SUMMARY
The food borne pathogen, Bacillus cereus, produces uronic acid-containing glycans that are secreted in a shielding biofilm environment, and certain alkaliphilic Bacillus deposit uronate-glycan polymers in the cell wall when adapting to alkaline environments. The source of these acidic sugars is unknown, and here we have described the functional identification of an operon in B. cerues subsp. cytotoxis NVH 391–98 that comprises genes involved in the synthesis of UDP-uronic acids in Bacillus spp. Within the operon, a UDP-glucose 6-dehydrognease (UGlcDH) converts UDP-glucose in the presence of NAD+ to UDP-glucuronic acid and NADH, and a UDP-GlcA 4-epimerase (UGlcAE) converts UDP-glucuronic acid to UDP-galacturonic acid. Interestingly, in vitro both enzymes can utilize the TDP-sugar forms as well, albeit at lower catalytic efficiency. Unlike most of the very few bacterial 4-epimerases that have been characterized, which are promiscuous, the B. cereus UGlcAE enzyme is very specific and cannot use UDP-Glc, UDP-GlcNAc, UDP-GlcNAcA or UDP-Xyl as substrates. Size exclusion chromatography suggests that UGlcAE is active as a monomer, unlike the dimeric form of plant enzymes; the Bacillus UGlcDH is also found as a monomer. Phylogenic analysis further suggests that the Bacillus UGlcAE may have evolved separately from other bacterial and plant epimerases. Our results provide insight into the formation and function of uronic acid-containing glycans in the lifecycle of B. cereus and related species containing homologous operons as well as the basis to determine the importance of these acidic glycans. We also discuss the ability to target UGlcAE as a drug candidate.
Keywords: Bacillus, hexuronic acid, UDP-glucuronic acid, UDP-galacturonic acid, biofilm, alkalinity
INTRODUCTION
B. cereus has garnered much notoriety as a food-poisoning bacterium, and similar to its close relatives, the human pathogen B. anthracis and the insecticidal B. thuringiensis, B. cereus has several life forms. For example, it can survive as a protective endospore in harsh conditions, in a shielding biofilm environment, or as a free-living cell in soil and water. An explanation of how changes in surface glycans confer advantages to Bacillus species during different stages of its life cycle is of great interest in understanding the pathogenesis of the organism. However, due to the infectious nature B. cereus and related Bacillus spp., it is understandable that many glycans have yet to be characterized, particularly those that may be made by the pathogen within the human body. Recently, polysaccharides isolated from spores of B. anthracis have been shown to be antigenic and are similar to cross-reactive epitopes that are found only in pathogenic strains of B. cereus, highlighting the role of carbohydrates in Bacillus spp. infections [1]. To identify new metabolic pathways involved in the formation of surface glycans in these pathogens, we sought after putative genes encoding enzymes involved in the synthesis of glycan precursors (i.e. nucleotide-sugars). These nucleotide-sugars are used by specific glycosyltransferases to make the different polysaccharide structures in an organism.
The occurrence of the sugar residue galacturonic acid (GalA) in cell surface glycans varies across different bacterial species. In Gram-negative bacteria, for example, the amount and distribution of GalA in lipopolysaccharides (LPS), the major glycan molecule in the outer cell surface membrane [2], shows significant variations. For instance, the core oligosaccharide portion of the LPS in the symbiotic nitrogen-fixing bacteria Rhizobium leguminosarum [3] and Bradyrhizobium japonicum [4], and in the pathogen Klebsiella pneumonia [5], consists of several GalA residues. However, the core oligosaccharides of E. coli and S. typhimurium lack GalA and instead consist of glucosamine residues. One reason for the variations in surface glycans could be that organisms form specific glycans in order to facilitate exclusive interactions with a particular host. Alternatively, specific glycans may display a mimicry-like structure to facilitate entry into the host, or perhaps to allow the bacteria to survive under different environmental conditions. It is also a possibility that the negative charge of acidic sugars (glycuronosyl residues) functions as an ionic barrier or to sequester metals required for normal organism function.
GalA-containing glycans have also been reported in Gram-positive bacteria. For example, GalA has been observed in the capsule of Streptococcus pneumoniae serotype 1 [6]. Interestingly, the bacterial capsular surface in S. pneumoniae has been suggested as the first barrier against the innate immune system as well as mediating attachment to cells/surfaces to resist clearance. Recent analyses of Bacillus cereus growing in biofilm have indicated that it secretes exo-polysaccharides (EPS) consisting of the glycuronosyl residues glucuronic acid (GlcA) and GalA [7]. GlcA and GalA residues, however, were not reported in B. cereus glycans isolated from spores or cultures grown in the lab [8–10].
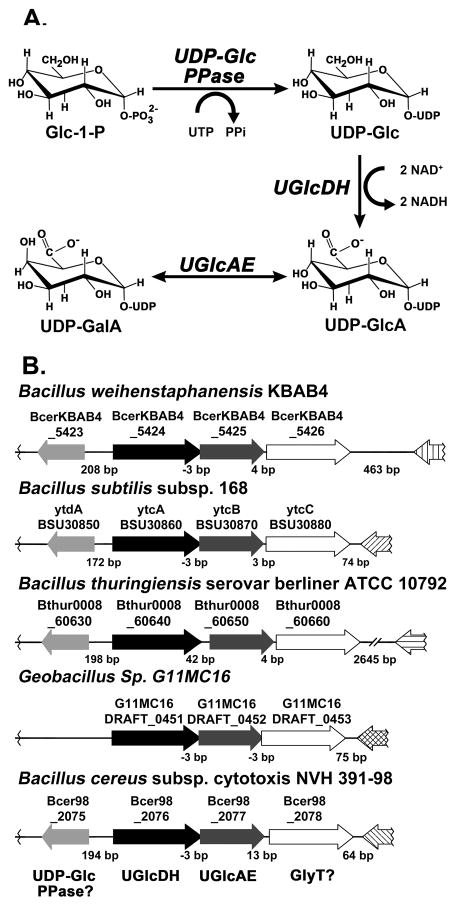
Synthesis of UDP-galacturonic acid (UDP-GalA) [11, 12] has been studied extensively in plants, where UDP-glucose (UDP-Glc) is first converted to UDP-glucuronic acid (UDP-GlcA) by UDP-glucose 6-dehydrogenase (annotated as UGDH, UGD or UGlcDH). Subsequently, a very specific membrane-bound 4-epimerase, UDP-GlcA 4-epimerase (UGlcAE) [13, 14] interconverts UDP-GlcA and UDP-GalA. Fewer bacterial UDP-GlcA 4-epimerases have been characterized, and questions still remain concerning how the specificity for UDP-GlcA of these epimerases varies across bacterial species. Studies of the Streptococcus pneumococcus type I epimerase [15] and Glakp from Klebsiella pneumoniae have shown that the enzymes are capable of interconverting several UDP-sugars including UDP-GlcA, UDP-Glc and UDP-GlcNAc [5]. On the other hand, the characterization of the type I S. pneumococcus Cap1J 4-epimerase demonstrated that the enzyme had specificity for the uronic acid moiety, as it was unable to interconvert UDP-Glc or UDP-Gal [16]. Here we report the identification and characterization of two genes (BcUGlcDH and BcUGlcAE) involved in the biosynthesis of UDP-GlcA and UDP-GalA (Fig. 1 A) from Bacillus cereus subsp. cytotoxis NVH 391–98. Interestingly, the operon for these two genes is not common in all Bacillus spp., which may provide insight into the roles of these charged-glycans within the lifecycle of this pathogen.
FIGURE 1. The biosyntheses of acidic sugar nucleotides in Bacillus.
A. In Bacillus, based on the present report, UDP-Glc pyrophosphorylase (UDP-Glc PPase) converts Glc-1-P and UTP to UDP-Glc. In the presence of NAD+, UDP-Glc is interconverted by UDP-glucose dehydrogenase (UGlcDH) into UDP-GlcA and NADH. UDP-GlcA can then be interconverted by the 4-epimerase, UGlcAE, into UDP-GalA.
