SUMMARY
Dengue viruses (DENV; family Flaviviridae, genus Flavivirus) are transmitted by Aedes aegypti mosquitoes and can cause dengue fever (DF), a relatively benign disease, or more severe dengue haemorrhagic fever (DHF). Arthropod saliva contains proteins delivered into the bite wound that can modulate the host haemostatic and immune responses to facilitate the intake of a blood meal. The potential effects on DENV infection of previous exposure to Ae. aegypti salivary proteins have not been investigated. We collected Ae. aegypti saliva, concentrated the proteins, and fractionated them by non-denaturing polyacrylamide gel electrophoresis (PAGE). By use of immunoblots we analysed reactivity with the mosquito salivary proteins (MSP) of sera from 96 Thai children diagnosed with secondary DENV infections leading either to DF or DHF, or with no DENV infection, and found that different proportions of each patient group had serum antibodies reactive to specific Ae. aegypti salivary proteins. Our results suggest that prior exposure to MSP might play a role in the outcome of DENV infection in humans.
Keywords: dengue, dengue haemorrhagic fever, Aedes aegypti, mosquito salivary proteins
INTRODUCTION
DF and DHF are caused by infection with one of the four serotypes of DENV (DENV 1–4) 1 transmitted principally by Ae. aegypti mosquitoes. After an incubation period of 3 to 14 days, a person bitten by an infected Ae. aegypti mosquito can experience acute fever accompanied by a variety of nonspecific signs and symptoms. In the last 50 years, the incidence of DEN disease has increased and hyperendemic transmission of DENV has been established 1, 2, resulting in a dramatic global increase in the most severe form of the disease, DHF, which was first described in Southeast Asia 3. A major risk factor for developing DHF is re-infection with a virus serotype different from the first infection 4. Molecular epidemiologic evidence has shown that there also is an increased risk of developing DHF when the infection is caused by a virulent DENV genotype 5, 6, 7.
Inoculation of MSP at the time of infection has been associated with enhanced pathogenesis of arboviruses 8, 9, 10, 11. Co-inoculation of salivary proteins or salivary gland extracts and arboviruses facilitates the dissemination of the pathogen due to modulation of the host immune response 12, 13, 14, 15. Other recognised functions of MSP that might potentiate pathogen transmission include vasodilation, inhibition of platelet activation, and suppression of inflammation 16, 17; for example, sequestration of inflammatory mediators such as biogenic amines and leukotrienes are important functions of the abundant D7 protein in Ae. aegypti saliva18, 19.
Exposure to MSP has been shown to elicit specific IgE and IgG1 antibodies 20. In experimental models, disease severity was reduced in mice pre-exposed to sandfly saliva before Leishmania infection, and to mosquito saliva prior to Plasmodium infection 21, 22, 23. In contrast, a report on the effect of pre-exposure to Ae. aegypti MSP on West Nile virus infection indicated that it enhances mortality in a mouse model 24.
In this retrospective study using well-characterized serum specimens from children who were hospitalized in Bangkok, Thailand, we examined the relationship between the human immune response to MSP and DEN disease severity.
MATERIALS AND METHODS
Patients
A total of 101 paired acute and convalescent coded serum specimens were obtained upon admission and 2–6 days later, respectively, from Thai patients who had been admitted to a Bangkok children’s hospital. These paired de-personalized serum specimens had been subjected to laboratory diagnosis, a service provided by the Armed Forces Research Institute of Medical Sciences (AFRIMS) to the Bangkok community. All sera were collected in compliance with Thai and U.S. regulations on human use research. Serologic diagnosis of acute DENV infection was made and Japanese encephalitis virus infection was excluded by IgM and IgG capture ELISA for both viruses. Dengue infection status (i.e., acute primary, acute secondary, or no DENV infection) was assigned to all patients based on established AFRIMS criteria for IgM and IgG capture ELISA results 25, 26. Reverse transcription-nested polymerase chain reaction (RT-PCR) was used to determine DENV serotype using primers described by Lanciotti et al. 27. Severity of infection was assigned using World Health Organization (WHO) criteria 28. Results reported in this study were limited to acute serum samples collected at the time of hospital admission from 96 patients whose subsequent laboratory diagnosis indicated they had DENV2 secondary infections or did not have DENV infections, determined after the patient identification code was broken, and whose sera reacted with Ae. aegypti salivary proteins in immunoblots. Of the 50 DHF patients, 46% were male; age range 3–28 yr, mean 8.5 yr; median 10 yr. Of the 28 DF patients, 36% were male; age range 3–17 yr, mean 9 yr, median 9 yr. Of the 18 non-DENV-infected (NI) patients, 50% were male; age range 2–10 yr, mean 5.7 yr, median 6 yr. All sera were collected between July and December 2001, inclusive of the peak transmission season.
