 11632 (37.6%) c | 3393 (34.5%) |
| Age at menarche ≥13 y, % | 9833 (53.9%) | 24478 (53.9%) | 6075 (53.9%) | 13816 (53.7%) | 4587 (54.7%) | 2380 (51.1%) | 16720 (54.1%) c | 5378 (54.7%) |
| Parous, % | 2600 (14.3%) | 4912 (10.8%) a | 1121 (10%) | 2743 (10.7%) b | 1048 (12.5%) | 543 (11.7%) | 3161 (10.2%) c | 1208 (12.3%) |
| Parent with diabetes, % | 5341 (29.3%) | 10597 (23.3%) a | 2537 (22.5%) | 5964 (23.2%) | 2096 (25%) | 1144 (24.6%) | 7073 (22.9%) c | 2380 (24.2%) |
| Smoker, % | 5282 (29%) | 14536 (32%) a | 3778 (33.5%) | 8120 (31.5%) b | 2638 (31.4%) | 1469 (31.6%) | 9964 (32.2%) c | 3103 (31.6%) |
| BMI (kg/m2) | 23.8 (3.8) | 22.9 (3.1) a | 22.7 (3.0) | 23.0 (3.1) b | 23.1 (3.1) | 23.4 (3.4) | 22.8 (3.0) c | 23.1 (3.1) |
| Breastfeeding >12 mo | 1254 (6.9%) | 2026 (4.5%) a | 432 (3.8%) | 1113 (4.3%) b | 481 (5.7%) | 213 (4.6%) | 1268 (4.1%) | 545 (5.5%) |
| Cholesterol >2g/l, % | 7604 (41.7%) | 20734 (45.7%) a | 4382 (38.9%) | 11997 (46.6%) b | 4355 (51.9%) | 2216 (47.6%) | 13518 (43.7%) c | 5000 (50.8%) |
| Hypertension, % | 2006 (11.0%) | 4031 (8.9%) a | 703 (6.2%) | 2398 (9.3%) b | 930 (11.1%) | 493 (10.6%) | 2484 (8.0%) c | 1054 (10.7%) |
| Physical activity (MET-h/wk) | 41.1 (28.9) | 39.8 (27.3) a | 39 (26.8) | 39.8 (27.3) b | 41 (28.1) | 40.5 (27.9) | 39.3 (27.0) c | 40.9 (27.8) |
| Alcool intake (g/day) | 10.5 (14.1) | 11.5 (14.1) a | 11.9 (14.5) | 11.4 (13.9) b | 11.2 (14) | 10.9 (13.5) | 11.6 (14.2) c | 11.3 (14.1) |
| Energy intake (kJ/day) | 8931 (2423) | 9051 (2362) a | 9077 (2342) | 9069 (2362) | 8958 (2386) | 9065 (2364) | 9071 (2359) | 8981 (2369) |
Significantly different from MHT never-users (P < 0.05);
Significantly different from oral route of estrogen administration (P < 0.05);
Significantly different from estrogen alone (P < 0.05);
A total of 8,863 women had missing data for this variable;
A total of 26,536 women had missing data for this variable;
Metabolic equivalent cost-hour/week.
Overall, the incidence of diabetes during follow-up was lower among MHT users than among never users (multivariate adjusted HR=0.82 [0.72 – 0.93]) (Table 2). Adjustment for BMI during follow-up instead of baseline BMI slightly enhanced the association (HR=0.75 [0.66 – 0.85]). Diabetes risk appeared not to be significantly related to the duration of MHT use. After one year of discontinuation of MHT, the protective effect was no longer significant when the model was adjusted for BMI during follow-up.
The decrease in risk of new-onset diabetes appeared significantly stronger for estrogen administered orally (whether used alone or in association with a progestagen) rather than through the skin, even after adjustment for BMI during follow-up (HR=0.61 [0.50–0.76] vs 0.78 [0.67–0.90], p=0.031) (Table 2).
