Summary
Shiga toxin-producing bacteria cause widespread outbreaks of bloody diarrhea that may progress to life-threatening systemic complications. Shiga toxins (Stxs), the main virulence factors expressed by the pathogens, are ribosome-inactivating proteins which inhibit protein synthesis by removing an adenine residue from 28S rRNA. Recently, Stxs were shown to activate multiple stress-associated signaling pathways in mammalian cells. The ribotoxic stress response is activated following the depurination reaction localized to the α-sarcin/ricin loop of eukaryotic ribosomes. The unfolded protein response (UPR) may be initiated by toxin unfolding within the endoplasmic reticulum, and maintained by production of truncated, misfolded proteins following intoxication. Activation of the ribotoxic stress response leads to signaling through MAPK cascades, which appears to be critical for activation of innate immunity and regulation of apoptosis. Precise mechanisms linking ribosomal damage with MAPK activation require clarification but may involve recognition of ribosomal conformational changes and binding of protein kinases to ribosomes, which activate MAP3Ks and MAP2Ks. Stxs appear capable of activating all ER membrane localized UPR sensors. Prolonged signaling through the UPR induces apoptosis in some cell types. The characterization of stress responses activated by Stxs may identify targets for the development of interventional therapies to block cell damage and disease progression.
Introduction: Shiga toxins
Shiga toxins (Stxs) are genetically and structurally related cytotoxins expressed by the enteric pathogens Shigella dysenteriae serotype 1 and an expanding number of Shiga toxin-producing Escherichia coli (STEC) serotypes (Gyles, 2007). Ingestion of small numbers of Stx-producing bacteria in contaminated food or water may lead to bloody diarrhea (bacillary dysentery or hemorrhagic colitis). Unfortunately, these patients are at risk for developing life-threatening extra-intestinal complications including acute renal failure and neurological abnormalities such as seizures and paralysis (Tarr et al., 2005; Proulx and Tesh, 2007; Obata, 2010). Shiga toxin, the prototypical member of the toxin family, is expressed by S. dysenteriae serotype 1. Stxs expressed by STEC may be categorized as Shiga toxin type 1 (Stx1), which is essentially identical to Shiga toxin, and Shiga toxin type 2 (Stx2), which is 56% homologous to Shiga toxin/Stx1 at the deduced amino acid sequence level (Jackson et al., 1987). Stx1 and Stx2 genetic variants have been described. Stxs are encoded by late genes of lambdoid bacteriophage (reviewed in Allison, 2007). The stx2 operon is under control of the PR and PR′ promoters, and toxin production is optimal under conditions that induce the phage lytic cycle. An additional iron-regulated promoter adjacent to the stx1 operon appears sufficient to induce stx1 transcription, although Stx1 translocates to the bacterial periplasmic space rather than being released into the environment (Wagner et al., 2002)
Stxs are AB5 holotoxins, consisting of an enzymatic A-subunit (~32-kDa) in non-covalent association with five B-subunits, each B-subunit protein being ~7.7 kDa. B-subunits pentamerize to form a ring, and the C-terminus of the A-subunit inserts into the central pore (Fraser et al., 2004). B-subunits of Stxs that cause disease in humans bind to the neutral globo-series glycolipid globotriaosylceramide (Gb3). Following toxin binding to Gb3-expressing cells, the holotoxin is internalized via a mechanism that may initially involve B-subunit-induced negative curvature of the host cell membrane leading to the formation of toxin-containing membrane invaginations (Römer et al., 2007). The toxin undergoes a process termed retrograde transport which involves routing via early/recycling endosomes to the trans-Golgi network, through the Golgi apparatus, to reach the ER lumen. During transport, the A-subunit is cleaved by furin or a furin-like protease to form a 27 kDa A1-fragment and a 4 kDa A2-fragment which remain associated via a disulfide bond (Sandvig et al., 1992; Garred et al., 1995). The ER is the site of disulfide bond reduction, unfolding of the A1-fragment, and retrotranslocation of the A1-fragment possibly via the Sec61 translocon into the cytoplasm (Lord et al., 2005; Tam and Lingwood, 2007). The A1-fragment possesses N-glycosidase activity and cleaves a single adenine residue (at position 4324 in the rat) from the 28S rRNA component of eukaryotic ribosomes (Endo et al., 1988). The target adenine residue is unpaired in a region of non-Watson-Crick base pairing called a GAGA tetraloop. This region of the 28S rRNA is also called the α-sarcin/ricin loop since the enzymatic action of these two ribosome-inactivating proteins (RIPs) is also directed to this loop. The depurination reaction blocks association with elongation factors, resulting in protein synthesis inhibition. Because Vero cells (African green monkey renal epithelial cells) are highly sensitive to protein synthesis inhibition and cell death by Stxs, the toxins are also referred to as verotoxins or verocytotoxins. Readers are directed to several reviews for more detailed information on toxin structure, receptor binding, internalization, retrograde transport, and retrotranslocation (Johannes and Römer, 2010; Lingwood et al., 2010; Sandvig et al., 2010).
While the enzymatic action of Stxs is well characterized, the precise relationship between rRNA depurination/protein synthesis inhibition and cell death remains unclear. It has become evident that Stxs induce apoptosis or programmed cell death in many cell types in vitro and in vivo (reviewed in Tesh, 2010). Thus, recent studies have focused on the exploration of cell death signaling mechanisms activated by the toxins. Stxs are effective signaling molecules activating multiple stress responses in eukaryotic cells. While protein synthesis inhibition may contribute to cell death, Stx-induced protein synthesis inhibition may be dissociated from cell death signaling in some cell types. This MicroReview examines cell stress responses activated by Stxs following the depurination reaction (ribotoxic stress response) or by the presence of unfolded proteins within the ER (unfolded protein response). Signaling through these pathways may be involved in the induction of cytokine/chemokine expression and programmed cell death, processes which contribute to the pathogenesis of disease caused by Stxs.