Mosquito saliva collection
Ae. aegypti, (Rex-D strain, Puerto Rico) were reared at 28°C, 80% relative humidity, and a 12 h light:12 h dark photocycle at the Arthropod-borne and Infectious Diseases Laboratory (AIDL), Colorado State University. After eclosion, 250 female mosquitoes were placed in containers and sugar and water were supplied ad libitum; 4 to 5 days later sugar and water were removed for 24 h to stimulate feeding. Salivation induction was modified from a technique previously described 29. Mosquitoes were allowed to feed for 1 h through a parafilm membrane on an artificial feeder containing 2 ml sterile salivation buffer, which consisted of 0.4 mM NaCl and 0.025 mM NaHCO3, maintained at 38°C. Collected saliva was stored at −20°C until use. This procedure yielded approximately 500 μg protein from 250 mosquitoes.
Mosquito salivary protein concentration and fractionation
MSP were precipitated from the buffer by the addition of trichloroacetic acid (TCA) to a final concentration of 10% (w/v). The mixture was incubated on ice for 30 min and centrifuged at 4 °C for 15 min at 15000 rpm. Protein pellets were washed with acetone (−20°C) and re-sedimented, vacuum dried and re-suspended in distilled water for storage at −20°C. Protein concentration was determined by bicinchoninic acid (BCA) assay (Pierce Chemicals, Rockford, IL). NuPAGE Bis-Tris 10% polyacrylamide gels (Invitrogen) were used to fractionate salivary proteins. Two hundred fifty ng of MSP were loaded per well in non-denaturing loading buffer (NuPAGE® LDS sample buffer, Invitrogen) and electrophoresed for 40 min at 200 volts. Proteins were fractionated under non-denaturing conditions so that both linear and conformational epitopes could be preserved for immunoblot analysis. Gels were stained using the SilverQuest kit (Invitrogen). The number of stained protein bands, mobility, and relative intensity of staining were reproducible following separate collections.
Detection of human antibodies against MSP by indirect ELISA
Total MSP were diluted in coating buffer (50 mM sodium carbonate and 50 mM sodium bicarbonate, pH 9.6), and 10 ng per well were added to 96-well plates. Individual serum samples diluted 1:100 were placed in wells, incubated at 37°C, washed, and pooled horseradish peroxidase (HRP)-labelled anti-human IgG, IgM and IgE (KPL, Gaithersburg, MD) diluted 1:1000 were added and incubated at room temperature. Reactivity was developed with 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS; KPL) and absorbance was read at 405 nm. Negative controls were human serum samples demonstrating no reactivity in immunoblot analysis. The cut-off for positive samples was the mean negative control absorbance value (0.0004) plus three standard deviations.
Detection of human antibodies against MSP by immunoblot analysis
After separation by PAGE, unstained MSP were transferred to a nitrocellulose membrane, the membrane was blocked with 5% non-fat milk in PBS/0.1% Tween 20 (blocking buffer), cut into strips, and each strip was incubated overnight at 4°C with an individual Thai patient serum sample diluted 1:200 in blocking buffer. Bound antibodies were detected with a mixture of HRP-labelled anti-human IgE, IgG, IgM, and IgA diluted 1:500 in blocking buffer. The small amounts of patient sera available necessitated testing each serum sample only once and detecting all immunoglobulin isotypes simultaneously. The nitrocellulose strips were developed with 3,3′-diaminobenzidine (DAB, Sigma Laboratories). Antibodies in individual sera were scored visually as reacting with one to seven MSP and no differences were noted in band intensities between blots.
Preparation of proteins for mass spectrometric analysis
To obtain sufficient quantities for identification of the major proteins detected in MSP immunoblots, 100 pairs of female Ae. aegypti salivary glands were dissected into lysis buffer containing 10 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5mM EDTA, 1% sodium deoxycholate, 1% Triton-X-100, 0.1% SDS and 1 mM PMSF. The salivary glands were mechanically disrupted and proteins were concentrated by TCA precipitation and fractionated by PAGE as described above. After Coomassie blue staining individual protein bands were marked and the gel destained. Marked protein bands were excised and half of each was submitted to the Proteomics and Metabolomics Facility, Colorado State University for analysis by mass spectrometry. Protein was eluted from the remaining gel, concentrated (PAGE Prep Advance Kit, Pierce Laboratories) and TCA precipitated. Concentrated proteins were re-suspended in distilled water, fractionated by PAGE and transferred to a nitrocellulose membrane. A pool of patient serum samples known to react with all major MSP was used in an immunoblot assay to verify the reactivity of individual proteins sent for analysis.