As some progestagens were combined only with oral or cutaneous estrogen, we could not exclude the possibility that differences in HRs of new-onset diabetes were due to differences between progestagens. Thus, analyses were also performed according to route of estrogen administration and type of progestagen (Table 3). For progestagens combined with either oral or cutaneous estrogen, we were not able to show significant differences in HRs according to estrogen route of administration. Similarly, for any given route of estrogen administration, we observed no significant differences in HRs among progestagens. However, only cutaneous estrogen combined with progesterone (it is rarely combined with oral estrogens), as well as oral estrogen combined with cyproterone acetate or norethisterone acetate (it is rarely combined with cutaneous estrogens) were associated with a statistically significant lower risk of diabetes than never use of MHT, even after controlling for BMI during follow-up (HR=0.67 [0.54–0.84], 0.44 [0.23–0.85], and 0.44 [0.26–0.75] respectively).
Table 3.
Hazard ratios [95% CI] for new-onset diabetes according to route of estrogen administration and type of progestagena, compared with MHT never-use, in the E3N-EPIC Study, France (1993–2007, n=63,624).
| Oral Estrogen | Cutaneous Estrogen | p-value for homogeneity among routes of estrogen treatment | |||
|---|---|---|---|---|---|
| cases / totalb | HR [95% CI] | cases / totalb | HR [95% CI] | ||
| Model 1c | |||||
| Estrogen aloned | 16 / 796 | 0.75 [0.44 – 1.28] | 72 / 3856 | 0.87 [0.67 – 1.13] | 0.62 |
| Estrogen combined with: | |||||
| Progesterone | 4 / 674 | -e | 112 / 7725 | 0.77 [0.62 – 0.97] | - |
| Dydrogesterone | 10 / 1571 | 0.62 [0.33 – 1.16] | 86 / 4455 | 0.93 [0.72 – 1.19] | 0.23 |
| Medroxyprogesterone acetate | 22 / 1453 | 0.85 [0.55 – 1.33] | 3 / 85 | -e | - |
| Cyproterone acetate | 12 / 1527 | 0.50 [0.26 – 0.97] | 2 / 159 | -e | - |
| Chlormadinone acetate | 5 / 424 | 0.49 [0.16 – 1.53] | 28 / 1748 | 0.95 [0.63 – 1.42] | 0.28 |
| Medrogestone | 2 / 272 | -e | 24 / 1167 | 1.20 [0.78 – 1.85] | - |
| Nomegestrol acetate | 12 / 627 | 1.07 [0.57 – 2.02] | 56 / 3272 | 0.97 [0.72 – 1.30] | 0.76 |
| Promegestone | 13 / 805 | 0.98 [0.53 – 1.78] | 37 / 2676 | 0.81 [0.57 – 1.15] | 0.58 |
| Norethisterone acetate | 17 / 2199 | 0.48 [0.28 – 0.81] | 0 / 25 | -e | - |
| P-value for homogeneity among progestagens | 0.35 | 0.57 | |||
| Model 2f | |||||
| Estrogen alone | 16 / 796 | 0.68 [0.40 – 1.17] | 72 / 3856 | 0.80 [0.61 – 1.04] | 0.59 |
| Estrogen combined with: | |||||
| Progesterone | 4 / 674 | -e | 112 / 7725 | 0.67 [0.54 – 0.84] | - |
| Dydrogesterone | 10 / 1571 | 0.58 [0.31 – 1.09] | 86 / 4455 | 0.84 [0.66 – 1.08] | 0.27 |
| Medroxyprogesterone acetate | 22 / 1453 | 0.77 [0.50 – 1.20] | 3 / 85 | -e | - |
| Cyproterone acetate | 12 / 1527 | 0.44 [0.23 – 0.85] | 2 / 159 | -e | - |
| Chlormadinone acetate | 5 / 424 | 0.45 [0.14 – 1.40] | 28 / 1748 | 0.86 [0.57 – 1.29] | 0.29 |
| Medrogestone | 2 / 272 | -e | 24 / 1167 | 1.01 [0.66 – 1.56] | - |
| Nomegestrol acetate | 12 / 627 | 0.95 [0.51 – 1.78] | 56 / 3272 | 0.88 [0.66 – 1.18] | 0.83 |
| Promegestone | 13 / 805 | 0.89 [0.49 – 1.63] | 37 / 2676 | 0.72 [0.51 – 1.03] | 0.54 |
| Norethisterone acetate | 17 / 2199 | 0.44 [0.26 – 0.75] | 0 / 25 | -e | - |
| P-value for homogeneity among progestagens | 0.37 | 0.47 | |||
corresponding to the MHT used for the greatest length of time);
The number of cases and total of women do not add up to the totals as data are not tabulated for weak estrogens (43 diabetes cases / 2376 total women), and other (intramusculary administered estrogen or progestogen; androgen; nasally administered estrogen; transdermally administered progestagen; or tibolone) or unknown MHT (126 diabetes cases / 7457 total women);
Model 1: adjusted for the same covariates as model 1 in Table 2;