Shiga toxins activate the ribotoxic stress response
The term ribotoxic stress response was introduced by Iordanov et al. (1997) who showed that site specific modifications to the ribosomal peptidyl transferase reaction center activated signaling through the c-Jun NH2-terminal (JNK) mitogen-activated protein kinase (MAPK) pathway. Rat-1 fibroblasts were treated with protein synthesis inhibitors acting at different ribosomal sites to disrupt translation. Anisomycin, which binds to domain V of 28S rRNA, rapidly activated the JNK1 isoform with a greater than 15-fold activation within 15 min of treatment. The response was sensitive to anisomycin with half maximal activation occurring at doses mediating < 10% reduction in [3H]-leucine incorporation into polypeptides. Other RIPs such as pactamycin and emetine effectively inhibited protein synthesis (>95%), but failed to activate JNK signaling. Thus, signal transduction and protein synthesis inhibitory activities could be dissociated. Anisomycin-specific signaling required alteration of functional ribosomes rather than directly activating the JNK pathway since pre-treatment of Rat-1 cells with pactamycin or emetine blocked the capacity of anisomycin, but not IL-1α, to activate JNK1. The analysis was extended to RIPs that act on domain VI (α-sarcin/ricin loop) of 28S rRNA including the fungal ribonuclease α-sarcin and the plant toxin ricin A-chain. While α-sarcin cleaved the phosphodiester bond adjacent to A4324, and ricin A-chain depurinated 28S rRNA at position A4324, both RIPs activated JNK1 and the upstream MAPK kinase (MAP2K) SEK1/MKK4. In contrast, toxins which ADP-ribosylated EF-2 but failed to mediate rRNA damage in the α-sarcin/ricin loop, failed to activate JNK1.
One might predict that Stxs, like ricin, activate the ribotoxic stress response since these toxins share identical enzymatic activities. Treatment of human macrophage-like (differentiated) THP-1 cells with Stx1, ricin or anisomycin activated JNK and p38 MAPK signaling cascades (Foster and Tesh, 2002; Cherla et al., 2006). Activation was rapid, with JNK1, JNK2 and p38 MAPK phosphorylation peaking 3–6 h after toxin exposure. Within this short timeframe, exposure of THP-1 cells to Stx1 transiently increased total protein synthesis and the cells were relatively resistant to the rapid onset of cytotoxicity characterized using other cell types such as Vero cells (Foster et al., 2000; Harrison, et al., 2005). Thus, Stx1 induction of the ribotoxic stress response in macrophage-like cells did not appear to require rapid protein synthesis inhibition or cell death. In contrast to stress-activated protein kinases, JNK and p38, Stx1 induced modest and transient activation of extracellular signal-regulated kinases (ERK). Patients infected with STEC may have elevated serum titers of anti-STEC lipopolysaccharide (LPS) antibodies (Karmali, 1998) and LPS bound to blood cells (Ståhl et al., 2009), suggesting that intestinal damage may be sufficient to allow LPS to enter the circulation. LPS are effective activators of innate immunity, and treatment of macrophage-like cells with Stx1 and LPS significantly increased activation of all three MAPK cascades (Cherla et al., 2006), suggesting that the presence of both Stxs and LPS in the circulation may be an important determinant in the progression of disease caused by Stx-producing bacteria.
Activation of the ribotoxic stress response by Stx1 played a critical role in the induction of cytokine/chemokine expression in macrophage-like cells. Inhibitors of JNK, p38 and ERK signaling partially blocked, and simultaneous use of all three inhibitors extensively blocked, toxin induced expression of soluble IL-1β and IL-8 (Cherla et al., 2006). An inhibitor of MAPK-interacting kinase 1 (Mnk1), a downstream substrate of p38 MAPK, also inhibited cytokine expression. Primary human peripheral blood monocytes treated with Stx1 or Stx2 showed a slightly different pattern of MAPK activation, with the JNK pathway being transiently activated and the ERK and p38 MAPK pathways more strongly activated (Cameron, et al., 2003). Inhibitors of p38 MAPK signaling were effective in blocking TNF-α and GM-CSF production in response to the toxins. Gray et al. (2008) showed that Stx1 treatment of the human monocytic cell line U937 increased IL-8 production, which was reduced ~80% by pretreatment of cells with PKR inhibitors. A similar phenomenon was noted using ribotoxic stress inducers ricin and deoxynivalenol (a trichothecene mycotoxin). When U937 cells stably transfected with a non-functional PKR mutant were used, elevated IL-8 levels were not detected following treatment with Stx1, ricin or deoxynivalenol. Optimal IL-8 expression induced by deoxynivalenol required a second kinase, hematopoietic cell kinase (Hck) which associates with the 40S ribosomal subunit and triggers activation of ASK1, MKK3/6, and p38 MAPK (Bae et al., 2010). Additional studies will be required to determine whether multiple kinases must interact with ribosomes for Stx activation of the ribotoxic stress response.
While the capacity of Stxs to activate MAPKs is well defined, and some of the upstream MAP2Ks and MAP3Ks involved in the ribotoxic stress response have been identified, proximal signaling events linking the depurination reaction with the initiation of signaling cascades remain to be fully characterized. Recently, Stx A1-fragments and the ricin A-chain have been shown to associate with acidic ribosomal phosphoproteins that comprise the ribosomal stalk, a protuberance of the large ribosomal subunit involved in the recruitment and binding of initiation and elongation factors. The interaction of Stxs and ricin with the ribosomal stalk appears to be required for the depurination reaction within the α-sarcin/ricin loop (Chiou et al., 2008; McCluskey et al., 2008). Gray et al. (2008) hypothesized that the interaction of Stx A1-fragments with ribosomes may alter ribosomal tertiary structure and/or toxin-mediated 28S rRNA damage may alter rRNA secondary structure. PKR is a serine/threonine kinase which binds to, and is activated by, damaged ribosomes via interaction with two dsRNA-binding domains (Nallagatla et al., 2011). Activated PKR phosphorylates eIF-2α at Ser51, leading to inhibition of overall translation, although the expression of genes involved in the host cell response to stress is maintained or up-regulated. Furthermore, there is evidence that the association of PKR with damaged ribosomes creates an activation scaffold for the direct binding and activation of JNK and p38 MAPKs (Iordanov et al., 2000; Alisi et al., 2008; Zang et al., 2009). Thus, the observation that PKR is activated in Stx1-treated U937 cells implicates this kinase as an immediate sensor of ribosomal damage and suggests that Stxs may inhibit protein synthesis through multiple mechanisms.