B. Organization of the three genes within the UGalA operon and the flanking regions across selective members of Bacillus spp. The locus number for each gene in the operon across the different species shown is indicated. Based on this report, Bcer98_2076 functions as a UGlcDH and Bcer98_2077 as a UGlcAE. The operon also comprises a putative glycosyltransferase (Bcer98_2078 in B. cereus) which may be involved in the synthesis of GlcA or GalA-containing glycans. Note: except for Geobacillus sp. G11MC16, in the 5′ region flanking the UGalA operon there is a single gene in the opposite direction of the operon encoding a putative UDP-Glc PPase (Bcer98_2075 in B. cereus, putative ytdA in B. subtilis).
RESULTS
Bioinformatic analyses of UDP-Glc 6-dehydrogenase and UDP-GlcA 4-epimerase in Bacillus
A BLAST analysis with characterized proteins from plants and bacteria was performed to identify enzymes involved in UDP-GalA production in B. cereus; and those with the greatest homology to known UGlcAE and UGlcDH were selected for further study. However, the validation of the selected enzymes based on the homology alone was challenging due to a relative low amino-acid sequence identity with the known enzymes. For example, the potential dehydrogenase from B. cereus, Bcer98_2076, shares only 35, 32, 31 and 32% amino-sequence identity with functional UDP-Glc 6-dehydrogenase from Drosophila (sugarless), plants (AtUGlcDH), Cryptococcus (Ugd1) and Gram-negative Sinorizobium (rkpK, SMc02641), respectively (Fig. S1A in the supplementary section). In addition, the Bcer98_2076 also shares 38% sequence identity with functional GDP-mannose dehydrogenase from Pseudomonas aeruginosa [17]. Similarly, the potential epimerase from B. cereus, Bcer98_2077, shares 36% and 32% with functional UDP-GlcA 4-epimerase (AtUGlcAE3) from plants, and LpsL from Sinorizobium (SMc02640), respectively (Supplemental Fig. S2B). Having such relative low sequence identities, it is impossible to predict function for these putative Bacillus spp. UGlcDH and UGlcAE homologs without further biochemical characterization. Interestingly, the putative dehydrogenase, Bcer98_2076, and epimerase, Bcer98_2077, from B. cereus are found in the same operon along with a putative glycosyltransferase, Bcer98_2078, that shares sequence similarity to an annotated lipopolysaccharide N-acetylglucosaminyltransferase. Similar operons (see Fig. 1B) with homologous genes are found in Bacillus subtilis subsp. 168, Bacillus weihenstephanensis KBAB4, Bacillus thuringiensis serovar berliner ATCC 10792 and Geobacillus sp. G11MC16, while such operon organization is not found in the genomes of other Bacillaceae, like B. anthracis strains Ames, A0248, or CDC 684. A putative nucleotidyl transferase, Bcer98_2075, is also found in proximity to the operon containing the putative dehydrogenase and epimerase, and homologous genes are found alongside the operon in B. subtilis subsp. 168, B. weihenstephanensis KBAB4, and B. thuringiensis serovar berliner ATCC 10792 but absent from Geobacillus sp. G11MC16. In B. subtilis subsp. 168, the homologous gene (ytdA BSU30850) is annotated as a putative UDP-glucose pyrophosphorylase, suggesting that Bcer98_2075 and its homologs may play a role in the synthesis of UDP-Glc (Fig. 1A) for subsequent interconversion to UDP-GlcA and UDP-GalA. To determine and elaborate upon the functions of the putative B. cereus UGlcDH and UGlcAE, we have cloned and expressed the genes as recombinant proteins in E. coli and examined their enzyme activities.
Biochemical characterization of Bc UDP-Glc 6-dehydrogenase and UDP-GlcA 4-epimerase
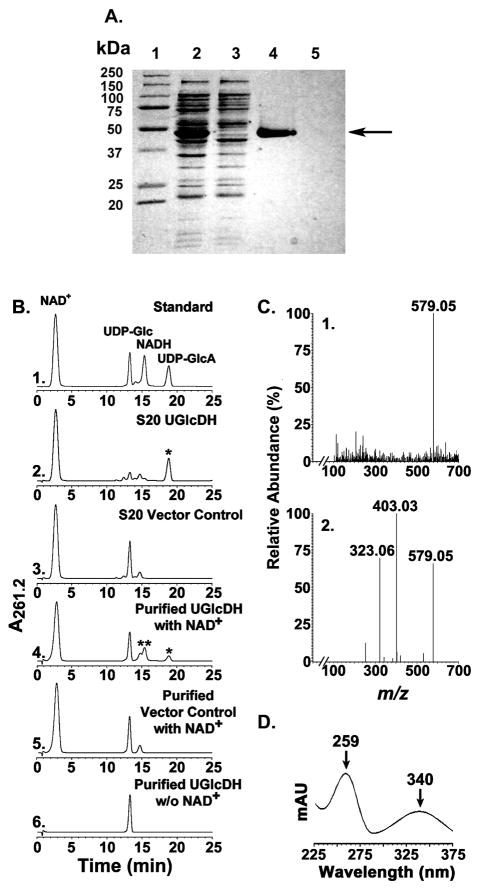
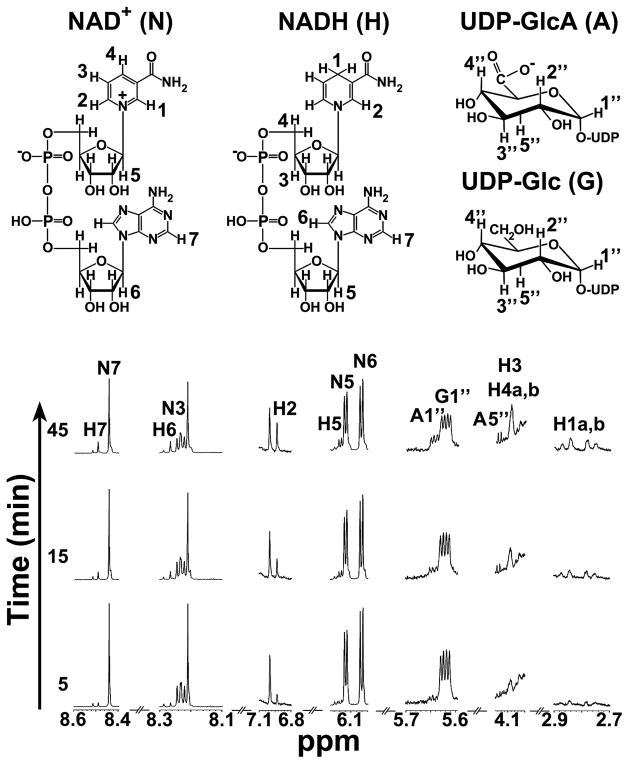
A distinct protein band migrating slightly lower than the 50 kDa standard, was detected by SDS-PAGE analysis of the extracts from E. coli cells expressing recombinant BcUGlcDH (Fig. 2A, lane 2) but was absent in E. coli cells expressing the control vector. The expressed UGlcDH protein (theoretical mass 49,792) was purified (Fig. 2A lane 4) and shown, using a HPLC-based assay, to convert UDP-Glc to a new UDP-sugar (Fig 2B, panel 2 and 4, see *) in the presence of NAD+ and to a product that migrated as NADH (Fig. 2B panel 4, see **) with a characteristic dual UV absorption maximum at 259 and 340 nm (Fig 2D). The newly formed UDP-sugar eluted from the Q15 ion-exchange column with a retention time (18.8 min), comparable to UDP-GlcA standard (Fig. 2B, panel 1). Analysis of the reaction product peak by ESI- mass spectrometer operated in the negative ion mode identified an ion product (Fig 2C, panel 1) of m/z 579.05, as expected for a UDP-hexuronic acid. Collision-induced ion fragmentation yielded two major ions at m/z 323.06 and 403.03 (Fig 2C panel 2), suggesting formation of UMP and UDP ions, as expected. The dehydrogenase enzymatic reaction was also followed with 1H-NMR (Fig. 3). As time advanced during the real-time NMR assay, a clear decrease of the peak corresponding to the anomeric proton (peak G1″, 5.62 ppm) of UDP-Glc was observed alongside a simultaneous increase of the peaks corresponding to the anomeric and H-5″ protons of UDP-GlcA (peaks A1″ and A5″, 5.64 and 4.16 ppm, respectively). Additionally, the signal from the protons added to the NAD+ nicotinamide ring after enzymatic reduction to NADH (peak H1a,b) appeared in the spectrum from 2.7 to 2.9 ppm as time advanced. Peaks from other diagnostic protons of NAD+ (N3, N5, N6, N7) were also diminished concomitantly with an increase of diagnostic peaks of NADH (H2, H3, H4a,b, H5, H6, H7). The ratio of the change in the diagnostic NADH proton signals compared with those of UDP-GlcA was found to be 2:1, validating that two moles of NAD+ are reduced per mole of UDP-GlcA made in the enzymatic reaction. Hence, we named Bcer98_2076 as UDP-Glc 6-dehydrogenase (abbr. UGlcDH, or dehydrogenase).