Conjugated equine estrogens were only marginally used by women in our cohort (0.7%), so separate estimates for conjugated equine estrogens and estradiol compounds are not provided;
Data are not presented as there are fewer than five cases in this MHT category;
Model 2: Model 1 with adjustment for BMI (<22 / [22–25[ / [25–27[ / [27–30[ / ≥30 kg/m2) as a time-dependent variable.
Compared to MHT never-use, ever-use of weak estrogens (orally or vaginally administered promestriene or estriol) was associated with a lower risk of incident diabetes when analyses were adjusted for BMI during follow-up (HR=0.70 [0.50–0.97]) but not when adjusted for baseline BMI (HR=0.74 [0.53–1.04]) (data not tabulated, 43 cases / 2374 women using weak estrogens).
All results were confirmed following sensitivity analyses including non-validated diabetes cases and estimates were consistent with the main results presented (data not shown).
Discussion
In this prospective cohort, we observed a lower risk of new-onset diabetes among women having ever used MHT, compared to MHT never users. Adjustment for BMI during follow-up rather than baseline BMI did not substantially modify the association. An oral route of estrogen administration was associated with a stronger decrease in diabetes risk than cutaneous administration. When further taking into account the type of progestagen in combined MHT, only cutaneous estrogen combined with progesterone, or oral estrogen combined with cyproterone acetate or norethisterone acetate were significantly associated with a lower risk or diabetes, although there was no statistically significant heterogeneity between progestagens with regard to diabetes risk.
The process underlying the change in glucose and insulin levels with exposure to estrogen is not fully understood, but several mechanisms have been hypothesized. Estrogen may have a direct effect on the pancreatic secretion of insulin, as estrogen receptors are present in pancreatic beta cells [32] and estrogen increases the release of insulin in beta cell models [33]. Previous studies have also found that estrogen may reduce peripheral vascular reactivity [34, 35]. A decreased peripheral blood flow may limit insulin delivery and promote insulin resistance. Results of the WHI trial suggested that a decrease in insulin resistance, induced by the MHT, may have been responsible for the lower incidence of diabetes observed in the women who received the active treatment [13, 14]; these results were confirmed in a meta-analysis [15]. As in a recent study [36], the relationship was no longer significant after discontinuation of MHT use, when model was adjusted for BMI during follow-up.
In the meta-analysis, MHT use was found to be associated with an increase in lean body mass and a decrease in abdominal fat [15], that could partially explain a reduced risk of diabetes in MHT users. Unfortunately, we were not able to assess changes in abdominal fat or lean mass, which could be induced by MHT, as waist circumference was measured only once during the follow-up. In our study, associations between MHT use and new-onset diabetes tended to be stronger when adjusting for BMI during follow-up. This result could be explained by a greater increase in BMI during the follow-up among MHT users. However, a recent systematic review concluded that there was no evidence for a BMI change according to MHT use different from that normally experienced at the time of menopause [37]. In the HERS trial [12], hormone therapy was associated with a significant decrease in waist circumference, but this change did not mediate the effect of MHT on diabetes risk. In the WHI trial, a greater non significant BMI increase was observed in treated women, probably due to an increase in lean body mass [28], but the effect of MHT on diabetes risk was also independent of BMI and waist circumference changes [13].