Once activated, the ribotoxic stress response regulates cytokine/chemokine expression at the transcriptional level through the activation of transcription factors such as NF-κB, AP-1 and Egr-1 (Sakiri, et al., 1998; Zoja et al., 2002; Leyva-Illades et al., 2010), and through post-transcriptional mechanisms involving mRNA stabilization, and increased ribogenesis and translation initiation (Thorpe, et al., 2001; Harrison et al., 2004; Cherla et al., 2006). Stxs also induce expression of dual specificity phosphatases (Kojima et al., 2000; Leyva-Illades et al., 2010). Thus, the ribotoxic stress response induced by Stxs may include the activation of mechanisms that ultimately down-regulate the response.
Smith et al. (2003) linked apoptosis with Stx-induced signaling through the ribotoxic stress response using the human epithelial cell line Hct8. Stx1, but not an enzymatic Stx1 mutant, triggered caspase-3 activation and DNA fragmentation. JNK and p38 MAPKs were activated by Stx1 and pharmacological inhibition of p38 MAPK signaling reduced caspase-3 activation and DNA fragmentation, and partially protected Hct8 cells from apoptosis. Anisomycin and UV-light are effective ribotoxic stress response inducers, and an inhibitor of the MAP3K zipper sterile-α-motif kinase (ZAK), or the knockdown of ZAK expression using small interfering (si)RNAs, protected COS-7 cells from apoptosis, and blocked JNK and p38 MAPK phosphorylation, induced by anisomycin or UV-light treatment. Neither ZAK inhibitor treatment nor transfection with ZAK siRNAs blocked JNK and p38 MAPK activation induced by TNF-α or IL-1β, specifically linking ZAK as an upstream signaling molecule in the ribotoxic stress response leading to apoptosis (Wang et al. 2005). The ZAK inhibitor and ZAK siRNAs blocked Stx2- and ricin-mediated activation of stress-activated protein kinases and partially protected Hct8 and Vero cells from apoptosis (Jandhyala et al. 2008). Inhibition of signaling through ZAK blocked Stx2-induced caspase-3 activation, but DNA fragmentation was not altered, suggesting that additional signaling pathways may be activated by Stxs to trigger DNA scission events. Treatment with the ZAK inhibitor did not alter Stx2-mediated protein synthesis inhibition. In contrast to intestinal epithelial cells, the Ramos Burkitt’s lymphoma cell line appears to express basal levels of activated p38 MAPK, and Stx1 treatment did not increase p38 MAPK activation above these levels. Inhibitors of p38 MAPKs actually increased apoptosis induced by Stx1 treatment of Burkitt’s lymphoma cells (Garibal et al., 2010). Thus, in lymphoid cells, prolonged signaling through p38 MAPKs may induce survival pathways that protect the cells from toxin-induced apoptosis. In summary, Stx-induced signaling through the ribotoxic stress response may differentially activate MAPK cascades leading to cell death or cell survival signaling in different cell types (Figure 1).
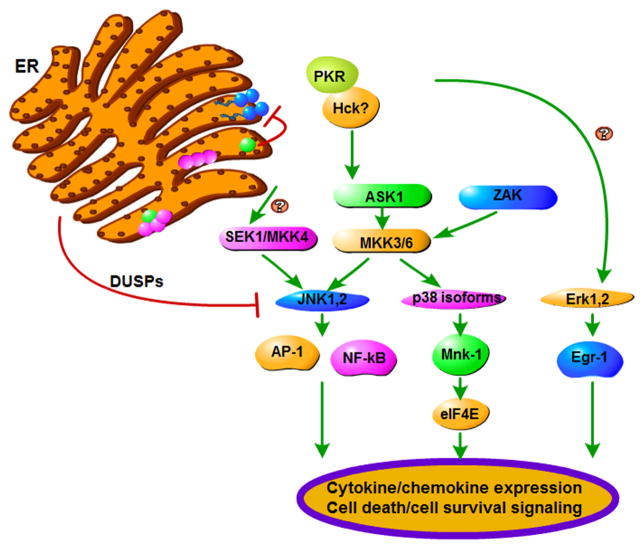
Fig. 1. Induction of the ribotoxic stress response by Stxs.
Following retrotranslocation of Stx A1-fragment (green circle) across the ER membrane, the depurination reaction involving a single adenine residue within the α-sarcin/ricin loop of the 28S rRNA ribosomal subunit (blue circles) may induce a sufficient conformational change to allow the serine/threonine protein kinase PKR to bind. Additional kinases may also recognize changes in ribosomal tertiary structure. Stxs appear to be capable of activating all three MAPKs: JNK1,2, the p38 MAPK isoforms, and ERK1,2. Upstream molecules transducing signals from intoxicated ribosomes to activate MAPKs remain to be fully characterized, but include the MAP3Ks, ASK-1 and ZAK, and the MAP2Ks, SEK1/MKK4 and MKK3/6. Downstream signaling molecules activated by the MAPKs regulate cytokine/chemokine gene expression at transcriptional and post-transcriptional levels, and activate apoptosis and cell survival pathways. Dual specificity phosphatases (DUSPs) are also activated by the ribotoxic stress response, suggesting that signals to ultimately down-regulate the response are initiated following intoxication.