Analysis of MSP by mass spectrometry (MS)
Polyacrylamide gel slices containing MSP were finely diced, washed with 100 mM NH4HCO3, 50% acetonitrile (ACN) and dried. Dried gel fragments were incubated in 10 mM dithiothreitol, 100 mM NH4HCO3, centrifuged, and the supernatant discarded. Gel fragments were washed in 55 mM iodoacetic acid in 100 mM NH4HCO3, proteins were digested in the gel with trypsin, extracted in 50% ACN, 0.1% trifluoroacetic acid and purified using a C18 Zip-tip (Millipore).
Analysis was performed with an UltraFlex-TOF/TOF mass spectrometer (Bruker Daltonics, Billerica, MA) in positive ion reflector mode with a 25 kV acceleration voltage, and data were processed using the SNAP algorithm in the FlexAnalysis software (version 2.4, Bruker Daltonics). A monoisotopic list was generated using a signal-to-noise threshold of 6 for MS spectra and 3 for MS/MS spectra. The combined MS and MS/MS spectra for each sample were searched using the Mascot (version 2.1) database search engine against the NCBInr database containing 16,885 sequence entries using a taxonomy filter for mosquito (Aedes, Taxonomy ID 7158). Parameters used in the database search were: peptide mass tolerance of 0.1 Da, fragment ion mass tolerance of 0.7 Da, trypsin peptides only allowing for 1 missed cleavage, variable modifications of cysteine carbamidomethylation and methionine oxidation.
Data analysis
ELISA results were tested for differences between patient groups using one-way analysis of variance (ANOVA) and group means were compared using a Students t-test. A chi-square test was used to analyze differences in patient group reactivities by immunoblot. All statistical analyses were carried out using the SAS program version 9.1 (SAS Institute, Inc., Cary NC, 2002).
RESULTS
PAGE protein fractionation and determination of immunoreactive MSP
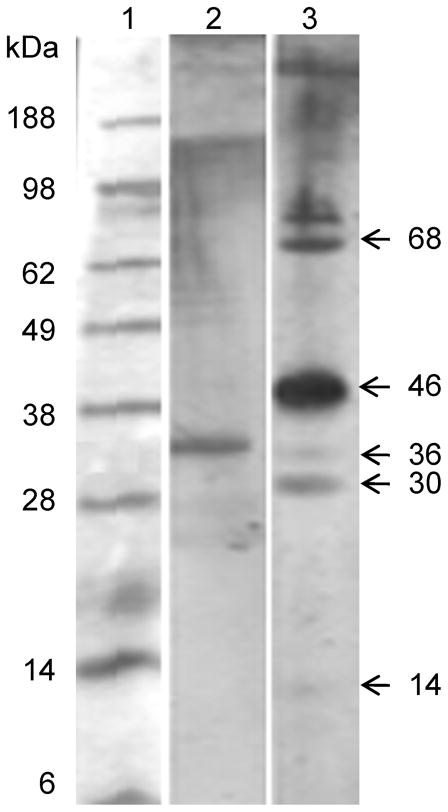
After PAGE fractionation of MSP, seven prominent bands were seen with approximate molecular masses of 68, 46, 36, 30, 19, 17, and 14 kDa (Figure 1). Because the proteins were separated under non-denaturing conditions, high molecular weight material, assumed to be aggregates, was seen at the top of both stained gels and immunoblots.
Figure 1. Fractionation of mosquito salivary proteins (MSP) by polyacrylamide gel electrophoresis (PAGE).
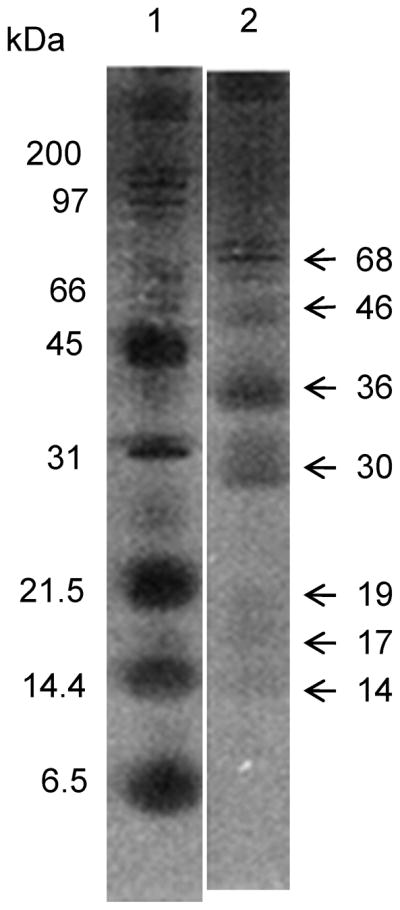
Lane 1, molecular markers; Lane 2, Ae. aegypti salivary proteins concentrated with trichloroacetic acid (TCA), fractionated by nondenaturing 10% PAGE and silver stained; approx. sizes in kDa and positions of major MSP are indicated at right.