Confirming our results of a difference between the oral and cutaneous route of estrogen administration on the risk of new-onset diabetes, a recent meta-analysis found a larger protective effect of oral MHT than transdermal MHT on metabolic syndrome components [15], possibly due to a stronger effect of oral than transdermal estradiol on peripheral vascular reactivity [34]. Few studies have examined associations with a given progestagen according to the route of administration of the associated estrogen. When we compared the route of estrogen administration within a category of MHT (either estrogen alone or according to the associated progestagen), we were not able to demonstrate any differences. However, we had too few cases to test the difference between routes of estrogens administration within some progestagens (progesterone, MPA, cyproterone acetate and norethisterone acetate).
Though our study is the first to consider different types of MHT combinations on diabetes risk, several authors have reported the impact of different types of hormone therapy on intermediate risk factors. In the WHI trial, conjugated equine estrogen combined with MPA was found to improve insulin sensitivity [14]. Low dose dydrogesterone associated with estradiol may lead to a decrease in circulating insulin concentrations [38]. Finally, estrogen plus norethisterone acetate was found to have no effect on glucose and insulin metabolism [39] while it seemed to improve insulin sensitivity. The meta-analysis by Salpeter [15] found no differences in insulin resistance between unopposed and combined treatments. In our study, we were not able to find any significant difference between the categories of MHT combinations on diabetes risk, but the number of cases of diabetes, within each category of MHT combination, for a given route of estrogen administration, may have be too small.
The major strength of our study is the range of MHTs evaluated and the fact that exposure was regularly updated during follow-up. This allowed us to limit misclassification of ever-, never-users, and users of a given MHT, which can occur in prospective studies with a single baseline assessment of exposure. However, we acknowledge that we have limited power to examine the effects of the route of estrogen administration within each category of progestagen, and the effects of different progestagens within each route of estrogen. Even if we cannot exclude the possibility that diabetes was not always reported, in particular for the women who were only given recommendations to improve their lifestyle, and that diabetes was not always diagnosed, we tried to limit the number of unreported diabetes cases, by identifying diabetes cases not only by self-report but also by drug reimbursement. Other strengths of our study were the prospective design, the large sample and the long duration of follow-up. We acknowledge, in contrast to randomized clinical trials, that the observational design of the study cannot control for unknown baseline differences between MHT users and MHT never-users or between route of estrogen administration or type of MHT used. Nevertheless, we were able to adjust for the major risk factor for diabetes in our analyses, in particular BMI during all of the follow-up, a measure that, although self-reported, was found to be valid [40].
Conclusion
In conclusion, the use of MHT was associated with a reduction in the incidence of diabetes in postmenopausal women, even after careful control for BMI. Oral administration of estrogen appeared to be associated with a lower risk of new-onset diabetes risk than cutaneous administration. However, due to the types of combinations prescribed, we were not able to assess the impact of routes of administration for each progestagen molecule.
Acknowledgments
The authors are indebted to all participants for providing the data used in the E3N Study and to practitioners for providing pathology reports. They are grateful to R. Chaït, M. Fangon, L. Hoang, M. Niravong and J. Sahuquillo for their technical assistance; and to the E3N group.
Funding
This work was carried out with the financial support of the “Mutuelle Générale de l’Education Nationale” (MGEN); European Community; French League against Cancer (LNCC); Gustave Roussy Institute (IGR); French Institute of Health and Medical Research (Inserm); 3M Company; and several General Councils of France. The validation of potential diabetes cases was supported by the European Union (Integrated Project LSHM-CT-2006-037197 in the Framework Program 6 of the European-Community), InterAct project. A Fabre was a recipient of a grant from “Canceropole – Region Ile-de-France”.