Shiga toxins activate the unfolded protein response (UPR)
The ER is the intracellular site for the correct folding and post-translational processing of proteins destined to be transported to locations within the cell or secreted. The ER is also involved in Ca2+ homeostasis and storage. Recently, an intracellular “quality control” mechanism has been defined which assesses the status of protein folding and Ca2+ storage (Bernales et al., 2006; Malhotra and Kaufman, 2007). Three ER-localized transmembrane proteins “sense” levels of unfolded proteins: RNA-dependent protein kinase-like ER kinase (PERK), inositol-requiring ER to nucleus signal kinase-1 (IRE1), and activating transcription factor-6 (ATF6). PERK is a serine/threonine kinase, IRE1 is a multifunctional protein with kinase and endoribonuclease activities, and ATF6 is a transcription factor. The sensor molecules associate with the chaperone binding immunoglobulin protein (BiP, also known as GRP78). When unfolded or improperly processed proteins accumulate in the ER, BiP dissociates from the sensor molecules (Bertolotti et al. 2000). Subsequently, PERK and IRE1 are activated by homo-oligomerization and proximity-dependent auto-phosphorylation, while ATF6 activation requires translocation from the ER membrane to the Golgi apparatus and proteolytic cleavage by site 1 and site 2 proteases. Activation of the sensor molecules leads to a transient, coordinated response involving the attenuation of overall protein translation coupled with the transcriptional activation of a subset of genes encoding chaperones (for correct protein folding) and proteins involved in degradation of unfolded proteins via the ER-associated protein degradation (ERAD) pathway. This coordinated response is called the unfolded protein response (UPR). Failure to correct protein folding defects and maintain Ca2+ homeostasis may lead to prolonged signaling through the UPR, which in turn, may activate apoptotic signaling events (Tabas and Ron, 2011). A key transcriptional factor in the UPR is C/EBP homologous protein (CHOP, also called Gadd 153). CHOP both positively and negatively regulates the expression of genes involved in apoptosis (McCullogh et al., 2001).
Stxs associate with ER-localized chaperone proteins HEDJ/ERdj3 and BiP (Yu and Haslam, 2005; Falguiéres and Johannes, 2006), suggesting that during retrotranslocation through the Sec61 translocon, Stx A1-fragments probably exist in a transient unfolded state. The induction of apoptosis by Stxs appears to require toxin enzymatic activity in most cell types examined (reviewed in Tesh, 2010). Based on these observations, Lee et al. (2008) reasoned that Stxs may activate the UPR via multiple mechanisms: the transient unfolding of Stx A1-fragments activates the UPR while the protein synthesis inhibitory activity of the toxins leads to the accumulation of unfolded host proteins within the ER and/or the alteration intracellular Ca2+ levels. Stxs may signal apoptosis, therefore, through prolonged UPR signaling. Human monocyte-like (undifferentiated) THP-1 cells are relatively sensitive to killing by Stxs, and Stx1 treatment of the cells activated all UPR sensors within 2 h of intoxication. Stx1 treatment led to the functional activation of the UPR: XBP-1, the mRNA transcript for X-Box Protein-1, was spliced by activated IRE1 to encode the functional transcription factor, eIF-2α was phosphorylated by activated PERK, and ATF6 was cleaved from the inactive 90kDa form to the active 50 kDa transcription factor. CHOP expression was up-regulated within hours of Stx1 treatment of THP-1 cells. CHOP is known to differentially regulate the expression of death receptor 5 (DR5, also known as TRAIL-R2) and the anti-apoptotic protein Bcl-2 (McCullogh et al., 2001; Yamaguchi and Wang, 2004). Stx1 treatment of monocyte-like THP-1 cells up-regulated the expression of DR5, and down-regulated the expression of Bcl-2. The capacity of Stxs to mediate the release of Ca2+ from intracellular stores was associated with the activation of calpains, which in turn, cleaved procaspase-8 and induced apoptosis. Stx1 enzymatic mutant and purified Stx1 B-subunits failed to trigger apoptosis or fully activate the UPR so that up-regulated expression of CHOP and the differential modulation of DR5 and Bcl-2 expression were not detected. However, there was evidence of IRE1 activation and XBP1 mRNA splicing using these inactive toxin preparations, suggesting that the presence of Stxs within the ER may be sufficient to initiate the UPR, but unfolded Stx A1-fragments and/or toxin enzymatic activity may be necessary to maintain the UPR leading to apoptosis. A summary of the UPR leading to apoptosis induced by Stx1 treatment of monocytic THP-1 cells is presented in Figure 2.
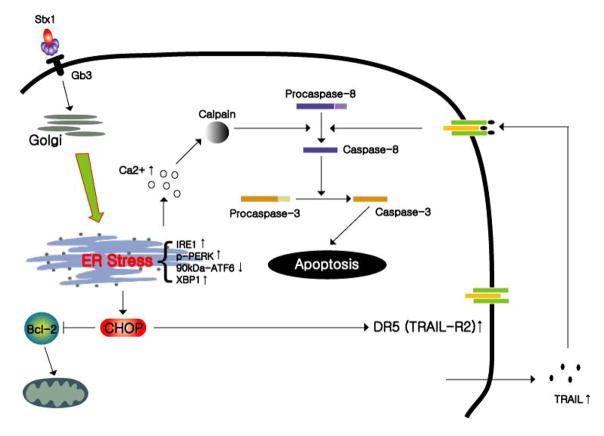
Fig. 2. Prolonged activation of the UPR by Stxs leads to apoptosis.
Unfolded Stx A1-fragments and/or the presence of truncated, misfolded proteins within the ER of intoxicated monocytic THP-1 cells leads to increased phosphorylation of ER stress sensors PERK and IRE1, and cleavage of the sensor ATF6 from the 90 kDa precursor form to the active 50 kDa transcription factor. Expression of the transcription factor CHOP is up-regulated, which in turn, down-regulates expression of the anti-apoptotic factor Bcl-2, and up-regulates expression of the apoptosis inducing factor TRAIL and its receptor DR5. Release of Ca2+ from ER stores also activates calpains which may directly cleave procaspase-8. Reproduced from Lee et al., Cellular Microbiology 10(3):770–780 [2008] with permission of the authors and Wiley-Blackwell Publishers.
