Abstract
Background
Black women often present with advanced-stage breast cancer compared with White women, which may result in the observed higher mortality among Black women. Age-related factors (e.g., comorbidity) also affect mortality. Whether racial disparities in mortality are evident within age and/or stage groups has not been reported, and risk factors for greater mortality among Black women are not well defined.
Methods
Using the 1988–2003 Surveillance, Epidemiology, and End Results (SEER) Program data, we conducted a retrospective, population-based cohort study to compare overall and stage-specific breast-cancer mortality between Black and White women within each age (<40, 40–49, 50–64, and 65+) and stage (stage 0–IV and unstaged) group at diagnosis. Cox regression models calculated unadjusted and adjusted hazard ratios (HR) and 95% confidence intervals (CI), the latter controlling for potential confounders of the relationship between race and survival.
Results
In the 1988–2003 SEER data, 20,424 Black and 204,506 White women were diagnosed with first primary breast cancer. In unadjusted models, Black women were more likely than White women to die from breast cancer (HR: 1.90, 95% CI: 1.83–1.96) and from all causes (HR: 1.52, 95% CI: 1.48–1.55) during follow-up. In models stratified by age and stage, Black women were at increased risk of breast-cancer-specific mortality within each stage group among women <65 years.
Conclusion
Racial disparities in breast-cancer-specific mortality were predominantly observed within each stage at diagnosis among women <65 years old. This greater mortality risk for Black women was largely not observed among women ≥65 years of age.
Keywords: Survival, Breast cancer, Race, SEER
INTRODUCTION
Breast cancer is the most common noncutaneous malignancy in American women and affects approximately one in eight women over the course of her lifetime (1). Despite overall improvements in breast cancer survival since the early 1990s, Black women with breast cancer are still more likely to die within five years of diagnosis than their White counterparts (1, 2). Numerous epidemiological studies have shown that Black women are more likely to be diagnosed at younger ages, at more advanced stages, with higher grade and larger tumor size, with greater nodal involvement, and with a higher proportion of hormone receptor negative tumors (2–7). The most important prognostic factor appears to be stage at diagnosis, and many studies have attributed racial differences in survival to more advanced stage at diagnosis among Black women (3–5, 8). While this may explain differences in overall survival rates, there appear to be racial disparities in survival even within stage (5).
Several factors might account for the stage-specific disparity in survival between Black and White women, including differences in distribution of comorbid conditions or other competing risks (9), differences in biologic characteristics of the tumor (3, 7), differences in treatment received (10, 11), lack of access to care (12) or inadequate follow-up after abnormal screening mammography or treatment (13, 14), and overall differences in income and insurance coverage (15). Given that there are significant differences by age in the prevalence of comorbid conditions, in insurance coverage, in access to care, and in mammography screening recommendations (16), and that survival after breast cancer diagnosis is dependent on these factors as well as stage at diagnosis, a better understanding of the role of race, age, and stage in breast cancer survival is needed. It is likely that what leads to racial disparities in breast cancer outcomes among young women may be different in specific ways than what leads to racial disparities in outcomes among older women. By identifying specific subgroups of the population of breast cancer patients that have disparate survival outcomes, we can target clinical and public health efforts to eliminate the disparities as well as develop a better understanding of the specific biologic, social and/or behavioral mechanisms that lead to disparities in survival in different age-race groups.
The objectives of this study were to determine whether disparities in survival between Black and White women exist within specific age and stage groups, and to determine if there are any discernable patterns by race in all-cause and breast-cancer-specific mortality across age and/or stage groups.
METHODS
Study population
We conducted a retrospective, population-based cohort study of women diagnosed with a first primary breast cancer between January 1, 1988 and December 31, 2003. We analyzed data from nine registries of the Surveillance, Epidemiology, and End Results (SEER) Program, including San Francisco-Oakland, Connecticut, Metropolitan Detroit, Hawaii, Iowa, New Mexico, Seattle (Puget Sound), Utah, and Metropolitan Atlanta. We included only White or Black women being treated for their first primary and only tumor at presentation (SEER Sequence Number = 00) recorded within the SEER public-use dataset (N=226,324). We further excluded death-certificate-only or autopsy-only cases (N=1,394). The total sample used in the analysis was 224,930 women.
Measures
In addition to SEER registry, demographic information included age, race, and marital status. Age at diagnosis was grouped into < 40 years, 40–49 years, 50–64 years and 65+ years to reflect differences in mammography-screening guidelines and access to Medicare. All women younger than 40 years of age were grouped together, as they represent a special high-risk population for which routine mammography screening is currently not recommended (16, 17). As described in the previous section, we included only women who were recorded as “Black” or “White” in the SEER registry. Marital status was grouped as married, not married (includes single, divorced, separated, widowed), or unknown.
Breast tumors were classified by stage at diagnosis (AJCC categories of Stage 0, I, II, III, IV and unstaged), size (in cm), grade (I, II, III, or unknown), and estrogen receptor (ER) and progesterone receptor (PR) status (positive, negative, or unknown), as well as laterality (left, right, or bilateral with unknown lateral origin and stated to be a single primary/unknown), and nodal involvement (yes or no). Tumor size was categorized for the analysis as <2 cm, 2–5 cm, >5–10 cm, >10–25 cm, diffuse/widespread, or unknown.
Treatment characteristics were classified by receipt of surgery and receipt of radiation therapy. Two SEER variables, “site-specific surgery” (for 1983–1997 records) and “surgery of the primary site” (for 1998–2003 records), were recoded to form a single, dichotomous variable categorizing women as receiving or not receiving any surgery on the primary site. Patients who received any type of surgical resection of the primary breast tumor (e.g., mastectomy or partial mastectomy) were categorized as having had surgery. Patients who did not receive any formal resection of their primary tumor or who only underwent breast biopsies for tissue diagnosis were categorized as not having surgery. Women whose surgery status was not recorded were categorized as ‘unknown’. Patients were categorized as having received or not received radiation therapy (including external-beam radiation, radioactive implants, radioisotopes, or other radiation). Margin status after primary tumor resection, location of and surgery on metastatic disease sites, and use of chemotherapy or hormonal therapy were not recorded in the SEER database.