The funding sources had no involvement in the present work.
Abbreviations
- E3N
prospective cohort (Etude Epidemiologique de Femmes de la Mutuelle Générale de l’Education Nationale)
- HERS
Heart and Estrogen/Progestin Replacement Study
- MGEN
national health insurance plan for teachers and co-workers (Mutuelle Générale de l’Education Nationale)
- MHT
menopausal hormone therapy, MPA, medroxyprogesterone acetate
- WHI
Women’s Health Initiative trial
Footnotes
Authorship
The authors declare that there is no duality of interest associated with this manuscript.
Dr Clavel-Chapelon had full access to all data in the study and accepts full responsibility for the integrity of the data and the conduct of the study. She controlled the decision to publish. Study concept and design: de Lauzon-Guillain, Fournier, Fabre, Clavel-Chapelon.
Acquisition of data: Clavel-Chapelon. Analysis and interpretation of data: de Lauzon-Guillain, Fournier, Fabre, Simon, Clavel-Chapelon. Drafting of the manuscript: de Lauzon-Guillain, Mesrine. Critical revision of the manuscript for important intellectual content de Lauzon-Guillain, Fournier, Fabre, Simon, Mesrine, Boutron-Ruault, Balkau, Clavel-Chapelon. Final approval of the version to be published: de Lauzon-Guillain, Fournier, Fabre, Simon, Mesrine, Boutron-Ruault, Balkau, Clavel-Chapelon.
References
- 1.Adeghate E, Schattner P, Dunn E. An update on the etiology and epidemiology of diabetes mellitus. Ann N Y Acad Sci. 2006;1084:1–29. doi: 10.1196/annals.1372.029. [DOI] [PubMed] [Google Scholar]
- 2.Kusnik-Joinville O, Weill A, Salanave B, Ricordeau P, Allemand H. Prevalence and treatment of diabetes in France: trends between 2000 and 2005. Diabetes Metab. 2008;34:266–272. doi: 10.1016/j.diabet.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Narayan KM, Boyle JP, Thompson TJ, Gregg EW, Williamson DF. Effect of BMI on lifetime risk for diabetes in the U.S. Diabetes Care. 2007;30:1562–1566. doi: 10.2337/dc06-2544. [DOI] [PubMed] [Google Scholar]
- 5.Wannamethee SG, Shaper AG, Walker M. Overweight and obesity and weight change in middle aged men: impact on cardiovascular disease and diabetes. J Epidemiol Community Health. 2005;59:134–139. doi: 10.1136/jech.2003.015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balkau B, Lange C, Fezeu L, et al. Predicting diabetes: clinical, biological, and genetic approaches: data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR) Diabetes Care. 2008;31:2056–2061. doi: 10.2337/dc08-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryzgalova G, Gao H, Ahren B, et al. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia. 2006;49:588–597. doi: 10.1007/s00125-005-0105-3. [DOI] [PubMed] [Google Scholar]
- 8.Ordonez P, Moreno M, Alonso A, Fernandez R, Diaz F, Gonzalez C. Insulin sensitivity in streptozotocin-induced diabetic rats treated with different doses of 17beta-oestradiol or progesterone. Exp Physiol. 2007;92:241–249. doi: 10.1113/expphysiol.2006.035006. [DOI] [PubMed] [Google Scholar]
- 9.Iozzo P, Beck-Nielsen H, Laakso M, Smith U, Yki-Jarvinen H, Ferrannini E. Independent influence of age on basal insulin secretion in nondiabetic humans. European Group for the Study of Insulin Resistance. J Clin Endocrinol Metab. 1999;84:863–868. doi: 10.1210/jcem.84.3.5542. [DOI] [PubMed] [Google Scholar]
- 10.Bentley-Lewis R, Koruda K, Seely EW. The metabolic syndrome in women. Nature clinical practice. 2007;3:696–704. doi: 10.1038/ncpendmet0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godsland IF. Oestrogens and insulin secretion. Diabetologia. 2005;48:2213–2220. doi: 10.1007/s00125-005-1930-0. [DOI] [PubMed] [Google Scholar]