FIGURE 2. Expression and characterization of recombinant of BcUGlcDH.
A. SDS-PAGE of total soluble protein isolated from E. coli cells expressing UGlcDH, (lane 2), negative vector control (lane 3), and of column-purified UGlcDH or control (lane 4 and 5, respectively). An arrow points to the purified protein.
B. High performance anion-exchange chromatography of the products formed by UGlcDH. Purified recombinant UGlcDH was reacted with UDP-Glc for 30 min in the presence (Panel 4) or absence (Panel 6) of NAD+. The corresponding column-purified protein isolated from cells expressing the control vector was reacted with UDP-Glc and NAD+ for 30 min (Panel 5) as a control. The reaction products were separated on a Q15 anion-exchange column. The distinct UDP-sugar peak marked by an asterisk (Panel 2 and 4 with retention time of 18.8 min) was collected and characterized by mass spectrometry and 1H-NMR spectroscopy. The activity of total soluble protein (denoted S20) isolated from cells expressing recombinant BcUGlcDH (Panel 2) or vector control (Panel 3) is also shown.
C. Analysis of BcUGlcDH enzymatic product by mass spectrometry operated in the negative ion mode. The HPLC peak (Panel 2B or 4B, marked by *) was collected and directly infused to ESI-MS. The spectrum of the full MS molecular ions (Panel 1, m/z of 579.05 for deprotonated [M-H]) and of the derived CID-fragments (Panel 2, m/z of 323.06 and 403.05) are shown.
D. The peak in Panel 4B marked by ** has major UV absorbance peaks at 259 and 340 nm, the characteristic absorbance signature for NADH.
FIGURE 3. Analysis of BcUGlcDH enzymatic reaction in time-resolved 1H-NMR.
Selected regions of the 600 mHz 1H-NMR spectrum show the interconversion of UDP-α-D-Glc and NAD+ to UDP-α-D-GlcA and NADH at 5, 15, and 45 min after the reaction was initiated. Diagnostic peaks of NAD+ and NADH are labeled N and H, respectively, while UDP-Glc peaks are labeled G and UDP-GlcA peaks are labeled A. Peaks are arbitrarily numbered to denote corresponding protons numbered as indicated in the molecular structures.
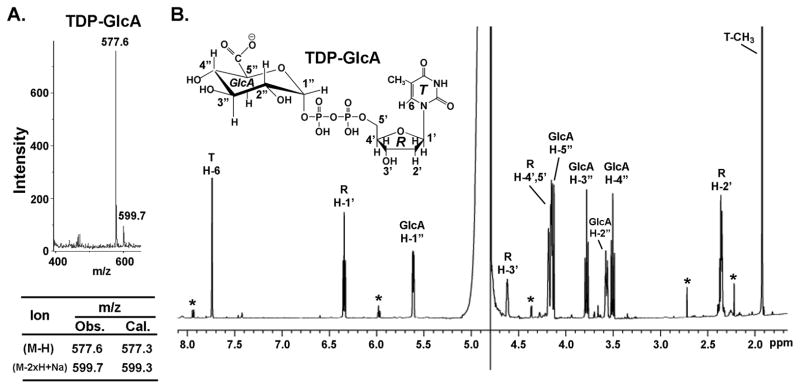
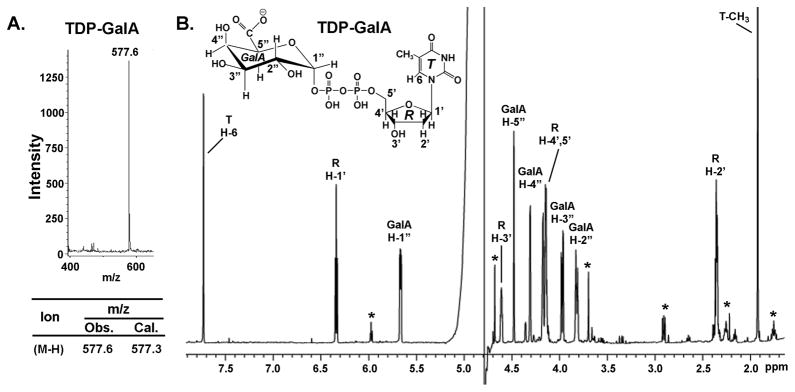
To determine substrate specificity, other nucleotide-glucose were tested. TDP-Glc was a substrate for the dehydrogenase as well, albeit with lower efficiency when compared with UDP-Glc (Table 1). The dehydrogenase, however, could not utilize ADP-Glc, GDP-Glc or GDP-Man as substrates. In addition, NADP+ could not substitute NAD+ as the cofactor. Following the reaction with TDP-Glc, the enzyme product was validated as TDP-GlcA as determined by MALDI-TOF MS in the negative mode with a major m/z ion at 577.6 and by 1H-NMR (Fig. 4, A and B). To determine the preferred in vivo substrate of dehydrogenase, E. coli harboring the expression plasmid were grown to mid-log phase, and 2 h after induction the cells were extracted and the amount of UDP-sugar produced by E. coli was determined by HPLC and by ESI-MS/MS. HPLC analyses reveal a major peak at 18.3 min (comparable to the UDP-GlcA standard peak) that is absent from NDP-sugars extracted from E. coli control (data not shown). Mass spectrometer analyses confirm the in vivo production of UDP-GlcA as the major ion as well. Interestingly, in vitro the dehydrogenase was unable to fully convert UDP-Glc to UDP-GlcA; this was later confirmed (see below) due to strong inhibition of the enzyme by its product NADH.
TABLE 1. Steady-state kinetics parameters of recombinant BcUGlcAE and BcUGlcDH.
Kinetics of UGlcAE were measured with varied concentrations of UDP-GlcA (0.1 – 1.0 mM) or TDP-GlcA (0.08 – 0.5 mM) and 1 mM NAD+ after 8 min at standard conditions. The reciprocal initial velocity was plotted against the reciprocal UDP-GlcA or TDP-GlcA concentration according to Lineweaver and Burk to calculate the corresponding Km values. The data presented are the average Km values from three experiments. Kinetics of BcUGlcDH were measured with 2 mM NAD+ and varied concentrations of UDP-Glc (0.1 – 1.0 mM) after 15 min or with varied concentrations of TDP-Glc (0.1–1.0 mM) after 25 min under standard conditions. Kinetics of BcUGlcDH were also measured with varied concentrations of NAD+ (0.1–2 mM) with 1 mM UDP-Glc after 15 min or 1 mM TDP-Glc after 25 min under standard conditions. The data presented are the average Km values from two experiments.
| Km (mM) | kcat (s−1) | kcat/Km (mM−1s−1) | |
|---|---|---|---|
| UDP-GlcAE activity | 0.12 ± 0.01 | 3.2 ± 0.1 | 26 ± 1.4 |
| TDP-GlcAE activity | 0.18 ± 0.01 | 2.1 ± 0.1 | 12 ± 0.4 |
| UDP-GlcDH Activity – (UDP-Glc) | 0.137 ± 0.019 | 0.974 ± 0.031 | 7.1 ± 1.0 |
| UDP-GlcDH Activity – (NAD+) | 0.042 ± 0.006 | 0.898 ± 0.014 | 21.4 ± 3.1 |
| TDP-GlcDH Activity* – (TDP-Glc) | 2.07 ± 0.39 | 0.646 ± 0.091 | 0.31 ± 0.073 |
| TDP-GlcDH Activity- (NAD+) | 0.664 ± 0.050 | 0.307 ± 0.012 | 0.46 ± 0.039 |
The kinetic value for TDP-Glc and NAD are an estimate and likely qualitative rather than quantitative, since the reaction with TDP-Glc could not be done at saturation. This in turn may affect the Kcat value for NAD. Nonetheless, the data provide sufficient information showing that the dehydrogenase is more efficient with UDP-Glc as the substrate.