Survival time was calculated in the SEER dataset using the date of diagnosis and one of the following: date of death, date last known to be alive, or date used as cut-off for this datafile (December 31, 2003). Survival time was recorded in months and years; we used these data to compute survival in months. Vital status was present in the SEER dataset as “dead” or “alive.” We also determined whether death was due to breast cancer or other causes using the “Cause of Death” recode variable in SEER (26000 for breast cancer). Breast-cancer-specific mortality was compared with being alive at the end of follow-up, lost to follow-up, and having died of other causes.
Statistical analyses
Chi-square tests were used to compare the distribution of patient demographic, tumor characteristics, and treatment characteristics between Black and White women within each age group. Cox regression models were generated to describe the relationship between race and risk of death among women by age and stage at diagnosis and to account for differences in follow-up/survival time; these models were used to calculate hazard ratios (HRs) and 95% confidence intervals (95% CIs). Separate models were generated for the outcomes of all-cause mortality and breast-cancer-specific mortality. We report both crude (cHR) and adjusted hazard ratios (aHR), the latter controlling for potential confounders of the relationship between race and survival Potential confounders were selected a priori based upon the scientific literature regarding variables associated with racial disparities in survival. In the event that a specific model did not converge, we used data-based methods to select potential confounders based on their specific relationships with both race and survival within this dataset. Death during the study period was the event of interest, and persons who were lost to follow-up during the study period or were still alive at the end of the study period (December 31, 2003) were considered censored. P values <0.05 were considered to be statistically significant. All analyses were conducted using SPSS 13.0™ statistical software.
RESULTS
Description of the study population
Characteristics of the study population, including demographics, tumor characteristics, treatment characteristics, and survival outcomes are summarized in Table 1. A total of 224,930 Black or White women with a first primary (and only) occurrence of breast cancer diagnosed between 1988 and 2003 were included in the analysis. Of these, 9.1% (n=20,424) of the sample was Black. In addition, 6.2% was < 40 years of age, 19.4% was 40–49 years, 33.2% was 50–64 years, and 41.2% was 65+ years of age at diagnosis. Black women were more likely than White women to be diagnosed in the < 40 (10.8% vs. 5.7%) and 40–49 (24.6% vs. 18.9%) age groups, while White women were more likely to be diagnosed in the 65+ age group (42.2% vs. 31.1%) (p<0.001). Within each age group, Black women were more likely to be unmarried, diagnosed at a more advanced stage, have larger tumors, higher grade tumors, and positive lymph nodes compared with White women (p<0.001 for each). Black women were less likely to have ER-or PR-receptor positive tumors and less likely to have received surgery or radiation therapy than White women (p<0.001 for each).
Table 1.
Prevalence (%) of Demographic, Tumor, Treatment and Outcome Characteristics of the Study Population at Diagnosis, by Age and Race, 1988–2003 SEER (N=224,930).
| < 40 yrs(N=13944) | 40–49 yrs(N=43589) | 50–64 yrs(N=74785) | 65+ yrs(N=92612) | |||||
|---|---|---|---|---|---|---|---|---|
| B | W | B | W | B | W | B | W | |
| N (%) | 2196(10.8) | 11748(5.7) | 5020(24.6) | 38569(18.9) | 6846(33.5) | 67939(33.2) | 6362(31.1) | 86250(42.2) |
| Demographics | ||||||||
| Marital Status | ||||||||
| Not Married | 56.7 | 27.0 | 51.7 | 26.3 | 54.9 | 28.0 | 67.9 | 53.7 |
| Married | 38.3 | 68.4 | 42.2 | 68.5 | 39.2 | 67.1 | 26.4 | 41.6 |
| Unknown | 5.0 | 4.6 | 6.2 | 5.2 | 5.9 | 4.9 | 5.7 | 4.7 |
| SEER Registry | ||||||||
| San Fran-Oak | 13.3 | 14.1 | 14.9 | 15.2 | 16.0 | 15.0 | 17.2 | 14.3 |
| Connecticut | 10.0 | 17.0 | 10.9 | 17.5 | 11.2 | 17.3 | 10.4 | 18.7 |
| Metro Detroit | 30.2 | 15.2 | 35.5 | 15.4 | 38.9 | 15.5 | 44.8 | 15.8 |
| Hawaii | 0.9 | 1.9 | 0.2 | 1.8 | 0.3 | 1.7 | 0.2 | 1.3 |