ELISA was used to determine reactivity of serum samples against total MSP and results are shown in Table 1. Mean reactivity was lowest for non-DENV infected patients (mean absorbance 0.659). Significantly higher values were obtained for the DF and DHF groups (mean values equivalent to maximum detectable absorbance of 1.80; p<0.001) (Table 1).
Table 1.
Reactivity of patients’ sera to total MSP in ELISA
| Patient group* (n) | ELISA titer** | Standard error |
|---|---|---|
| DF (28) | 1.80a | 0.00059 |
| DHF (50) | 1.80a | 0.00044 |
| NI (18) | 0.659b | 0.00073 |
In analysis of differences between groups by t-test, means with common superscripts are not significantly different (P>0.05).
DF = dengue fever, DHF = dengue haemorrhagic fever, NI = not DENV infected
Mean absorbance at 450 nm
Immunoblots with each of the 96 patients’ acute sera were scored blindly for reactivity with each of the seven major MSP identified by PAGE. Each blot was scored visually for either presence or absence of each reactive band; differences in band intensities were not noted. Examples of immunoblots with two patients’ sera, one of whom reacted with only p36 and the other with 5 major MSP, are shown in Figure 2, lanes 2 and 3 respectively. At that point the key to the disease status of each patient was broken. Proportions of sera from each of the three groups (DHF, DF or NI) reacting with each protein were determined and patient groups were statistically compared pairwise using a chi-square test. The proportion of non-DENV infected (NI), uncomplicated DEN fever (DF), and DEN haemorrhagic fever (DHF) patients’ sera that had antibodies reactive with each of the seven major MSP are shown in Table 2. Significantly more DF patients’ than DHF patients’ sera reacted with p30 (9/28, 32.1%; 4/50, 8%, respectively) (p<0.001). Conversely, significantly more DHF than DF patients’ sera reacted with p68 (18/50, 36%; 4/28, 14.3%, respectively) (p<0.005). Sera from both infected groups (DF and DHF) reacted with p36 in significantly higher percentages (20/28, 71.4%; 31/50, 62%, respectively), than sera from non-DENV infected patients (3/18, 16.7%, p<0.005), although there was no significant difference between the p36 reactivity of the two infected groups, as detailed in Table 2.
Figure 2. Reactivity of patients’ sera in immunoblots.
Lane 1, molecular markers; Lanes 2 and 3, Immunoblots showing reactivity of 2 different patients’ sera with MSP. Approximate sizes in kDa of immunoreactive proteins shown at right.
Table 2.
Reactivity of patients’ sera to individual mosquito salivary proteins in immunoblots
| Mosquito salivary protein | |||||||
|---|---|---|---|---|---|---|---|
| p14 | p17 | p19 | p30 | p36 | p46 | p68 | |
| Patient group* | Number of patients reactive/number tested (% reactive) | ||||||
| DF | 5/28 (17.9)a | 4/28 (14.3)a | 3/28 (10.7)a | 9/28 (32.1)a | 20/28 (71.4)a | 22/28 (78.5)a | 4/28 (14.3)a |
| DHF | 11/50 (22)a | 7/50 (14)a | 5/50 (10)a | 4/50 (8)b | 31/50 (62)a | 40/50 (80)a | 18/50 (36)b |
| NI | 2/18 (11.1)a | 2/18 (11.1)a | 0/18 (0)a | 3/18 (16.7)ab | 3/18 (16.7)b | 14/18 (77.7)a | 0/18 (0)a |
Percent patients’ sera reactive with each protein in immunoblots. Percentages with common superscripts are not significantly different (P>0.05).