FIGURE 4. BcUGlcDH can convert TDP-Glc to TDP-GlcA as determined by MALDI and NMR.
A. MALDI-MS analysis - Negative-ion MALDI mass spectra were recorded using a Microflex LT mass spectrometer (Bruker Daltonik, Bremen, Germany). 1 μl aqueous samples of 1 mM TDP-GlcA (produced from the dehydrogenase reaction with TDP-Glc/NAD+ and collected by HPLC) were mixed with an equalvolume of matrix solution (1 μg/μl 2,5-dihydroxybenzoic acid in 50% methanol) and driedon the plate. Spectra from 500 laser(N2, 337nm) shots were summed to generate a mass spectrum. The ion mass, calculated (abbr. Cal.) or observed (abbr. Obs.), are listed below the spectrum.
B. 1H-NMR spectroscopy at 600-MHz of HPLC collected peak after reaction of BcUGlcDH with TDP-Glc and NAD+. The assignments of each proton are indicated on the spectrum: peaks from protons on the thymidine (T) ring are indicated by H, the ribose (R) protons are indicated by H′, and the GlcA (G) protons protons are indicated by H″. The peaks marked by * are resonances from impurities.
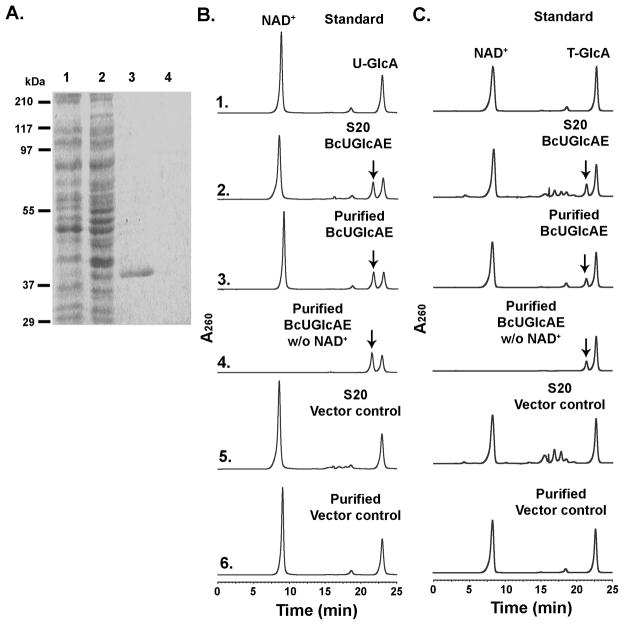
We next proceeded to characterize the function of the second gene in the operon, Bcer98_2077. Expression of this recombinant protein in E. coli yielded a highly expressed protein migrating alongside a 37 kDa marker as expected from the theoretical mass (36,734) (Fig. 5A, lane3). The enzyme was tested against various commercially available ADP- UDP- and GDP-sugars in the presence or absence of co-factors. The recombinant Bcer98_2077 converted a portion of UDP-GlcA to a new peak migrating at 22.5 min when separated on the HPLC column (Fig. 5B, panel 2, 3 marked by arrow). The peak was collected from the column and 1H-NMR confirmed the enzyme product as UDP-GalA as it had identical proton assignments as described [14]. Thus, the enzyme is a true 4-epimerase, as it is capable of interconverting UDP-GalA to UDP-GlcA (not shown) as well. Hence, we named Bcer98_2077 as UDP-GlcA 4-Epimerase, BcUGlcAE. Based on the sensitivity of our assay, this B. cereus 4-epimerase was unable to convert UDP-glucose or UDP-xylose. The characterization of a 4-epimerase in P. aeruginosa capable of epimerizing UDP-GlcNAc and UDP-GlcNacA [18] and the recent identification of a UDP-GlcNAc 6-dehydrogenase in B. cereus subsp. cytotoxis NVH 391–98 [19] motivated us to test the epimerase with UDP-GlcNAc and UDP-GlcNAcA as substrates, but no conversion was observed. However, the fact that the recombinant dehydrogenase (described above) was capable of utilizing TDP-Glc prompted us to test if TDP-GlcA could be utilized as a substrate. Indeed, the recombinant BcUGlcAE converted a portion of TDP-GlcA to a new product as observed after separation by HPLC (Fig. 5C, panel 2, 3 marked by arrow). To further validate the nature of this new TDP-sugar, the peak was collected and its mass was analyzed by MALDI-TOF MS and the structure by NMR. The major mass ion at m/z 577.6 suggested a TDP-hexuronic acid (Fig. 6A) and 1H-NMR (Fig. 6B) confirmed the epimerase product as TDP-GalA.
FIGURE 5. Expression and characterization of recombinant BcUGlcAE.
A. SDS-PAGE of total soluble protein isolated from E. coli cells expressing BcUGlcAE (lane 1), control vector (lane 2), and of column-purified BcUGlcAE or control (lane 3 and 4, respectively).
B. HPLC analysis of the products formed by BcUGlcAE reacting with UDP-GlcA. Total soluble proteins (denoted S20) or purified recombinant UGlcAE was reacted with UDP-GlcA for 30 min in the presence (Panel 2, 3), or absence (Panel 4) of NAD+. The crude or purified protein isolated from cells expressing control vector was incubated with UDP-GlcA for 60 min (Panel 5,6) as a control. The distinct UDP-sugar peak marked by the arrow (Panel 2, 3, and 4 with the same retention time) was collected and analyzed by 1H-NMR spectroscopy.
C. HPLC analysis of BcUGlcAE that was reacted with TDP-GlcA to yield a product (marked by an arrow in panels 2, 3, and 4) that was collected and analyzed by NMR and MALDI-TOF.
FIGURE 6. 1H-NMR spectroscopic and MALDI-TOF analyses of BcUGlcAE reaction products indicates formation of TDP-α-D-galacturonic acid.
A. The HPLC peaks (Fig. 5C, Panel 2, 3, or 4) corresponding to the product formed by BcUGlcAE was collected and analyzed by MALDI-TOF.
B. 1H-NMR spectroscopy at 600-MHz of HPLC peak from Fig. 5C panel 3. The assignments of each proton are indicated on the spectrum: peaks from proto ns on the thymidine (T) ring are indicated by H, the ribose (R) protons are indicated by H′, and the GalA (G) protons protons are indicated by H″. The peaks marked by * are resonances from impurities.
Enzymatic characterization of UGlcDH and UGlcAE
The B. cereus UDP-glucose 6-dehydrogenase (BcUGlcDH) had the highest activity at a temperature of 42 °C (Supplemental Table S1). Additionally, the dehydrogenase was found to have substantial activity at high pH with its optimal pH at 9.0 in sodium phosphate buffer (Table S1). Relatively little UDP-GlcA was produced in enzymatic assays conducted at pH of 4 or lower. Optimal epimerase activity was observed at 55 °C and, like the dehydrogenase, was also most active above pH 7. Epimerase assays conducted at pH 8.2 in sodium phosphate buffer yielded the highest levels of activity. Kinetic analyses of the enzymes are summarized in Table 1. The data from kinetic experiments of dehydrogenase with UDP-Glc as the variable substrate and NAD+ as the fixed substrate (or vice versa) fit well to the Michaelis-Menten model. The apparent Km value for the dehydrogenase utilizing UDP-Glc was 137.2 μM for UDP-Glc and 42.3 μM for NAD+, with Vmax values of 27.1 pmol s−1. The kcat/Km (mM−1 s−1) values for the dehydrogenase were found to be 7.1 (UDP-Glc) and 21.4 (NAD+). When TDP-Glc was used as the substrate, the apparent Km values for the dehydrogenase were 2067 μM (TDP-Glc) and 644 μM (NAD+). The Vmax was found to be 19.5 pmol s−1 when TDP-Glc was the variable substrate and 9.26 pmol s−1 when variable concentrations of NAD+ were used, and the kcat/Km (mM−1 s−1) values were 0.31 for TDP-Glc and 0.46 for NAD+. The epimerase had apparent Km values of 120 μM (UDP-GlcA) and 180 μM when TDP-GlcA was used as the substrate. The kcat/Km (mM−1 s−1) values were 26 (UDP-GlcA) and 12 (TDP-GlcA). Inhibition studies (Supplementary Tables S2 and S3) have shown that the Bacillus dehydrogenase is strongly inhibited by its product NADH, while on the other hand NADH had only a marginal inhibitory effect on the epimerase activity. However, the epimerase was highly inhibited by UDP and to a lesser extent by UDP-Xyl.