| Iowa | 1.9 | 12.6 | 1.3 | 11.4 | 1.4 | 12.5 | 1.7 | 16.1 |
| New Mexico | 0.5 | 6.9 | 0.6 | 6.4 | 0.8 | 6.5 | 0.7 | 6.0 |
| Seattle | 5.5 | 16.3 | 4.5 | 17.1 | 4.0 | 16.9 | 3.7 | 15.9 |
| Utah | 0.2 | 6.4 | 0.2 | 5.7 | 0.2 | 6.1 | 0.2 | 5.8 |
| Metro Atlanta | 37.6 | 9.7 | 32.0 | 9.4 | 27.1 | 8.6 | 21.1 | 6.0 |
| Tumor Characteristics | ||||||||
| Stage at Diagnosis | ||||||||
| In Situ | 9.1 | 12.0 | 16.3 | 19.5 | 17.0 | 17.3 | 14.2 | 11.6 |
| I | 17.5 | 27.6 | 24.1 | 32.2 | 28.0 | 39.7 | 30.4 | 43.4 |
| II | 48.2 | 44.4 | 40.1 | 35.5 | 34.8 | 30.5 | 30.6 | 28.6 |
| III | 12.4 | 7.7 | 8.8 | 5.8 | 8.3 | 4.6 | 8.3 | 5.0 |
| IV | 6.1 | 3.1 | 5.1 | 2.6 | 6.8 | 3.6 | 7.9 | 4.9 |
| Unstaged | 6.7 | 5.2 | 5.5 | 4.4 | 5.1 | 4.2 | 8.4 | 6.5 |
| Tumor Size | ||||||||
| <2 cm | 27.6 | 40.9 | 36.6 | 49.1 | 41.6 | 55.5 | 40.4 | 52.6 |
| 2–5 cm | 44.4 | 39.1 | 39.0 | 31.8 | 34.2 | 27.3 | 32.9 | 29.8 |
| >5–10 cm | 11.3 | 6.1 | 7.4 | 4.4 | 6.9 | 3.4 | 7.3 | 3.8 |
| >10–25 cm | 1.0 | 0.6 | 1.1 | 0.5 | 1.1 | 0.4 | 1.2 | 0.4 |
| Diffuse | 2.4 | 1.6 | 2.1 | 1.2 | 1.8 | 1.1 | 1.8 | 0.9 |
| Unknown | 13.3 | 11.7 | 13.9 | 13.1 | 14.3 | 12.3 | 16.3 | 12.4 |
| Grade | ||||||||
| 1 | 2.9 | 5.2 | 6.8 | 10.2 | 8.9 | 13.7 | 10.3 | 14.5 |
| 2 | 18.4 | 23.8 | 22.7 | 28.4 | 24.8 | 30.5 | 26.1 | 31.0 |
| 3 | 50.5 | 41.3 | 41.4 | 30.5 | 36.4 | 26.0 | 28.5 | 21.8 |
| Unknown | 28.2 | 29.7 | 29.0 | 30.8 | 29.9 | 29.8 | 35.1 | 32.7 |
| Node Involvement | ||||||||
| No | 47.1 | 55.8 | 57.0 | 65.4 | 59.7 | 68.7 | 57.6 | 66.1 |
| Yes | 44.8 | 38.5 | 36.0 | 30.3 | 32.3 | 26.3 | 26.7 | 21.1 |
| Unknown | 8.0 | 5.7 | 7.1 | 4.3 | 8.0 | 5.0 | 15.7 | 12.8 |
| Laterality | ||||||||
| Right | 48.9 | 49.4 | 48.5 | 49.4 | 47.1 | 48.5 | 48.4 | 48.1 |
| Left | 50.5 | 50.1 | 51.0 | 50.2 | 52.3 | 51.0 | 50.5 | 50.7 |
| Bilateral/Unknown | 0.5 | 0.5 | 0.5 | 0.4 | 0.6 | 0.5 | 1.1 | 1.2 |
| ER status | ||||||||
| Neg | 34.6 | 27.0 | 28.8 | 18.1 | 24.9 | 14.7 | 16.0 | 10.2 |
| Pos | 31.5 | 39.9 | 34.1 | 46.0 | 37.0 | 50.8 | 41.4 | 53.6 |
| Unknown | 33.8 | 33.0 | 37.2 | 35.9 | 38.1 | 34.5 | 42.5 | 36.2 |
| PR status | ||||||||
| Neg | 36.9 | 29.3 | 30.6 | 20.2 | 30.6 | 21.0 | 23.4 | 18.5 |
| Pos | 28.6 | 36.7 | 31.6 | 42.8 | 30.5 | 42.9 | 33.0 | 43.7 |
| Unknown | 34.5 | 34.0 | 37.8 | 37.0 | 38.9 | 36.1 | 43.6 | 37.8 |
| Treatment Characteristics | ||||||||
| Radiation | ||||||||
| No | 56.5 | 54.4 | 55.4 | 52.0 | 54.2 | 50.6 | 65.6 | 64.1 |
| Yes | 39.1 | 42.9 | 40.2 | 45.5 | 41.9 | 47.5 | 32.0 | 34.6 |
| Unknown | 4.5 | 2.7 | 4.4 | 2.5 | 3.9 | 1.9 | 2.4 | 1.3 |
| Surgery | ||||||||
| No | 4.6 | 1.9 | 3.6 | 1.9 | 4.5 | 2.3 | 8.2 | 4.6 |
| Yes | 78.9 | 81.2 | 81.3 | 82.6 | 81.1 | 85.2 | 79.0 | 86.0 |
| Unknown | 16.5 | 16.9 | 15.1 | 15.5 | 14.4 | 12.5 | 12.8 | 9.4 |
| Outcomes | ||||||||
| Died | ||||||||
| Yes | 31.7 | 18.9 | 23.5 | 12.3 | 26.6 | 14.9 | 45.1 | 38.4 |
| No | 68.3 | 81.1 | 76.5 | 87.7 | 73.4 | 85.1 | 54.9 | 61.6 |
| Breast Cancer-Specific Death | ||||||||
| Yes | 27.7 | 17.0 | 19.2 | 10.5 | 19.0 | 10.5 | 19.6 | 13.3 |
| No | 72.3 | 83.0 | 80.8 | 89.5 | 81.0 | 89.5 | 80.4 | 86.7 |
| Other Cause of Death | ||||||||
| Yes | 4.0 | 1.9 | 4.3 | 1.8 | 7.6 | 4.4 | 25.5 | 25.1 |
| No | 96.0 | 98.1 | 95.7 | 98.2 | 92.4 | 95.6 | 74.5 | 74.9 |
Note. B = Black; W = White; All chi-square comparisons were statistically significant except for laterality within each of the age groups, and for other cause of death within the 65+ age group.
Overall and breast-cancer-specific mortality
A total of 56,773 women died of all causes and 28,802 (51%) died of breast cancer. Across all age groups, a greater percentage of Black women died due to all causes and due to breast cancer specifically compared to White women (Table 1 and Figures 1–6). Interestingly, in each of the <65 age groups, Black women were more likely to die of other causes (although mortality due to other causes was generally uncommon in both racial groups in women <65 years of age), but there was no significant racial difference in mortality due to other causes among women in the 65+ age group. Overall, for all age and stage groups combined, the crude risk of death due to all causes and the crude risk of death due to breast cancer specifically was higher in Black women compared to White women [cHRall-cause = 1.52 (95% CI 1.48–1.55); cHRbreastcancer = 1.90 (95% CI 1.83–1.96)].