The response of macrophage-like (differentiated) THP-1 cells to Stxs is more complex: protein synthesis is transiently up-regulated after intoxication, proinflammatory cytokine and chemokine expression is induced, and both survival and apoptosis pathways appear to be simultaneously activated (Foster et al.,2000; Harrison et al., 2005; Lee et al., 2007). However, over time macrophage-like cells die in response to intoxication, a phenomenon termed the delayed apoptosis phenotype. Treatment of the cells with Stxs led to the rapid activation of PERK and IRE1, and eIF-2α was phosphorylated and XBP1 mRNA was spliced. However, ATF6 activation was not detected in Stx1 treated macrophage-like THP-1 cells, suggesting that cell maturation correlates with loss of processing and signaling through ATF6. The anti-apoptotic protein Bcl-2 emerged as a critical regulator determining the kinetics of cell death induced by Stxs in THP-1 cells. In contrast to monocyte-like cells, where Bcl-2 expression was down-regulated by Stx1, treatment of macrophage-like THP-1 cells with Stx1 increased Bcl-2 expression and mediated an increased translocation of Bcl-2 to mitochondria (Lee et al., 2009). This study also identified “cross-talk” between the ribotoxic stress response and the UPR activated by Stxs. The anti-apoptotic function of Bcl-2 requires JNK-mediated phosphorylation of Bcl-2 at Ser70 (Deng et al., 2001). Alternative Bcl-2 phosphorylation reactions, including p38 MAPK-directed phosphorylation of Bcl-2 at amino acid Thr56, inhibit Bcl-2 function (DeChiara et al., 2006). Bcl-2 was differentially phosphorylated by Stx1 treatment of monocyte- vs. macrophage-like THP-1 cells. Levels of anti-apoptotic Ser70-phospho-Bcl-2 molecules were transiently increased in macrophage-like cells, while levels declined in monocyte-like cells. In contrast, levels of Thr56-phospho-Bcl-2 declined in toxin treated monocyte-like cells, and Bcl-2 phosphorylated at position Thr56 was not detected in Stx1-treated macrophage-like THP-1 cells (Lee et al., 2009). Thus, the ribotoxic stress response induced by Stxs may regulate the activation of the Bcl-2 family of proteins that, in turn, control apoptosis.
Studies to characterize the role of the UPR induced by Stxs in apoptosis in other cell types are limited. Stx1 and Stx2 appear to differentially activate UPR sensors in the human renal tubule epithelial cell line HK-2 so that Stx1 is more effective at activating ATF6, while Stx2 primarily triggered the phosphorylation of PERK and IRE1 (Lentz et al., 2011). Microarray analysis of genes modulated by Stx2 treatment of human brain microvascular endothelial cells revealed that transcripts for genes involved in the UPR were up-regulated, including the genes encoding PERK, CHOP and ATF4 (Fujii et al., 2008). Subtilase cytotoxin, a newly described AB5 holotoxin expressed by STEC, that undergoes retrograde transport to the ER where it selectively cleaves the chaperone BiP, has been shown to activate all UPR stress sensors in Vero cells (Wolfson et al., 2008). In contrast to these studies, Parikh et al. (2008) showed that the transfection of plasmids expressing mature ricin A-chain into yeast expressing an UPR element::lacZ gene reporter construct resulted in decreased β-galactosidase expression, suggesting that the enzymatic activity of ricin may suppress the UPR. Yeast cells lack the ER membrane sensors PERK and ATF-6, so that Ire1 is the sole resident ER membrane protein involved in the UPR (Kohno, 2010). However, Wang et al. (2011) used bovine and human epithelial cell lines to show that ricin A-chain failed to activate Ire1, to mediate XBP-1 mRNA splicing, and to phosphorylate eIF-2α. Furthermore, ricin A-chain inhibited tunicamycin-induced XBP-1 splicing and DTT-induced eIF-2α phosphorylation. The cleavage of procaspase-7 and -3 was dramatically increased in cells treated with ricin A-chain plus the UPR inducers tunicamycin or DTT compared to treatment with ricin A-chain alone, suggesting that ricin-induced inhibition of the UPR sensitized the cells to cytotoxicity. These results highlight the need for caution in formulating generalizations on the role of the UPR in cell death signaling, and justify the need for additional studies to characterize the roles the UPR in the host response to Stxs and other RIPs.
Role of Shiga toxin-activated cell stress responses in pathogenesis
There is limited information available in the literature on the relationship between Stx-induced activation of stress responses characterized in multiple cells types in vitro, and pathogenesis of disease caused by Stxs in humans or in animal models. Furthermore, the cell types which specifically respond to Stxs by inducing the ribotoxic stress response or the UPR have not been characterized in vivo. Psotka et al. (2009) showed that the administration of a caspase inhibitor in mice challenged with Stx2 and LPS reduced the numbers of TUNEL-positive cells detected in renal tissue sections, and reduced indicators of renal failure (BUN and urine osmolality). Stxs are known to activate MAPK cascades and the UPR in epithelial cells in vitro (Smith et al., 2003; Lentz et al., 2011) and it would be interesting to correlate activation of these stress responses with tissue-specific production of cytokines and chemokines and the induction of apoptosis in vivo. Studies employing animal models of ricin intoxication may be informative in the design of these experiments. For example, Korcheva et al (2005) showed that the intravenous administration of ricin to mice resulted in the activation of all three MAPK cascades. MAPK activation was localized to renal glomerular and peritubular microvascular endothelial cells and nuclei of proximal and distal convoluted tubules, cardiac myocytes, hepatocytes, and splenic lymphocytes. Ricin-induced signaling through MAPKs was associated with the significant up-regulation of genes encoding cytokines/chemokines and transcriptional regulators, and the development of thrombocytopenia, hemolytic anemia and renal failure. Reagents necessary to test the role of the ribotoxic stress response and UPR in pathogenesis in vivo are becoming available, and studies to assess the role of cell stress responses in disease are clearly warranted.