Size-exclusion chromatography analysis of the epimerase gave an estimated mass 46.5 kDa, suggesting that the BcUGlcAE is active in a monomeric form (Table S1). Active dehydrogenase eluted from the size-exclusion column (Table S1) consistently with two distinctive peaks of activity – the first (with approximately 20% of total activity) corresponded to a molecular mass of 152.1 kDa and the second corresponded to 80.8 kDa. Considering the theoretical mass of the dehydrogenase (49 kDa) and the 2:1 ratio of the estimated masses for the two active forms of the enzyme, size-exclusion chromatography suggested that the enzyme was most likely active as a monomer. Performing the size-exclusion chromatography of the dehydrogenase in the presence of UDP, UDP-Glc and NAD+, or only with NADH, yielded the same elution pattern (data not shown). The elution behavior of the dehydrogenase from the column is not sufficient to draw conclusion for its size. It could be function as a monomer or as a dimer and current efforts to crystalize the dehydrogenase is underway to determine its form(s) in solution.
DISCUSSION
In this study, we have demonstrated that the food borne pathogenic bacterium B. cereus subsp. cytotoxis NVH 391–98 contains an operon that functions to generate acidic sugar-precursors (UDP-GlcA, UDP-GalA, and possibly their TDP-analogs). The operon organization (Bcer98_2076, Bcer98_2077, Bcer98_2079) is conserved in few terrestrial Bacillus spp. including Bacillus weihenstephanensis KBAB4, Bacillus thuringiensis IBL 200, Geobacillus sp. G11MC16, and Bacillus subtilis subsp. subtilis str. 168. Similar genes are also found in sea-dwelling bacteria such as Bacillus halodurans C-125 and others, although the operon organization slightly varies.
The UGalA operon also contains a putative glycosyltransferase, which may utilize the UDP-GlcA and/or UDP-GalA as substrates for the biosynthesis of acidic sugar-containing glycans, for example, the acidic glycan secreted by B. cereus living in biofilm. Glycans containing GlcA and GalA have been also noted in the cell walls of alkaliphilic Bacillus species B. halodurans C-125 and Oceanobacillus iheyensis. The amount of polymers containing these acidic sugars increases when such Bacillus spp. are grown in an alkaline environment. It has been demonstrated that alkaline conditions (up to pH = 9) do not substantially inhibit the growth of B. cereus [20, 21], and certain Bacillus spp., like the deep sea dwelling O. iheyensis, can grow at pH 10 [22]. It has been hypothesized that the presence of these acidic sugars in the outer layers of the cell wall serve to permit growth in an alkaline pH [23, 24]. The enzymatic activity of the dehydrogenase (see Fig. 1A) could also help to promote survival in high pH environments, as three mol protons are released per mol of UDP-GlcA formed: two protons are released to form NADH, and at pH of the cytosolic environment above 3.8 the carboxylic moiety of UDP-GlcA is deprotonated. Oxidation of the produced NADH could further generate free protons for the cell. An additional mechanism that may facilitate growth at high pH is the fact that a number of Bacillus spp. produce ‘pectinases’ and ‘lyases’ in alkaline conditions. Such hydrolytic enzymes are capable of hydrolyzing polygalacturonan [25] and perhaps the cell’s own uronic-acid glycan. Such enzymes from B. cereus, B. subtilis, and Bacillus sp. BP-23, for instance, have been found to be optimally active at pH 8.5, 9.5, and 10, respectively [26, 27]. Genes for the transport and catabolism of GlcA and GalA have been identified in Bacillus spp., and the release of these free acidic sugars may help to permit Bacillus spp. growth in alkaline environments [28]. At high pH, Bacillus spp. can hydrolyze polyanionic oligosaccharide structures (i.e., GlcA- and GalA-containing glycans) and the free acidic sugars can be transported to the cell where GlcA and GalA metabolites can serve as a source of protons to acidify the cytoplasm despite the high outside pH.
The balance between the oxidized and reduced forms of nicotinamide adenine dinucleotide is believed to reflect the metabolic activities of the cell, and the fact that the recombinant B. cereus dehydrogenase is strongly inhibited in the presence of NADH may point to the role of UGalA operon in the B. cereus lifecycle. It is possible that the dehydrogenase serves as a checkpoint in the regulation of downstream metabolic pathways utilizing UDP-GlcA or UDP-GalA, as high NADH levels will likely strongly inhibit UDP-GlcA formation and therefore also hinder UDP-GalA production in vivo. This suggests that B. cereus, in a state of abundant intracellular energy (i.e., a high NADH/NAD+ ratio), may produce less GlcA and/or GalA-containing glycans. On the other hand, one function of the dehydrogenase could be to support the formation of NADH for various metabolic pathways, especially under conditions when the bacterium is deprived of essential nutrients and required to form a protective spore. This may reflect a possible role of the operon in the biosynthesis of GlcA and GalA-containing glycans in a nutrient-starved cell, for example during sporulation. The intracellular concentration of NADH may act to modulate UDP-GlcA and UDP-GalA production in vegetative cells. In fact, NADH inhibition may be a conserved feature of some UDP-glucose 6-dehydrogenases and has been reported in spore and non-spore forming bacteria alike. For instance, NADH has been shown to inhibit a UDP-glucose 6-dehydrogenase (TuaD BSU35580) of B. subtilis subsp. 168 that is distinct from the BcUGlcDH homolog as well as UDP-glucose 6-dehydrogenases from Pseudomonas aeruginosa PAO1 [29] and Cryptococcus neoformans [30].
The enzyme specificity and the nature of their evolution are fundamental questions regarding bacterial UGlcAE. For instance, the K. pneumoniae UGlcAE can 4-epimerize UDP-GlcA, UDP-Glc and UDP-GlcNAc, while the Bacillus enzyme has a strict specificity for the uronic acid moiety. The Cap1J UGlcAE from S. pneumoniae has also been shown to have specificity for the uronic acid moiety, and is capable of 4-epimerizing UDP-GlcA and UDP-GalA while being unable to interconvert UDP-Glc and UDP-Gal; unfortunately, whether Cap1J can utilize UDP-GlcNAc, UDP-GlcNAcA or other nucleotide sugars has not been tested [16]. We have tried to address the promiscuity of some 4-epimerases by analyzing the origin of UGlcAE proteins (from the known UGlcAE and homologs that share high amino acid sequence identity). Phylogenic analysis of eukaryotic and bacterial UGlcAE clearly separates the Bacillus spp. UGlcAE into a separate and distinct clade (Fig. S2A). While plant and promiscuous bacterial UGlcAE are related (for example, AtUGlcAE2, Cap1J and Glakp), the Bacillus spp. appear to belong to a different group that is well separated from other bacterial and eukaryotic UGlcAE. The phylogeny suggests that UGlcAE enzymes might share the same ancestor since those enzymes are well divided from UGEs and decarboxylases. However, currently we cannot predict the evolution of Bacillus UGlcAE.