- 12.Kanaya AM, Herrington D, Vittinghoff E, et al. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138:1–9. doi: 10.7326/0003-4819-138-1-200301070-00005. [DOI] [PubMed] [Google Scholar]
- 13.Margolis KL, Bonds DE, Rodabough RJ, et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women's Health Initiative Hormone Trial. Diabetologia. 2004;47:1175–1187. doi: 10.1007/s00125-004-1448-x. [DOI] [PubMed] [Google Scholar]
- 14.Bonds DE, Lasser N, Qi L, et al. The effect of conjugated equine oestrogen on diabetes incidence: the Women's Health Initiative randomised trial. Diabetologia. 2006;49:459–468. doi: 10.1007/s00125-005-0096-0. [DOI] [PubMed] [Google Scholar]
- 15.Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 2006;8:538–554. doi: 10.1111/j.1463-1326.2005.00545.x. [DOI] [PubMed] [Google Scholar]
- 16.Rossi R, Origliani G, Modena MG. Transdermal 17-beta-estradiol and risk of developing type 2 diabetes in a population of healthy, nonobese postmenopausal women. Diabetes Care. 2004;27:645–649. doi: 10.2337/diacare.27.3.645. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Howard BV, Cowan LD, et al. The effect of estrogen use on levels of glucose and insulin and the risk of type 2 diabetes in american Indian postmenopausal women: the strong heart study. Diabetes Care. 2002;25:500–504. doi: 10.2337/diacare.25.3.500. [DOI] [PubMed] [Google Scholar]
- 18.Gabal LL, Goodman-Gruen D, Barrett-Connor E. The effect of postmenopausal estrogen therapy on the risk of non-insulin-dependent diabetes mellitus. Am J Public Health. 1997;87:443–445. doi: 10.2105/ajph.87.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manson JE, Nathan DM, Krolewski AS, Stampfer MJ, Willett WC, Hennekens CH. A prospective study of exercise and incidence of diabetes among US male physicians. Jama. 1992;268:63–67. [PubMed] [Google Scholar]
- 20.Fournier A, Fabre A, Mesrine S, Boutron-Ruault MC, Berrino F, Clavel-Chapelon F. Use of different postmenopausal hormone therapies and risk of histology- and hormone receptor-defined invasive breast cancer. J Clin Oncol. 2008;26:1260–1268. doi: 10.1200/JCO.2007.13.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. World Health Organisation / International Diabetes Federation; Geneva, Switzerland: 2006. [Google Scholar]
- 22.Kilpatrick ES. Haemoglobin A1c in the diagnosis and monitoring of diabetes mellitus. J Clin Pathol. 2008;61:977–982. doi: 10.1136/jcp.2007.054304. [DOI] [PubMed] [Google Scholar]
- 23.Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB. A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab. 2008;93:2447–2453. doi: 10.1210/jc.2007-2174. [DOI] [PubMed] [Google Scholar]
- 24.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39(Suppl 1):5–41. [PubMed] [Google Scholar]
- 25.Thiebaut AC, Benichou J. Choice of time-scale in Cox's model analysis of epidemiologic cohort data: a simulation study. Stat Med. 2004;23:3803–3820. doi: 10.1002/sim.2098. [DOI] [PubMed] [Google Scholar]
- 26.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Medicine and science in sports and exercise. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 27.Kopelman PG, Albon L. Obesity, non-insulin-dependent diabetes mellitus and the metabolic syndrome. Br Med Bull. 1997;53:322–340. doi: 10.1093/oxfordjournals.bmb.a011616. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z, Bassford T, Green SB, et al. Postmenopausal hormone therapy and body composition--a substudy of the estrogen plus progestin trial of the Women's Health Initiative. Am J Clin Nutr. 2005;82:651–656. doi: 10.1093/ajcn.82.3.651. [DOI] [PubMed] [Google Scholar]