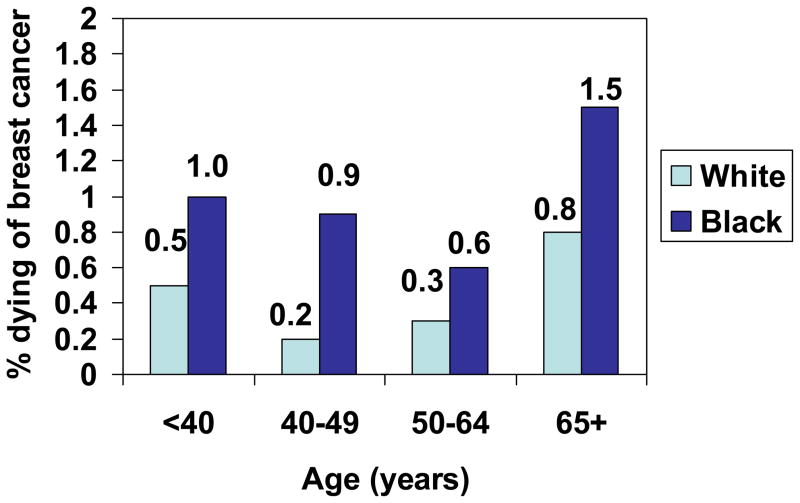
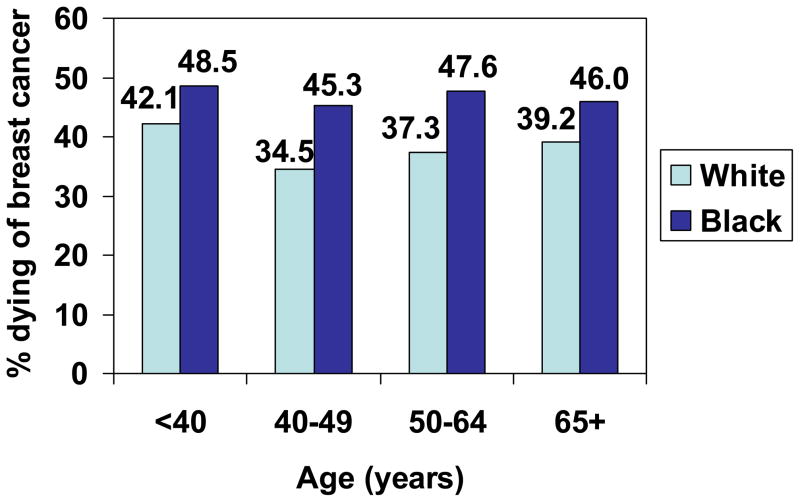
Figure 1.
Proportion of women with In Situ breast cancer who died of breast cancer, by age and race, SEER, 1988–2003.
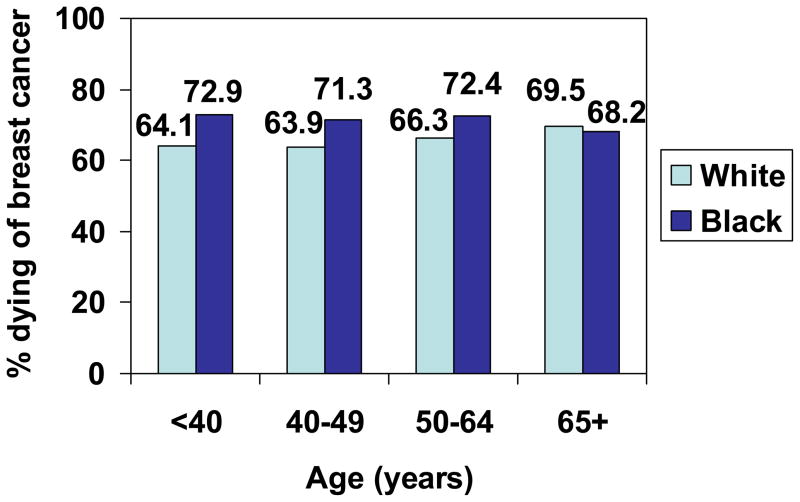
Fig 6.
Proportion of women with Unstaged breast cancer who died of breast cancer, by age and race, SEER, 1988–2003.
Racial disparities in all-cause and breast-cancer-specific mortality by age and stage
Crude and adjusted hazard ratios for the associations between race and risk of death for all-cause mortality and for breast-cancer-specific mortality are provided by age and stage in Table 2. The aHR are presented in bold font in the table.
Table 2.
Crude and Adjusted* Hazard Ratios and 95% Confidence Intervals for the Relationship of Race (Black vs. White) with All-cause and Breast Cancer-specific Mortality by Age and Stage, 1988–2003 SEER.