The ability of the Bacillus dehydrogenase and epimerase to utilize TDP-Glc and TDP-GlcA, respectively, may point to a separate or additional role of the operon in the B. cereus life cycle. TDP-Glc is a product formed in B. anthracis for synthesis of TDP-rhamnose [33], and homologous proteins for TDP-Rha production are also found in the B. cereus genome [34]. In B. anthracis, Rha residues are incorporated into the spore glycoprotein bclA, and Rha residues has been shown to be major components of the glycans in the exosporium of B. cereus [35] and Rha is also a minor constituent of the biofilm EPS of B. cereus [7]. However, the capability of the enzymes to interconvert TDP-Glc and TDP-GlcA may be the result of the structural similarity between the thymine and uracil moieties of the TDP- and UDP-sugars.
At present, the function of glucuronic and galacturonic acid at different points of the B. cereus lifecycle remains to be determined, and the identification of the UGalA operon will help to allow the elucidation of their importance. Our laboratory is unfortunately not licensed to work with this human pathogen, as necessary for genetic deletion of the operon, however, gene knockouts in non-pathogenic Bacillus spp. also possessing the UGalA operon (Fig. 1B) could be used to reveal its role.. Current efforts are underway to examine the function of these genes and perhaps their utilization in adapting to high pH in different Bacillus spp. Determination of the expression of the genes of the UGalA operon in response to various environment stimuli (e.g., alkaline pH, nutrient limitation, or temperature extremes) may also be advantageous in developing an understanding of the operon’s biological role.
If the GalA-containing glycans are shown to be necessary for survival of the organism, the Bacillus UGlcAE may prove to be a potential target for drugs combating this pathogen. As far as we are aware, humans do not synthesize GalA-containing glycans and are not thought to possess UDP-GlcA 4-epimerases. Indeed, the specificity of the Bacillus enzyme may permit the development of inhibitors restricted to BcUGlcAE that do not affect similar enzymes necessary for normal human metabolism.
MATERIALS AND METHODS
Cloning of BcUGlcDH and BcUGlcAE
The Bcer98_2076 and Bcer98_2077 genes from Bacillus cereus subsp. cytotoxis NVH 391–98, herein named, UDP-Glc 6-dehydrogenase (BcUGlcDH) and UDP-glucuronic acid 4-epimerase (BcUGlcAE), respectively, were cloned by high-fidelity PCR from genomic DNA. P C R p rimers were: sense 5′-CCatggtgaatatatgcattataggatctg and antisense 5′-AAGCTTtgagcgaccaactcctacatagc for BcUGlcDH; and sense 5′-TCatgaaaatacttgttactggagcag and antisense 5′-AAGCTTatataattgcttcatatactc for BcUGlcAE. Genomic DNA was kindly provided by the A. Sorokin laboratory. The agarose gel-purified PCR fragments were cloned to yield the plasmids: pCR4-gmdh#2, and pCR4-gmad#1, respectively. The corresponding DNA sequences were deposited in Genbank (with respective accession numbers HM581980 and HM581979). The Nco1-HindIII fragment of BcUGlcDH (1289 bp) and BspH1-HindIII fragment of BcUGlcAE (944 bp) were cloned into an E. coli expression vector to form pET28b:BcUGlcDH#1 and pET28b:BcUGlcAE#1, respectively. The recombinant enzymes were designed to have a six-histidine extension at their C-terminus to facilitate affinity-purification.
Expression and purification of recombinant proteins
E. coli cells harboring pET28b:BcUGlcAE#1 or a control vector (pET28) were cultured for 16 h at 37 °C (or 30 °C for pET28b:BcUGlcDH#1 or a control vector) in 20 ml LB medium supplemented with kanamycin (50 μg/ml) and chloramphenicol (34 μg/ml). A portion (5–7 ml) of the cultured cells was transferred into fresh LB liquid medium (250 ml) supplemented with the same antibiotics, and the cells were grown at 37 °C (BcUGlcAE) or 30 °C (BcUGlcDH) at 250 rpm until cell density reached OD600 = 0.8 (BcUGlcAE) or OD600=0.6 (BcUGlcDH). Isopropyl β-D-thiogalactoside (0.5 mM) was added to induce gene expression. The cells harboring pET28b:BcUGlcDH#1 were transferred to an 18 °C incubator and grown for 20–24h while shaking at 250 rpm; cell harboring pET28b:BcUGlcAE#1 were incubated at 30 °C and grown for 4h at 250 rpm. After induction the cells were harvested by centrifugation (6,000 × g for 10 min at 4 °C) and suspended in 20 ml lysis buffer (For the dehydrogenase the buffer was 50 mM Tris-HCl, pH 8, 20% glycerol (v/v), 1 mM EDTA, 100 mM NaCl, 25 mM KCl, 25 mM (NH4)2SO4 supplemented with fresh 5 mM DTT and 0.5 mM phenylmethylsulfonyl fluoride; For BcUGlcAE the lysis buffer was 50 mM sodium-phosphate, pH 7.6, containing 10% (v/v) glycerol, 1 mM EDTA, and fresh 1 mM DTT and 0.5 mM phenylmethylsulfonyl fluoride). Cells were lysed by sonication [36], and centrifuged (6,000 × g for 10 min at 4 °C). The supernatant was supplemented with fresh DTT (1 mM for BcUGlcAE, 10 mM for BcUGlcDH), and centrifuged again (30 min at 20,000 × g). The resulting supernatant (termed S20) was kept at −20°C. His-tagged proteins were purified on a Ni-Sepharose fast-flow column (GE Healthcare Life Sciences, NJ, 2 ml resin packed in 1 cm id × 15 cm polypropylene column). The column was pre-equilibrated with loading buffer (For BcUGlcAE 50 mM sodium-phosphate (pH 7.6), 0.1 M NaCl; For BcUGlcDH 50 mM sodium-phosphate (pH 8), 0.3 M NaCl). The bound BcUGlcAE-6His was eluted with the same buffer containing an increased amount of imidazole (0–250 mM). Likewise, the bound BcUGlcDH-6His was eluted from Ni-column with solution adjusted to pH 8, which was composed of 0.3 M NaCl, 50 mM sodium phosphate, and imidazole (0- to 250 mM). The fraction containing BcUGlcAE activity, typically eluted with 250 mM imidazole, was supplemented with 1 mM DTT and 10% glycerol, and was dialyzed (6,000–8,000 molecular weight cut-off, Spectrum Laboratories, Inc., CA) 3 times (twice for 30 min, and once for ~1h), each time against 800 ml of cold dialysis buffer (50 mM sodium-phosphate pH 7.6 containing 0.1 M NaCl, 10% (v/v) glycerol, 1 mM DTT). The protein after dialysis was divided into small aliquots, flash frozen in liquid nitrogen and stored at −80°C. The Ni-column fraction containing UDP-Glc 6-dehydrogenase activity, typically eluted with 250 mM imidazole, was supplemented with 20% glycerol, divided into aliquots, flash frozen and kept at −80°C. Proteins extracted from E. coli cells expressing the control vector were obtained using the same purification protocol and were used as controls in enzyme assays and SDS-PAGE analyses. Proteins concentration was determined using the Bradford reagent with bovine serum albumin as standard. The native molecular weight of the dehydrogenase and the epimerase was estimated by size-exclusion chromatography using a Superdex-75 column as described [36].
UGlcDH and UGlcAE enzyme assays, HPLC and product analyses by NMR
Standard UGlcDH reactions (50 μl final volume) contained 100 mM sodium phosphate (pH 8), 20 mM KCl, 1 mM NAD+, 0.5 mM UDP-Glc and 1.1 μg purified recombinant BcUGlcDH. UGlcAE reactions (50 μl final volume) contained 50 mM sodium phosphate (pH 7.6), 1 mM NAD+, 1 mM UDP-GlcA and 0.2 μg purified recombinant BcUGlcAE. UGlcAE or UGlcDH reactions were kept for up to 15 or 30 min, respectively, at 37 °C and then terminated (100 °C water bath for 45 sec). Reaction products were extracted with chloroform and chromatographed [36] on a Q15 anion-exchange column (1 mm × 250 mm, Amersham) using an Agilent 1200 Series HPLC system equipped with an autosampler, diode-array detector and ChemStation software. Nucleotides were detected by their UV absorbance and the maximum absorbance for UDP-sugars, NAD+ and NADH were 261, 259 and 259/340 nm, respectively. The amount of product formed was determined using calibration curves of standard UDP-GlcA. The product formed by the specific reaction (eluted from Q-column) was collected, lyophilized, dissolved in 99.9% D2O, and analyzed by 1H-NMR spectroscopy [36]. TDP-Glc, UDP-Glc, NADH, NAD+ were obtained from Sigma.