- 29.Chmouliovsky L, Habicht F, James RW, Lehmann T, Campana A, Golay A. Beneficial effect of hormone replacement therapy on weight loss in obese menopausal women. Maturitas. 1999;32:147–153. doi: 10.1016/s0378-5122(99)00037-7. [DOI] [PubMed] [Google Scholar]
- 30.Gambacciani M, Ciaponi M, Cappagli B, Genazzani AR. Effects of low-dose continuous combined conjugated estrogens and medroxyprogesterone acetate on menopausal symptoms, body weight, bone density, and metabolism in postmenopausal women. Am J Obstet Gynecol. 2001;185:1180–1185. doi: 10.1067/mob.2001.117669. [DOI] [PubMed] [Google Scholar]
- 31.Thorneycroft IH, Lindsay R, Pickar JH. Body composition during treatment with conjugated estrogens with and without medroxyprogesterone acetate: analysis of the women's Health, Osteoporosis, Progestin, Estrogen (HOPE) trial. Am J Obstet Gynecol. 2007;197:e131–137. doi: 10.1016/j.ajog.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 32.Sutter-Dub MT. Rapid non-genomic and genomic responses to progestogens, estrogens, and glucocorticoids in the endocrine pancreatic B cell, the adipocyte and other cell types. Steroids. 2002;67:77–93. doi: 10.1016/s0039-128x(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 33.Nadal A, Ropero AB, Fuentes E, Soria B, Ripoll C. Estrogen and xenoestrogen actions on endocrine pancreas: from ion channel modulation to activation of nuclear function. Steroids. 2004;69:531–536. doi: 10.1016/j.steroids.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Vehkavaara S, Hakala-Ala-Pietila T, Virkamaki A, et al. Differential effects of oral and transdermal estrogen replacement therapy on endothelial function in postmenopausal women. Circulation. 2000;102:2687–2693. doi: 10.1161/01.cir.102.22.2687. [DOI] [PubMed] [Google Scholar]
- 35.Zegura B, Keber I, Sebestjen M, Borko E. Orally and transdermally replaced estradiol improves endothelial function equally in middle-aged women after surgical menopause. Am J Obstet Gynecol. 2003;188:1291–1296. doi: 10.1067/mob.2003.326. [DOI] [PubMed] [Google Scholar]
- 36.Pentti K, Tuppurainen M, Honkanen R, et al. Hormone therapy protects from diabetes: the Kuopio Osteoporosis Risk Factor and Prevention Study. Eur J Endocrinol. 2009 doi: 10.1530/EJE-09-0151. [DOI] [PubMed] [Google Scholar]
- 37.Kongnyuy EJ, Norman RJ, Flight IH, Rees MC. Oestrogen and progestogen hormone replacement therapy for peri-menopausal and post-menopausal women: weight and body fat distribution. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD001018. [DOI] [PubMed] [Google Scholar]
- 38.Crook D, Godsland IF, Hull J, Stevenson JC. Hormone replacement therapy with dydrogesterone and 17 beta-oestradiol: effects on serum lipoproteins and glucose tolerance during 24 month follow up. Br J Obstet Gynaecol. 1997;104:298–304. doi: 10.1111/j.1471-0528.1997.tb11457.x. [DOI] [PubMed] [Google Scholar]
- 39.Luotola H, Pyorala T, Loikkanen M. Effects of natural oestrogen/progestogen substitution therapy on carbohydrate and lipid metabolism in post-menopausal women. Maturitas. 1986;8:245–253. doi: 10.1016/0378-5122(86)90032-0. [DOI] [PubMed] [Google Scholar]
- 40.Tehard B, van Liere MJ, Com Nougue C, Clavel-Chapelon F. Anthropometric measurements and body silhouette of women: validity and perception. J Am Diet Assoc. 2002;102:1779–1784. doi: 10.1016/s0002-8223(02)90381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