| All-cause Mortality | ||||
|---|---|---|---|---|
| < 40 yrs | 40–49 yrs | 50–64 yrs | 65+ yrs | |
| Stage at Diagnosis | ||||
| In Situ | 1.59 (0.61–4.17) 1.31 (0.49–3.51) | 3.46 (2.13–5.63) 3.42 (1.98–5.92) | 2.99 (2.30–3.88) 2.61 (1.96–3.47) | 1.39 (1.20–1.62) 1.27 (1.09–1.49) |
| I | 2.01 (1.49–2.70) 2.01 (1.44–2.82) | 2.64 (2.19–3.19) 2.19 (1.78–2.70) | 1.84 (1.60–2.12) 1.43 (1.23–1.66) | 1.14 (1.05–1.24) 1.00 (0.91–1.09) |
| II | 1.48 (1.30–1.68) 1.45 (1.26–1.68) | 1.81 (1.64–1.99) 1.53 (1.37–1.70) | 1.65 (1.53–1.79) 1.39 (1.27–1.52) | 1.29 (1.20–1.38) 1.13 (1.05–1.21) |
| III | 1.67 (1.39–2.01) 1.70 (1.35–2.13) | 1.83 (1.58–2.12) 1.48 (1.26–1.74) | 1.80 (1.59–2.03) 1.59 (1.39–1.83) | 1.32 (1.19–1.47) 1.09 (0.97–1.22) |
| IV | 1.72 (1.37–2.15) 1.56 (1.23–1.98)** | 1.40 (1.20–1.64) 1.23 (1.02–1.48) | 1.38 (1.24–1.55) 1.23 (1.08–1.39) | 1.10 (0.99–1.21) 0.95 (0.85–1.06) |
| Unstaged | 2.38 (1.78–3.20) 1.88 (1.29–2.74) | 2.11 (1.68–2.66) 1.56 (1.17–2.07) | 2.49 (2.10–2.97) 1.80 (1.48–2.19) | 1.13 (1.01–1.27) 0.93 (0.82–1.05) |
| Breast-Cancer-specific Mortality | ||||
| Stage at Diagnosis | ||||
| In Situ | 2.16 (0.45–10.42) 1.42 (0.29–7.02) | 4.31 (1.77–10.48) 7.25 (2.52–20.80) | 2.83 (1.25–6.44) 3.07 (1.23–7.68) | 2.22 (1.25–3.91) 2.26 (1.21–4.21) |
| I | 2.03 (1.48–2.80) 2.08 (1.45–2.98) | 2.30 (1.83–2.90) 1.90 (1.47–2.46) | 1.74 (1.40–2.17) 1.25 (0.99–1.58) | 1.45 (1.19–1.77) 1.21 (0.99–1.49) |
| II | 1.44 (1.26–1.64) 1.44 (1.24–1.67) | 1.69 (1.52–1.88) 1.44 (1.28–1.62) | 1.56 (1.42–1.72) 1.34 (1.21–1.49) | 1.44 (1.29–1.60) 1.20 (1.07–1.34) |
| III | 1.52 (1.25–1.85) 1.53 (1.20–1.95) | 1.81 (1.54–2.11) 1.43 (1.21–1.71) | 1.73 (1.51–1.97) 1.54 (1.33–1.79) | 1.38 (1.21–1.58) 1.09 (0.94–1.27) |
| IV | 1.66 (1.31–2.11) 1.50 (1.17–1.93)** | 1.44 (1.23–1.70) 1.26 (1.03–1.53) | 1.39 (1.24–1.56) 1.24 (1.08–1.41) | 1.06 (0.95–1.18) 0.95 (0.84–1.07) |
| Unstaged | 2.49 (1.82–3.41) 2.10 (1.40–3.14) | 2.01 (1.56–2.60) 1.43 (1.05–1.96) | 2.73 (2.22–3.35) 1.86 (1.47–2.36) | 1.26 (1.07–1.48) 1.07 (0.89–1.27) |
Note. Adjusted hazard ratios are presented in bold font.
All models are adjusted for marital status, SEER registry, tumor size, tumor grade, node involvement, ER status, PR status, receipt of radiation treatment, and receipt of surgery unless otherwise noted
Adjusted for marital status and SEER registry only, because model failed to converge with other covariates added
In unadjusted and adjusted models, Black women were at increased risk of both all-cause mortality and breast-cancer-specific mortality at each stage among women <65 years of age. In the 65+ age group, Black women diagnosed with in situ or stage II breast cancer were at greater risk of dying from breast cancer than White women diagnosed at the same stage of disease. The disparities in survival between Black and White women in the 65+ age group for all other stages were not statistically significant.
DISCUSSION
Even as recent studies indicate that the overall rate of breast cancer mortality is declining nationwide, socioeconomic and racial disparities not only persist but are, in fact, worsening (18). Chu et al. (19) recently examined racial and ethnic disparities in cancer survival by measuring excess cancer burden and trends in cancer rates for nine cancer sites, including breast. Racial disparities in cancer mortality continued to increase during the 1995–2000 time period compared to the 1990–1994 time period (calculated as a ratio of the average annual cancer mortality rate for 1995–2000 relative to the rate for 1990–1994) for colorectal cancer, prostate cancer, and breast cancer for each racial/ethnic minority (19).
The greater likelihood that Black women are diagnosed with late-stage disease has been shown to be a major contributor to their lower survival rates (2–5, 10). The reasons for this phenomenon are likely complex and multifactorial. Some have attributed racial differences in stage at diagnosis to racial differences in the utilization of mammography screening (20). Smith-Bindman et al. (20) found that 34% of African-American women received inadequate mammographic screening prior to their diagnosis of breast cancer. When they stratified their analysis by frequency of previous mammography screening, the observed greater likelihood of being diagnosed with advanced cancers among African-American women compared with White women was attenuated or eliminated. In contrast, other surveys have found only small differences in mammography use between White and non-White women (21, 22), and in their analysis of Behavioral Risk Factor Surveillance System data, Schootman et al. (12) reported that, by 2000, racial/ethnic disparities in the prevalence of mammography utilization had disappeared. However, these latter studies were based on self-reported mammography screening and inquired only about recent use, not adherence to recommended screening guidelines over time. Self-reported data about mammography screening are prone to recall bias, particularly among low-income African-American women (23). Inadequate diagnostic follow-up after abnormal screening results also might result in a delay in presentation (13) resulting in a late-stage diagnosis.