Acknowledgments
The author thanks Sang-Yun Lee and Dinorah Leyva-Illades for artwork and careful review of the text. Funding provided by grant RO1 AI034530 from NIAID, NIH, Bethesda, MD
References
- Alisi A, Spaziani A, Anticoli S, Ghidinelli M, Balsano C. PKR is a novel functional direct player that coordinates skeletal muscle differentiation via p38MAPK/AKT pathways. Cell Signal. 2008;20:534–542. doi: 10.1016/j.cellsig.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Allison HE. Stx-phages: drivers and mediators of the evolution of STEC and STEC-like pathogens. Future Microbiol. 2007;2:165–174. doi: 10.2217/17460913.2.2.165. [DOI] [PubMed] [Google Scholar]
- Bae H, Gray JS, Li M, Vines L, Kim J, Pestka JJ. Hematopoietic cell kinase associates with the 40S ribosomal subunit and mediates the ribotoxic stress response to deoxynivalenol in mononuclear phagocytes. Toxicol Sci. 2010;115:444–452. doi: 10.1093/toxsci/kfq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernales S, Papa FR, Walter P. Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol. 2006;22:487–508. doi: 10.1146/annurev.cellbio.21.122303.120200. [DOI] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- Cameron P, Smith SJ, Giembycz MA, Rotondo D, Plevin R. Verotoxin activates mitogen-activated protein kinase in human peripheral blood monocytes: role in apoptosis and proinflammatory cytokine release. Brit J Pharmacol. 2003;140:1320–1330. doi: 10.1038/sj.bjp.0705560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherla RP, Lee SY, Mees PL, Tesh VL. Shiga toxin 1-induced cytokine production is mediated by MAP kinase pathways and translation initiation factor eIF4E in the macrophage-like THP-1 cell line. J Leukoc Biol. 2006;79:397–407. doi: 10.1189/jlb.0605313. [DOI] [PubMed] [Google Scholar]
- Chiou JC, Li XP, Remacha M, Ballesta JPG, Tumer NE. The ribosomal stalk is required for ribosome binding, depurination of the rRNA and cytotoxicity of ricin A chain in Saccharomyces cerevisiae. Mol Microbiol. 2008;70:1441–1452. doi: 10.1111/j.1365-2958.2008.06492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChiara G, Marcocci ME, Torcia M, Lucibello M, Rosini P, Bonini P, et al. Bcl-2 phosphorylation by p38 MAPK: identification of target sites and biological consequences. J Biol Chem. 2006;281:21353–21361. doi: 10.1074/jbc.M511052200. [DOI] [PubMed] [Google Scholar]
- Deng X, Xiao L, Lang W, Gao F, Ruvolo P, May WS. Novel role for JNK as a stress-activated Bcl2 kinase. J Biol Chem. 2001;276:23681–23688. doi: 10.1074/jbc.M100279200. [DOI] [PubMed] [Google Scholar]
- Endo Y, Tsurugi K, Yutsudo T, Takeda Y, Ogasawara T, Igarashi K. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur J Biochem. 1988;171:45–50. doi: 10.1111/j.1432-1033.1988.tb13756.x. [DOI] [PubMed] [Google Scholar]
- Falguiéres T, Johannes L. Shiga toxin B-subunit binds to the chaperone BiP and the nucleolar protein B23. Biol Cell. 2006;98:125–134. doi: 10.1042/BC20050001. [DOI] [PubMed] [Google Scholar]
- Foster GH, Tesh VL. Shiga toxin 1-induced activation of c-Jun NH2-terminal kinase and p38 in the human monocytic cell line THP-1: possible involvement in the production of TNF-α. J Leukoc Biol. 2002;71:107–114. [PubMed] [Google Scholar]
- Foster GH, Armstrong CS, Sakiri R, Tesh VL. Shiga toxin-induced tumor necrosis factor alpha expression: requirement for toxin enzymatic activity and monocyte protein kinase C and protein tyrosine kinases. Infect Immun. 2000;68:5183–5189. doi: 10.1128/iai.68.9.5183-5189.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser ME, Fujinaga M, Cherney MM, Melton-Celsa AR, Twiddy EM, O’Brien AD, James MNG. Structure of Shiga toxin type 2 (Stx2) from Escherichia coli O157:H7. J Biol Chem. 2004;279:27511–27517. doi: 10.1074/jbc.M401939200. [DOI] [PubMed] [Google Scholar]
- Fujii J, Wood K, Matsuda F, Carneiro-Filho BA, Schlegel KH, Yutsudo T, et al. Shiga toxin 2 causes apoptosis in human brain microvascular endothelial cells via C/EBP homologous protein. Infect Immun. 2008;76:3679–3689. doi: 10.1128/IAI.01581-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garibal J, Hollville E, Renouf B, Tétaud C, Wiels J. Caspase-8-mediated cleavage of Bid and protein phosphatase 2A-mediated activation of Bax are necessary for verotoxin-1-induced apoptosis in Burkitt’s lymphoma cells. Cell Signal. 2010;22:467–475. doi: 10.1016/j.cellsig.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Garred Ø, van Deurs B, Sandvig K. Furin-induced cleavage and activation of Shiga toxin. J Biol Chem. 1995;270:10817–10821. doi: 10.1074/jbc.270.18.10817. [DOI] [PubMed] [Google Scholar]
- Gray JS, Bae HK, Li JCB, Lau AS, Pestka JJ. Double-stranded RNA-activated protein kinase mediates induction of interleukin-8 expression by deoxynivalenol, Shiga toxin 1, and ricin in monocytes. Toxicol Sci. 2008;105:322–330. doi: 10.1093/toxsci/kfn128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyles CL. Shiga toxin-producing Escherichia coli: an overview. J Anim Sci. 2007;85:E45–E62. doi: 10.2527/jas.2006-508. [DOI] [PubMed] [Google Scholar]