Characterization of the recombinant enzymes
UGlcDH and UGlcAE activities were determined using different buffers, at different temperatures, and in the presence of selected cations or potential inhibitors. For optimal pH studies, solutions of recombinant BcUGlcDH or BcUGlcAE were first mixed in 100 mM of each individual buffer. Subsequently, NAD+ (1 mM) and UDP-Glc (0.5 mM) were added to BcUGlcDH reactions while 1 mM NAD+ and UDP-GlcA were added to BcUGlcAE reactions. Assays were then carried out for 15 min (UGlcAE) or 30 min (UGlcDH) at 37 °C. Assays to determine the optimal temperature of recombinant BcUGlcDH or BcUGlcAE were similarly run under standard UGlcDH or UGlcAE reaction conditions and incubated at variable temperatures for 15 min (UGlcAE) or 30 min (UGlcDH) before termination. UGlcDH or UGlcAE inhibition assays were performed by first mixing the enzyme with 100 mM phosphate buffer (pH 8) or 50 mM phosphate buffer (pH 7.6), respectively, with various additives (e.g., nucleotides) on ice for 10 min. UDP-Glc and NAD+ (0.5 and 1 mM, respectively) were then added to UGlcDH assays while 1mM UDP-GlcA and NAD+ were added to UGlcAE reactions. After 15 min (UGlcAE) or 30 min (UGlcDH) at 37 °C, the reactions were terminated and the amount of UDP-sugar formed was determined by Q-HPLC.
The steady-state kinetic experiments of BcUGlcAE were determined at 37 °C for 8 min in 50 mM sodium-phosphate pH 7.6, 1 mM NAD+, with variable concentrations (0.1 to 1 mM) of UDP-GlcA (for UGlcAE activity), or with variable concentrations (0.08 to 0.5 mM) of TDP-GlcA (for TGlcAE activity), and 0.1 μg purified protein. Steady-state kinetic of BcUGlcDH was similarly determined at 37 °C for 15 min in 0.1 M sodium phosphate buffer (pH 8), 20 mM KCl, and 1.5 μg protein. In calculating the Km for UDP-Glc or TDP-Glc, UGlcDH assays were carried out with fixed amount of NAD+ (2 mM) and variable concentrations of UDP-Glc or TDP-Glc (0.1 to 1 mM); for the Km value for NAD+, BcUGlcDH was incubated with variable concentration of NAD+ (0.1 to 2 mM) and a fixed amount of UDP-Glc or TDP-Glc (1mM). The Prism program (Deltagraph version 5) was used to generate a best-fit curve calculated by nonlinear regression analyses. The reciprocal initial velocity was plotted against the reciprocal nucleotide-sugar concentration according to Lineweaver and Burk to calculate Km values.
Acknowledgments
We thank Sung Lee and James A. Smith for technical help. This research was supported in part by NSF: IOB-0453664 (MB-P) and by the BioEnergy Science Center (Grant DE-PS02-06ER64304) that is supported by the Office of Biological and Environmental Research in the DOE Of ce of Science. This research also benefited from activities at the Southeast Collaboratory for High-Field Biomolecular NMR, a research resource at the University of Georgia, funded by the National Institute of General Medical Sciences (NIGMS grant number GM66340) and the Georgia Research Alliance.
Abbreviations
- DTT
Dithiothreitol
- Gal
Galactose
- GalA
Galacturonic acid
- GlcA
Glucuronic acid
- GlcNAc
N-acetylglucosamine
- GlcNAcA
N-acetylglucosaminuronic acid
- kDa
Kilodalton
- Man
Mannose
- MHz
Megahertz
- m/z
Mass-to-charge ratio
- ppm
Parts per million
- Rha
Rhamnose
- UGlcDH
UDP-glucose 6-dehydrogenase
- UGlcAE
UDP-glucuronic acid 4-epimerase
- Xyl
Xylose
Footnotes
Database: Nucleotide sequence data are available in the Genbank database with accession numbers HM581979 and HM581980.
SUPPORTING INFORMATION
Table S1 – Temperature and pH optima of BcUGlcAE and BcUGlcDH and their masses estimated from size-exclusion chromatography, Table S2 - The effects of NAD+, NADH, NADP+ and NADPH on BcUGlcDH Activity, Table S3 - The effects of nucleotides and nucleotide sugars on BcUGlcAE activity, Figure S1 - Sequence comparison and Phylogenetic analysis of Bacillus UGlcDH, Figure S2 - Sequence comparison Phylogenetic analysis of Bacillus UGlcAE.
References
- 1.Leoff C, Saile E, Rauvolfova J, Quinn CP, Hoffmaster AR, Zhong W, Mehta AS, Boons GJ, Carlson RW, Kannenberg EL. Secondary cell wall polysaccharides of Bacillus anthracis are antigens that contain specific epitopes which cross-react with three pathogenic Bacillus cereus strains that caused severe disease, and other epitopes common to all the Bacillus cereus strains tested. Glycobiology. 2009;19:665–73. doi: 10.1093/glycob/cwp036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muszynski A, Laus M, Kijne JW, Carlson RW. Structures of the lipopolysaccharides from Rhizobium leguminosarum RBL5523 and its UDP-glucose dehydrogenase mutant (exo5) Glycobiology. 2011;21:55–68. doi: 10.1093/glycob/cwq131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quelas JI, Mongiardini EJ, Casabuono A, Lopez-Garcia SL, Althabegoiti MJ, Covelli JM, Perez-Gimenez J, Couto A, Lodeiro AR. Lack of galactose or galacturonic acid in Bradyrhizobium japonicum USDA 110 exopolysaccharide leads to different symbiotic responses in soybean. Molecular plant-microbe interactions: MPMI. 2010;23:1592–604. doi: 10.1094/MPMI-05-10-0122. [DOI] [PubMed] [Google Scholar]
- 5.Frirdich E, Whitfield C. Characterization of Gla(KP), a UDP-galacturonic acid C4-epimerase from Klebsiella pneumoniae with extended substrate specificity. J Bacteriol. 2005;187:4104–15. doi: 10.1128/JB.187.12.4104-4115.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arrecubieta C, Lopez R, Garcia E. Type 3-specific synthase of Streptococcus pneumoniae (Cap3B) directs type 3 polysaccharide biosynthesis in Escherichia coli and in pneumococcal strains of different serotypes. The Journal of experimental medicine. 1996;184:449–55. doi: 10.1084/jem.184.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratto M, Suihko ML, Siika-aho M. Polysaccharide-producing bacteria isolated from paper machine slime deposits. J Ind Microbiol Biotechnol. 2005;32:109–14. doi: 10.1007/s10295-005-0210-9. [DOI] [PubMed] [Google Scholar]
- 8.Slock JA, Stahly DP. Polysaccharide that may serve as a carbon and energy storage compound for sporulation in Bacillus cereus. Journal of bacteriology. 1974;120:399–406. doi: 10.1128/jb.120.1.399-406.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh SY, Budzik JM, Garufi G, Schneewind O. Two capsular polysaccharides enable Bacillus cereus G9241 to cause anthrax-like disease. Mol Microbiol. 2011;80:455–470. doi: 10.1111/j.1365-2958.2011.07582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forsberg LS, Choudhury B, Leoff C, Marston CK, Hoffmaster AR, Saile E, Quinn CP, Kannenberg EL, Carlson RW. Secondary cell wall polysaccharides from Bacillus cereus strains G9241, 03BB87, and 03BB102 causing fatal pneumonia share similar glycosyl structures with the polysaccharides from Bacillus anthracis. Glycobiology. 2011;21:934–948. doi: 10.1093/glycob/cwr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bar-Peled M, O’Neill MA. Plant nucleotide sugar formation, interconversion, and salvage by sugar recycling*. Annual review of plant biology. 2011;62:127–55. doi: 10.1146/annurev-arplant-042110-103918. [DOI] [PubMed] [Google Scholar]