Although the overall impact of mammography screening on stage at diagnosis is not discernible using the SEER data, we observed that Black women were more likely to be diagnosed with late-stage breast cancer compared with White women and that this difference was seen across all age groups, including women <40 years (who are not likely to have routine screening mammography) and women >40 years (who are recommended to undergo yearly screening mammography). However, we did observe that the percentage of women, both Black and White, who were diagnosed at Stage II or III generally decreased as age increased (more women diagnosed at Stage I) which may indicate that older women in both racial groups are more likely to get screened than their younger counterparts. Despite the finding that Black women are more likely to be diagnosed at more advanced stage, late stage at diagnosis alone does not account for the differences in breast-cancer-specific mortality between Black and White women. For the most part, Black women <65 years of age were more likely to die from their breast cancer within each stage of disease and within each age category. This disparity was attenuated and of borderline significance among women with stage I breast cancer in the 50–64 age group. Among women >65 years of age, Black women were at greater risk of dying from breast cancer compared with White women if diagnosed with in situ or stage II breast cancer but not at other stages of disease. For older women diagnosed with stage III or IV disease, in particular, one might expect an extremely poor clinical prognosis regardless of race, and with the greater likelihood of co-morbidities in the face of regional or metastatic disease, a racial disparity in survival outcomes would not be expected. Unfortunately, comorbidity information is not available in the SEER database, therefore we could not include comorbidity in our analysis. Information about comorbid conditions is available in the SEER-Medicare linked data, though analysis would be limited to patients >65 years old.
Socioeconomic status and health insurance coverage also have been shown to be an important factor in cancer disparities (12–15). Socioeconomic status impacts not only access to cancer screening programs, but can also affect access to optimal treatment and follow-up. Socioeconomic barriers can exist at the patient, provider, and health system levels and are pervasive among racial and ethnic minorities because of existing public health policies that impact populations of lower educational attainment, lower rates of employment, and reduced health insurance coverage (24, 25). Patient factors include perceived lack of urgency, competing health or other problems, lack of time, and cancer fear and anxiety (26). Kerner et al. (27) found that Black women with high cancer anxiety were 50% less likely to complete a diagnostic evaluation within 90 days compared with Black women with lower cancer anxiety scores. Physician behaviors and quality of care also can contribute to disparities in breast cancer mortality, and minority women are more likely to receive a course of surgical and radiation treatment that does not meet the 2000 National Comprehensive Cancer Network standards (28). Indeed, we observed in these SEER data that Black women in each age group were less likely to have had surgical excision of their breast cancer (Table 1). Finally, health system barriers, including patient self-pay costs, lack of access to a primary care physician, and complex treatment schedules lead to fragmentation of care and worse outcomes among racial and ethnic minorities (29). Although we were unable to account for these specific factors in the current study, future research might be designed to measure their contribution to poorer survival among Black women within each stage at diagnosis.
Tumor characteristics certainly play a role in breast-cancer survival. Black women are more likely than White women to have breast cancers with unfavorable biologic parameters, including negative ER and PR status, high nuclear grade, and high S-phase fraction (3, 7). Our results corroborate these findings across all age categories. Gene expression analysis has identified several breast cancer subtypes, including basal-like, human epidermal growth factor receptor-2 positive/estrogen receptor negative (HER2+/ER−), luminal A, and luminal B (30). These subtypes differ markedly in prognosis and in the repertoire of therapeutic targets they express, with the luminal A subtype having the best prognosis (31). The basal-like subtype has been associated with poor clinical outcome, which is likely attributable to its high proliferative capacity, as well as the lack of directed therapies since basal-like tumors do not typically express ER or over-express HER2 (31). Carey et al. (32) recently examined the prevalence of breast cancer subtypes within racial and menopausal subsets in 496 incident cases of invasive breast cancer from the Carolina Breast Cancer Study. The basal-like breast cancer subtype was more prevalent among pre-menopausal African-American women compared with post-menopausal African-American women and non-African-American women of any age, whereas the luminal A subtype was less prevalent (32). This finding could account for our observed disparities in breast-cancer-specific mortality, especially in the <40 year group where the rate of ER/PR negative tumors was highest.
One of the most interesting findings of our analysis was that our highest within-stage disparity in breast-cancer-specific mortality was observed between Black and White women with early-stage breast cancer. The adjusted hazard ratios were highest in the 40–49 age group with in situ disease. Although the absolute proportions of patients dying with in situ disease are small (Figure 1), this is still likely to be clinically relevant given that 15–20% of all mammographically detected cancers are ductal carcinoma in situ (33). The reasons for this disparity in survival for non-invasive breast cancer are not clearly known. Pocock et al. (34) found that Black women with ductal carcinoma in situ were 64% more likely to undergo a delay to surgery (>50 days) compared with White women. Joslyn (35) analyzed women with ductal carcinoma in situ in the 1973–2000 SEER database and found that Black and Asian/Pacific island women were significantly more likely to receive breast conservation surgery, although Black women were less likely to receive follow-up radiation therapy. This translated to a significantly increased risk of death for Black women (35). Black women in our study were less likely to undergo radiation therapy compared to White women across all age categories, and this could contribute to the survival disparity for in situ and early-stage invasive disease, since the increased local recurrence rate after lumpectomy without radiotherapy translates to a decrease in 15-year survival (36).
Suboptimal systemic treatment also may account for the poorer survival observed in Black women with early-stage disease. Hershman et al. (37) analyzed the association of early systemic treatment termination and treatment duration with all-cause mortality in patients with stage I and II breast cancer. A substantial fraction (28%) of women with early-stage breast cancer terminated their chemotherapy prematurely, and that early termination was associated with Black race and poorer survival (37). One major limitation of our study is the inability to control for variations in systemic treatment regimens. Types of systemic regimens, receipt of fewer than the expected number of cycles, and delay in receiving the expected number of cycles could potentially account for the observed racial disparities in survival, but the SEER program does not collect this type of information.
In spite of this lack of potentially relevant information in the SEER database, our analysis, which controlled for several variables associated with survival, demonstrated significantly poorer survival for Black women <65 years of age at all stages of disease within each age group. The differences were most pronounced for younger women with early-stage disease, and this should be the focus of future prospective studies. A better understanding of the patient, physician, tumor, and treatment factors contributing to the disparity in survival outcomes between Black and White women may lead to interventions that reduce racial disparities in breast cancer survival.