- Harrison LM, Cherla RP, van den Hoogen C, van Haaften WCE, Lee SY, Tesh VL. Comparative evaluation of apoptosis induced by Shiga toxin 1 and/or lipopolysaccharides in human monocytic and macrophage-like cells. Microb Pathog. 2005;38:63–76. doi: 10.1016/j.micpath.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Harrison LM, van Haaften WCE, Tesh VL. Regulation of pro-inflammatory cytokine expression by Shiga toxin 1 and/or lipopolysaccharides in the human monocytic cell line THP-1. Infect Immun. 2004;72:2628–2627. doi: 10.1128/IAI.72.5.2618-2627.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanov MS, Paranjape JM, Zhou A, Wong J, Williams BRG, Meurs EF, et al. Activation of p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase by double-stranded RNA and encephalomyocarditis virus: involvement of RNase L, protein kinase R, and alternative pathways. Mol Cell Biol. 2000;20:617–627. doi: 10.1128/mcb.20.2.617-627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanov MS, Pribnow D, Magun JL, Dinh TH, Pearson JA, Chen SLY, Magun BE. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the α-sarcin/ricin loop in the 28S rRNA. Mol Cell Biol. 1997;17:3373–3381. doi: 10.1128/mcb.17.6.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MP, Neill RJ, O’Brien AD, Holmes RK, Newland JW. Nucleotide sequence analysis and comparison of the structural genes for Shiga-like toxin I and Shiga-like toxin II encoded by bacteriophages from Escherichia coli 933. FEMS Microbiol Lett. 1987;44:109–114. doi: 10.1016/0882-4010(87)90106-9. [DOI] [PubMed] [Google Scholar]
- Jandhyala DM, Ahluwalla A, Obrig T, Thorpe CM. ZAK: a MAP3kinase that transduces Shiga toxin- and ricin-induced proinflammatory cytokine expression. Cell Microbiol. 2008;10:1466–1477. doi: 10.1111/j.1462-5822.2008.01139.x. [DOI] [PubMed] [Google Scholar]
- Johannes L, Römer W. Shiga toxins – from cell biology to biomedical applications. Nat Rev Microbiol. 2010;8:105–116. doi: 10.1038/nrmicro2279. [DOI] [PubMed] [Google Scholar]
- Karmali MA. Human immune response and immunity to Shiga toxin (verocytotoxin)-producing Escherichia coli infection. In: Kaper JB, O’Brien AD, editors. Escherichia coli O157:H7 and Other Shiga toxin-Producing E. coli Strains. Washington, D.C: ASM Press; 1998. pp. 236–248. [Google Scholar]
- Kojima S, Yanagihara I, Kono G, Sugahara T, Nasu H, Kijima M, et al. mkp-1 encoding mitogen-activated protein kinase phosphatase 1, a verotoxin 1 responsive gene, is detected by differential display reverse transcription-PCR in Caco-2 cells. Infect Immun. 2000;68:2791–2796. doi: 10.1128/iai.68.5.2791-2796.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno K. Stress-sensing mechanisms in the unfolded protein response: similarities and differences between yeast and mammals. J Biochem. 2010;147:27–33. doi: 10.1093/jb/mvp196. [DOI] [PubMed] [Google Scholar]
- Korcheva V, Wong J, Corless C, Iordanov M, Magun B. Administration of ricin induces a severe inflammatory response via nonredundant stimulation of ERK, JNK and p38 MAPK and provides a mouse model of hemolytic uremic syndrome. Am J Pathol. 2005;166:323–339. doi: 10.1016/S0002-9440(10)62256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Cherla RP, Leyva-Illades D, Tesh VL. Bcl-2 regulates the onset of Shiga toxin 1-induced apoptosis in THP-1 cells. Infect Immun. 2009;77:5233–5244. doi: 10.1128/IAI.00665-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Cherla RP, Tesh VL. Simultaneous induction of apoptotic and survival signaling pathways in macrophage-like THP-1 cells by Shiga toxin 1. Infect Immun. 2007;75:1291–1302. doi: 10.1128/IAI.01700-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Lee MS, Cherla RP, Tesh VL. Shiga toxin 1 induces apoptosis through the ER stress response in human monocytic cells. Cell Microbiol. 2008;10:770–780. doi: 10.1111/j.1462-5822.2007.01083.x. [DOI] [PubMed] [Google Scholar]
- Lentz EK, Leyva-Illades D, Lee MS, Cherla RP, Tesh VL. Differential response of the human renal proximal tubule epithelial cell line HK-2 to Shiga toxins type 1 and type 2. Infect Immun. 2011;79:3527–3540. doi: 10.1128/IAI.05139-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyva-Illades D, Cherla RP, Galindo CL, Chopra AK, Tesh VL. Global transcriptional response of macrophage-like THP-1 cells to Shiga toxin type 1. Infect Immun. 2010;78:2454–2465. doi: 10.1128/IAI.01341-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood CA, Binnington B, Manis A, Branch DR. Globotriaosyl ceramide receptor function – where membrane structure and pathology intersect. FEBS Lett. 2010;584:1879–1886. doi: 10.1016/j.febslet.2009.11.089. [DOI] [PubMed] [Google Scholar]
- Lord JM, Roberts LM, Lencer WI. Entry of protein toxins into mammalian cells by crossing the endoplasmic reticulum membrane: co-opting basic mechanisms of endoplasmic reticulum-associated degradation. Curr Topics Microbiol Immunol. 2005;300:149–168. doi: 10.1007/3-540-28007-3_7. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskey AJ, Poon GMK, Bolewska-Pedyczak E, Srikumar T, Jeeram SM, Raught B, Gariépy J. The catalytic subunit of Shiga-like toxin 1 interacts with ribosomal stalk proteins and is inhibited by their conserved C-terminal domain. J Mol Biol. 2008;378:375–386. doi: 10.1016/j.jmb.2008.02.014. [DOI] [PubMed] [Google Scholar]
- McCullogh KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd 153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallagatla SR, Toroney R, Bevilacqua PC. Regulation of innate immunity through RNA structure and the protein kinase PKR. Curr Opin Struct Biol. 2011;21:119–127. doi: 10.1016/j.sbi.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata F. Influence of Escherichia coli Shiga toxin on the mammalian central nervous system. Adv Appl Microbiol. 2010;71:1–19. doi: 10.1016/S0065-2164(10)71001-7. [DOI] [PubMed] [Google Scholar]