- 12.Molhoj M, Verma R, Reiter WD. The biosynthesis of D-Galacturonate in plants. functional cloning and characterization of a membrane-anchored UDP-D-Glucuronate 4-epimerase from Arabidopsis. Plant Physiol. 2004;135:1221–30. doi: 10.1104/pp.104.043745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu X, Bar-Peled M. The biosynthesis of UDP-galacturonic acid in plants. Functional cloning and characterization of Arabidopsis UDP-D-glucuronic acid 4-epimerase. Plant Physiol. 2004;136:4256–64. doi: 10.1104/pp.104.052365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu X, Wages CJ, Davis KE, Guyett PJ, Bar-Peled M. Enzymatic characterization and comparison of various poaceae UDP-GlcA 4-epimerase isoforms. J Biochem. 2009;146:527–34. doi: 10.1093/jb/mvp099. [DOI] [PubMed] [Google Scholar]
- 15.Smith EE, Mills GT, Bernheimer HP, Austrian R. The presence of an uronic acid epimerase in a strain of pneumococcus type I. Biochim Biophys Acta. 1958;29:640–1. doi: 10.1016/0006-3002(58)90023-4. [DOI] [PubMed] [Google Scholar]
- 16.Munoz R, Lopez R, de Frutos M, Garcia E. First molecular characterization of a uridine diphosphate galacturonate 4-epimerase: an enzyme required for capsular biosynthesis in Streptococcus pneumoniae type 1. Mol Microbiol. 1999;31:703–13. doi: 10.1046/j.1365-2958.1999.01211.x. [DOI] [PubMed] [Google Scholar]
- 17.Kimmel JL, Tipton PA. Inactivation of GDP-mannose dehydrogenase from Pseudomonas aeruginosa by penicillic acid identifies a critical active site loop. Archives of biochemistry and biophysics. 2005;441:132–40. doi: 10.1016/j.abb.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 18.Miller WL, Matewish MJ, McNally DJ, Ishiyama N, Anderson EM, Brewer D, Brisson JR, Berghuis AM, Lam JS. Flagellin glycosylation in Pseudomonas aeruginosa PAK requires the O-antigen biosynthesis enzyme WbpO. J Biol Chem. 2008;283:3507–18. doi: 10.1074/jbc.M708894200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu X, Glushka J, Lee SG, Bar-Peled M. Biosynthesis of a new UDP-sugar, UDP-2-acetamido-2-deoxyxylose, in the human pathogen Bacillus cereus subspecies cytotoxis NVH 391–98. J Biol Chem. 2010;285:24825–33. doi: 10.1074/jbc.M110.125872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raevuori M, Genigeorgis C. Effect of pH and sodium chloride on growth of Bacillus cereus in laboratory media and certain foods. Applied microbiology. 1975;29:68–73. doi: 10.1128/am.29.1.68-73.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valero M, Fernandez PS, Salmeron MC. Influence of pH and temperature on growth of Bacillus cereus in vegetable substrates. International journal of food microbiology. 2003;82:71–9. doi: 10.1016/s0168-1605(02)00265-9. [DOI] [PubMed] [Google Scholar]
- 22.Lu J, Nogi Y, Takami H. Oceanobacillus iheyensis gen. nov., sp. nov., a deep-sea extremely halotolerant and alkaliphilic species isolated from a depth of 1050 m on the Iheya Ridge. FEMS microbiology letters. 2001;205:291–7. doi: 10.1111/j.1574-6968.2001.tb10963.x. [DOI] [PubMed] [Google Scholar]
- 23.Aono R, Uramoto M. Presence of fucosamine in teichuronic acid of the alkalophilic Bacillus strain C-125. The Biochemical journal. 1986;233:291–4. doi: 10.1042/bj2330291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takami H, Takaki Y, Uchiyama I. Genome sequence of Oceanobacillus iheyensis isolated from the Iheya Ridge and its unexpected adaptive capabilities to extreme environments. Nucleic acids research. 2002;30:3927–35. doi: 10.1093/nar/gkf526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Namasivayam E, Ravindar J, Mariappan K. Production of Extracellular Pectinase by Bacillus Cereus Isolated From Market Solid Waste. J Bioanal Biomed. 2011;3:070–075. [Google Scholar]
- 26.Soriano M, Blanco A, Diaz P, Pastor FI. An unusual pectate lyase from a Bacillus sp. with high activity on pectin: cloning and characterization. Microbiology. 2000:146–89. doi: 10.1099/00221287-146-1-89. [DOI] [PubMed] [Google Scholar]
- 27.Ahlawat S, Mandhan R, Dhiman SS, Kumar R, Sharma J. Potential application of alkaline pectinase from Bacillus subtilis SS in pulp and paper industry. Applied biochemistry and biotechnology. 2008;149:287–293. doi: 10.1007/s12010-007-8096-9. [DOI] [PubMed] [Google Scholar]
- 28.Pujic P, Dervyn R, Sorokin A, Ehrlich SD. The kdgRKAT operon of Bacillus subtilis: detection of the transcript and regulation by the kdgR and ccpA genes. Microbiology. 1998;144(Pt 11):3111–8. doi: 10.1099/00221287-144-11-3111. [DOI] [PubMed] [Google Scholar]
- 29.Hung RJ, Chien HS, Lin RZ, Lin CT, Vatsyayan J, Peng HL, Chang HY. Comparative analysis of two UDP-glucose dehydrogenases in Pseudomonas aeruginosa PAO1. J Biol Chem. 2007;282:17738–48. doi: 10.1074/jbc.M701824200. [DOI] [PubMed] [Google Scholar]
- 30.Bar-Peled M, Griffith CL, Ory JJ, Doering TL. Biosynthesis of UDP-GlcA, a key metabolite for capsular polysaccharide synthesis in the pathogenic fungus Cryptococcus neoformans. The Biochemical journal. 2004;381:131–6. doi: 10.1042/BJ20031075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell RE, Mosimann SC, van De Rijn I, Tanner ME, Strynadka NC. The first structure of UDP-glucose dehydrogenase reveals the catalytic residues necessary for the two-fold oxidation. Biochemistry. 2000;39:7012–23. [PubMed] [Google Scholar]
- 32.Egger S, Chaikuad A, Kavanagh KL, Oppermann U, Nidetzky B. UDP-glucose dehydrogenase: structure and function of a potential drug target. Biochemical Society transactions. 2010;38:1378–85. doi: 10.1042/BST0381378. [DOI] [PubMed] [Google Scholar]
- 33.Dong S, McPherson SA, Wang Y, Li M, Wang P, Turnbough CL, Jr, Pritchard DG. Characterization of the enzymes encoded by the anthrose biosynthetic operon of Bacillus anthracis. Journal of bacteriology. 2010;192:5053–62. doi: 10.1128/JB.00568-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graninger M, Kneidinger B, Bruno K, Scheberl A, Messner P. Homologs of the Rml enzymes from Salmonella enterica are responsible for dTDP-beta-L-rhamnose biosynthesis in the gram-positive thermophile Aneurinibacillus thermoaerophilus DSM 10155. Applied and environmental microbiology. 2002;68:3708–15. doi: 10.1128/AEM.68.8.3708-3715.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox A, Black GE, Fox K, Rostovtseva S. Determination of carbohydrate profiles of Bacillus anthracis and Bacillus cereus including identification of O-methyl methylpentoses by using gas chromatography-mass spectrometry. Journal of clinical microbiology. 1993;31:887–94. doi: 10.1128/jcm.31.4.887-894.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu X, Glushka J, Yin Y, Xu Y, Denny T, Smith J, Jiang Y, Bar-Peled M. Identification of a bifunctional UDP-4-keto-pentose/UDP-xylose synthase in the plant pathogenic bacterium Ralstonia solanacearum strain GMI1000, a distinct member of the 4,6-dehydratase and decarboxylase family. J Biol Chem. 2010;285:9030–40. doi: 10.1074/jbc.M109.066803. [DOI] [PMC free article] [PubMed] [Google Scholar]