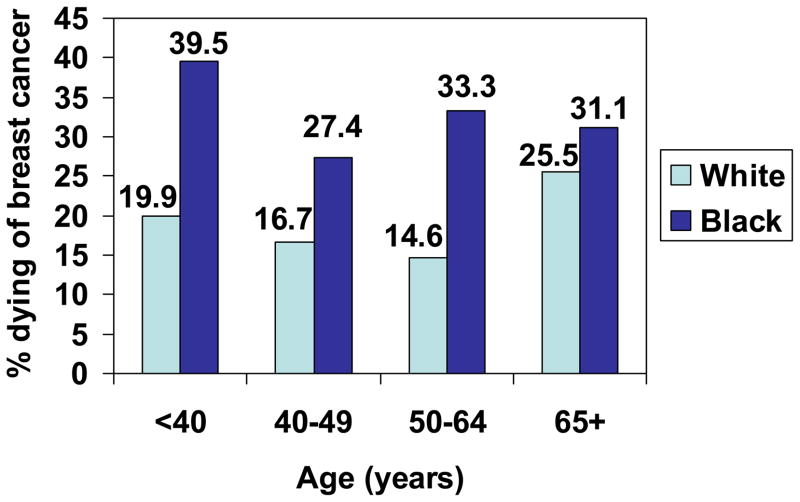
Figure 2.
Proportion of women with Stage I breast cancer who died of breast cancer, by age and race, SEER, 1988–2003.
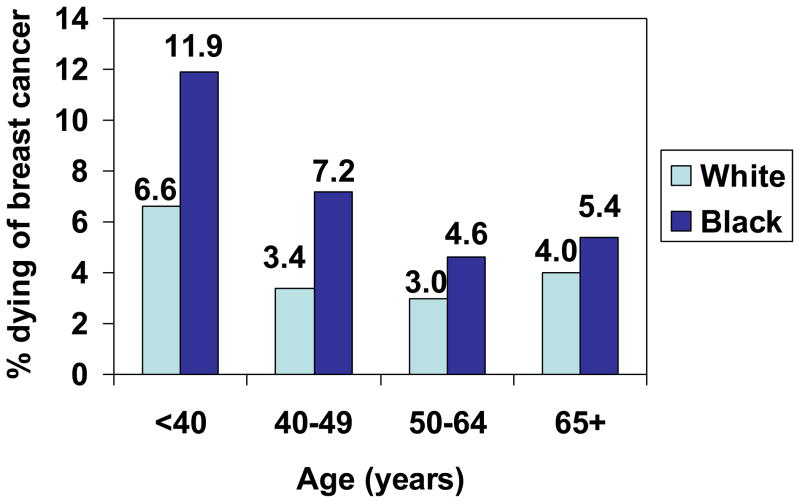
Figure 3.
Proportion of women with Stage II breast cancer who died of breast cancer, by age and race, SEER, 1988–2003.
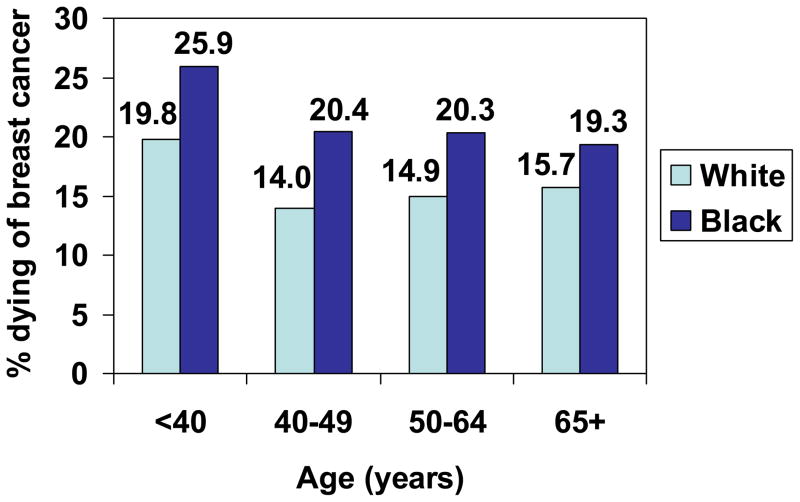
Figure 4.
Proportion of women with Stage III breast cancer who died of breast cancer, by age and race, SEER, 1988–2003.
Fig 5.
Proportion of women with Stage IV breast cancer who died of breast cancer, by age and race, SEER, 1988–2003.
Acknowledgments
We thank the Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis, Missouri, for the use of the Health Behavior and Outreach Core, especially James Struthers for data management services. The Siteman Cancer Center is supported in part by the National Cancer Institute Cancer Center Support Grant #P30 CA91842.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Curtis E, Quale C, Haggstrom D, Smith-Bindman R. Racial and ethnic differences in breast cancer survival: How much is explained by screening, tumor severity, biology, treatment, comorbidities, and demographics? Cancer. 2008;112:171. doi: 10.1002/cncr.23131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg J, Chia YL, Plevritis S. The effect of age, race, tumor size, tumor grade, and disease stage on invasive ductal breast cancer survival in the U.S. SEER database. Cancer Res Treat. 2005;89:47. doi: 10.1007/s10549-004-1470-1. [DOI] [PubMed] [Google Scholar]
- 4.Joslyn SA, West MM. Racial differences in breast carcinoma survival. Cancer. 2000;88:114. doi: 10.1002/(sici)1097-0142(20000101)88:1<114::aid-cncr16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 5.McBride R, Hershman D, Tsai W-Y, Jacobson JS, Grann V, Neugut AI. Within-stage racial differences in tumor size and number of positive lymph nodes in women with breast cancer. Cancer. 2007;110:1201. doi: 10.1002/cncr.22884. [DOI] [PubMed] [Google Scholar]
- 6.Joslyn SA. Hormone receptors in breast cancer: racial differences in distribution and survival. Breast Cancer Res Treat. 2002;73:45. doi: 10.1023/a:1015220420400. [DOI] [PubMed] [Google Scholar]
- 7.Li CI, Malone KE, Daling JR. Differences in breast cancer hormone receptor status and histology by race and ethnicity among women 50 years of age and older. Cancer Epidemiol Biomarkers Prev. 2002;11:601. [PubMed] [Google Scholar]
- 8.Chu KC, Lamar CA, Freeman HP. Racial disparities in breast carcinoma survival rates: Separating factors that affect diagnosis from factors that affect treatment. Cancer. 2003;97:2853. doi: 10.1002/cncr.11411. [DOI] [PubMed] [Google Scholar]
- 9.Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005;294(14):1765. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 10.Du W, Simon MS. Racial disparities in treatment and survival of women with stage I-III breast cancer at a large academic medical center in metropolitan Detroit. Breast Cancer Res Treat. 2005;91:243. doi: 10.1007/s10549-005-0324-9. [DOI] [PubMed] [Google Scholar]
- 11.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 12.Schootman M, Jeffe DB, Reschke AH, Aft R. Disparities related to socioeconomic status and access to medical care remain in the United States among women who never had a mammogram. Cancer Causes and Control. 2003;14:419. doi: 10.1023/a:1024941626748. [DOI] [PubMed] [Google Scholar]
- 13.Schootman M, Jeffe DB, Gillanders W, Yan Y, Jenkins B, Aft R. Geographic clustering of adequate diagnostic follow-up after abnormal screening results for breast cancer among low-income women in Missouri. Ann Epidem. 2007;17(9):704. doi: 10.1016/j.annepidem.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Schootman M, Jeffe DB, Lian M, Aft R, Gillanders WE. Surveillance mammography and the risk of death among elderly breast cancer patients. Breast Cancer Res Treat. doi: 10.1007/s10549-007-9795-1. in press. [DOI] [PubMed] [Google Scholar]