- Parikh BA, Tortora A, Li XP, Tumer NE. Ricin inhibits activation of the unfolded protein response by preventing splicing of the HAC1 mRNA. J Biol Chem. 2008;283:6145–6153. doi: 10.1074/jbc.M707981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx F, Tesh VL. Renal diseases in the Pediatric Intensive Care Unit: thrombotic microangiopathy, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura. In: Wheeler DS, Wong HR, Shanley TP, editors. Pediatric Critical Care Medicine: Basic Science and Clinical Evidence. London: Springer-Verlag; 2007. pp. 1198–1204. [Google Scholar]
- Psotka MA, Obata F, Kolling GL, Gross LK, Saleem MA, Satchell SC, et al. Shiga toxin 2 targets the murine renal collecting duct epithelium. Infect Immun. 2009;77:959–969. doi: 10.1128/IAI.00679-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römer W, Berland L, Chambon V, Gaus K, Windschiegl B, Tenza D, et al. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature. 2007;450:670–675. doi: 10.1038/nature05996. [DOI] [PubMed] [Google Scholar]
- Sakiri R, Ramegowda B, Tesh VL. Shiga toxin type 1 activates tumor necrosis factor-α gene transcription and nuclear translocation of the transcriptional activators nuclear factor-κB and activator protein-1. Blood. 1998;92:558–566. [PubMed] [Google Scholar]
- Sandvig K, Garred Ø, Prydz K, Kozlov JV, Hansen SH, van Deurs B. Retrograde transport of endocytosed Shiga toxin to the endoplasmic reticulum. Nature. 1992;358:510–511. doi: 10.1038/358510a0. [DOI] [PubMed] [Google Scholar]
- Sandvig K, Bergan J, Dyve AB, Skotland T, Torgersen ML. Endocytosis and retrograde transport of Shiga toxin. Toxicon. 2010;56:1181–1185. doi: 10.1016/j.toxicon.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Smith WE, Kane AV, Campbell ST, Acheson DWK, Cochran BH, Thorpe CM. Shiga toxin 1 triggers a ribotoxic stress response leading to p38 and JNK activation and induction of apoptosis in intestinal epithelial cells. Infect Immun. 2003;71:1497–1504. doi: 10.1128/IAI.71.3.1497-1504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ståhl A, Sartz L, Nelsson A, Békássy ZD, Karpman D. Shiga toxin and lipopolysaccharide induce platelet-leukocyte aggregates and tissue factor release, a thrombotic mechanism in hemolytic uremic syndrome. PLoS One. 2009;4:e6990. doi: 10.1371/journal.pone.0006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Rev Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam PJ, Lingwood CA. Membrane – cytosolic translocation of verotoxin A1 subunit in target cells. Microbiology. 2007;153:2700–2710. doi: 10.1099/mic.0.2007/006858-0. [DOI] [PubMed] [Google Scholar]
- Tarr PI, Gordon CA, Chandler WI. Shiga toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365:1073–1086. doi: 10.1016/S0140-6736(05)71144-2. [DOI] [PubMed] [Google Scholar]
- Tesh VL. Induction of apoptosis by Shiga toxins. Future Microbiol. 2010;5:431–453. doi: 10.2217/fmb.10.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe CM, Smith WE, Hurley BP, Acheson DWK. Shiga toxins induce, superinduce, and stabilize a variety of C-X-C chemokine mRNAs in intestinal epithelial cells, resulting in increased chemokine expression. Infect Immun. 2001;69:6140–6147. doi: 10.1128/IAI.69.10.6140-6147.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner PL, Livny J, Neely MN, Acheson DWK, Friedman DI, Waldor MK. Bacteriophage control of Shiga toxin 1 production and release by Escherichia coli. Mol Microbiol. 2002;44:957–970. doi: 10.1046/j.1365-2958.2002.02950.x. [DOI] [PubMed] [Google Scholar]
- Wang CT, Jetzt AE, Cheng JS, Cohick WS. Inhibition of the unfolded protein response by ricin A-chain enhances its cytotoxicity in mammalian cells. Toxins. 2011;3:453–468. doi: 10.3390/toxins3050453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Mader MM, Toth JE, Yu X, Jin N, Campbell RM, et al. Complete inhibition of anisomycin and UV irradiation but not cytokine induced JNK and p38 activation by an aryl-substituted dihydropyrrolopyrazole quinoline and mixed lineage kinase 7 small interfering RNA. J Biol Chem. 2005;280:19298–19305. doi: 10.1074/jbc.M413059200. [DOI] [PubMed] [Google Scholar]
- Wolfson JJ, May KL, Thorpe CM, Jandhyala DM, Paton JC, Paton AW. Subtilase cytotoxin activates PERK, IRE1 and ATF6 endoplasmic reticulum stress-signaling pathways. Cell Microbiol. 2008;10:1775–1786. doi: 10.1111/j.1462-5822.2008.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem. 2004;279:45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- Yu M, Haslam DB. Shiga toxin is transported from the endoplasmic reticulum following interaction with the luminal chaperone HEDJ/ERdj3. Infect Immun. 2005;73:2524–2532. doi: 10.1128/IAI.73.4.2524-2532.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang P, Langland JO, Jacobs BL, Samuel CE. Protein kinase PKR-dependent activation of mitogen-activated protein kinases occurs through mitochondrial adapter IPS-1 and is antagonized by Vaccinia virus E3L. J Virol. 2009;83:5718–5725. doi: 10.1128/JVI.00224-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoja C, Angioletti S, Donadelli R, Zanchi C, Tomasoni S, Binda E, et al. Shiga toxin-2 triggers endothelial leukocyte adhesion and transmigration via NF-κB dependent up-regulation of IL-8 and MCP-1. Kidney Int. 2002;62:846–856. doi: 10.1046/j.1523-1755.2002.00503.x. [DOI] [PubMed] [Google Scholar]