- 15.Schootman M, Walker M, Rohrer J, Jeffe DB, Baker EA. Breast cancer screening and incidence in communities with high proportion uninsured. Am J Prev Med. doi: 10.1016/j.amepre.2007.07.032. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leitch AM, Dodd GD, Costanza M, et al. American Cancer Society guidelines for the early detection of breast cancer: Update 1997. CA Cancer J Clin. 1997;47:150. doi: 10.3322/canjclin.47.3.150. [DOI] [PubMed] [Google Scholar]
- 17.U S. Preventive Services Task Force. Screening for breast cancer: recommendations and rationale. Ann Intern Med. 2002;137:344. doi: 10.7326/0003-4819-137-5_part_1-200209030-00011. [DOI] [PubMed] [Google Scholar]
- 18.Blackman DJ, Masi CM. Racial and ethnic disparities in breast cancer mortality: Are we doing enough to address the root causes? J Clin Oncol. 2006;24:2170. doi: 10.1200/JCO.2005.05.4734. [DOI] [PubMed] [Google Scholar]
- 19.Chu KC, Miller BA, Springfield SA. Measures of racial/ethnic health disparities in cancer mortality rates and the influence of socioeconomic status. J Natl Med Assoc. 2007;99(10):1092. [PMC free article] [PubMed] [Google Scholar]
- 20.Smith-Bindman R, Miglioretti DL, Lurie N, et al. Does utilization of screening mammography explain racial and ethnic differences in breast cancer? Ann Intern Med. 2006;144:541. doi: 10.7326/0003-4819-144-8-200604180-00004. [DOI] [PubMed] [Google Scholar]
- 21.Blackman DK, Bennett EM, Miller DS. Trends in self-reported use of mammograms (1989–1997) and Papanicolaou tests (1991–1997) – Behavioral Risk Factor Surveillance System. MMWR CDC Surveill Summ. 1999;48:1. [PubMed] [Google Scholar]
- 22.Breen N, Wagener DK, Brown ML, Davis WW, Ballard-Barbash R. Progress in cancer screening over a decade: results of cancer screening from the 1987, 1992, and 1998 National Health Interview Surveys. J Natl Cancer Inst. 2001;93:1704. doi: 10.1093/jnci/93.22.1704. [DOI] [PubMed] [Google Scholar]
- 23.Champion VL, Menon U, McQuillen DH, Scott C. Validity of self-reported mammography in low-income African-American women. Am J Prev Med. 1998;14:111. doi: 10.1016/s0749-3797(97)00021-4. [DOI] [PubMed] [Google Scholar]
- 24.Wherry L, Finegold K. Changes in health insurance coverage and health status by race and ethnicity, 1997–2002. J Natl Med Assoc. 2004;96:1577. [PMC free article] [PubMed] [Google Scholar]
- 25.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94:490. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 26.Caplan L, Helzlsouer KJ, Shapiro S, et al. Reasons for delay in breast cancer diagnosis. Prev Med. 1996;25:218. doi: 10.1006/pmed.1996.0049. [DOI] [PubMed] [Google Scholar]
- 27.Kerner JF, Yedidia M, Padgett D, et al. Realizing the promise of breast cancer screening: Clinical follow-up after abnormal screening among black women. Prev Med. 2003;37:92. doi: 10.1016/s0091-7435(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 28.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163:49. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 29.Bickell NA, Young GJ. Coordination of care for early-stage breast cancer patients. J Gen Intern Med. 2001;16:737. doi: 10.1111/j.1525-1497.2001.10130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sotiriou C, Neo SY, McShane LM, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci USA. 2003;100:10393. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 33.Sakorafas G, Farley D. Optimal management of ductal carcinoma in situ of the breast. Surg Oncol. 2003;17:221. doi: 10.1016/S0960-7404(03)00031-8. [DOI] [PubMed] [Google Scholar]
- 34.Pocock B, Nash S, Klein L, El-Tamer M, Schnabel FR, Joseph KA. Disparities in time to definitive surgical treatment between black and white women diagnosed with ductal carcinoma in situ. Am J Surg. 2007;194(4):521. doi: 10.1016/j.amjsurg.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Joslyn SA. Ductal carcinoma in situ: trends in geographic, temporal, and demographic patterns of care and survival. Breast J. 2006;12(1):20. doi: 10.1111/j.1075-122X.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- 36.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med. 1997;337:949. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 37.Hershman D, McBride R, Jacobson JS, et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23:6639. doi: 10.1200/JCO.2005.12.633. [DOI] [PubMed] [Google Scholar]