DF = dengue fever, DHF = dengue haemorrhagic fever, NI = not DENV infected
Identification of mosquito salivary gland proteins by mass spectrometry (MS)
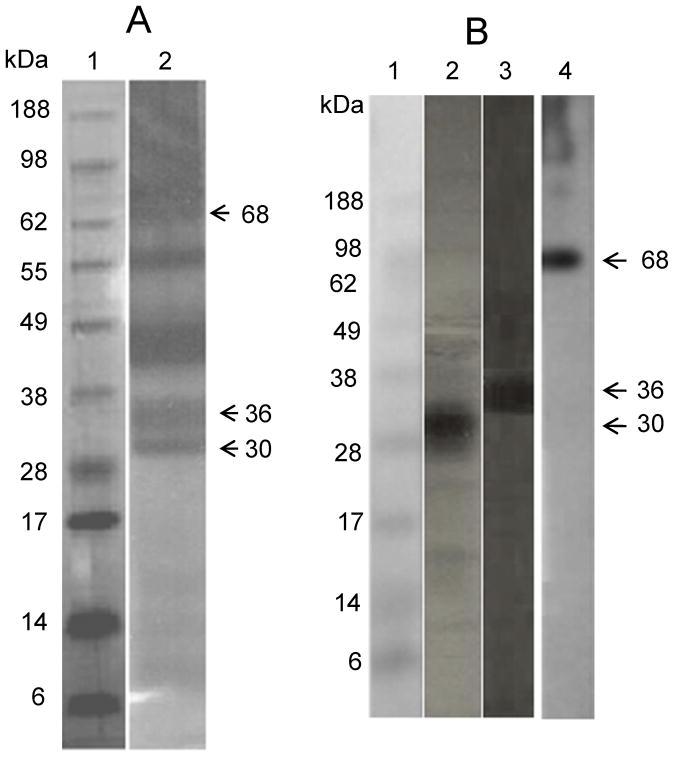
We observed significantly different reactivities between patient groups’ sera to MSP p68, p36, and p30 in immunoblots. Proteins with these molecular masses previously have been shown to be allergens designated Aed a 1, Aed a 2, and Aed a 3 respectively 30. Mass spectrometric (MS) analysis identified these proteins as follows: the 30 kDa salivary gland allergen was recently characterized as aegyptin, a collagen binding protein 31, 32; the 36 kDa protein is from the D7 family, which bind host biogenic amines and leukotrienes 18, 19, 33; and the 68 kDa protein was previously shown to be apyrase, a 5′-nucleotidase 17, 34 (Table 3). To ensure that the proteins detected by immunoblot were the same as the ones that were analysed by MS, each of the three salivary gland extract proteins was re-fractionated and blotted as described. Each of the three proteins reacted with pooled patients’ sera, as shown in Figure 3.
Table 3.
Protein identification from MS/MS spectra by MASCOT ion score.
| Protein designation (Accession number) | Mascot protein ion score (cutoff score) | Sequence coverage (No. of matched peaks) | Peptide sequences identified by MS/MS spectra | Mascot MS/MS score (cutoff score) | Putative identity |
|---|---|---|---|---|---|
| 30 kDa (gi|14423642) | 101(55) | 12% (3) | K.VDHIQSEYLR.S R.SALNNDLQSEVR.V |
57(28) 32(28) |
Aegyptin |
| 36 kDa (gi|118216) | 124(55) | 37% (14) | R.SQIYAFNLPK.K K.FDASVIQEQFK.A |
32(27) 37(27) |
D7 |
| 68 kDa (gi|1703351) | 311(55) | 44% 16) | K.LFPLTLIHINDLHAR.F K.GADIWDVAEHSFALDDEGR.T K.NPIYLNAGDNFQGTLWYNLLR.W |
49(24) 122(24) 23(24) |
Apyrase |
Data from MS and MS/MS analysis of tryptic peptides were processed using the SNAP algorithm in the FlexAnalysis software as described in Materials and Methods and a monoisotopic list was generated for each sample. Combined MS and MS/MS results for each sample were searched using the Mascot (version 2.1) search engine against the NCBInr mosquito protein database. Protein ion scores that exceeded the cutoff scores for each sample were used in identification and are shown here.
Figure 3. Identity of salivary gland homogenate proteins and immunoreactive MSP.
A. Lane 1, molecular markers; Lane 2, Salivary gland homogenate fractionated by 10% PAGE and Coomassie blue stained. Bands indicated by arrows were excised from the gel for MS analysis. B. Lane 1, molecular markers; Lanes 2, 3, 4, Immunoblot analysis of individual proteins excised and eluted from gel and fractionated by PAGE using pooled patient sera. Lane 2 = 30 kDa, Lane 3 = 36 kDa, Lane 4 = 68 kDa.
DISCUSSION
Our study demonstrated that proteins in collected Ae. aegypti saliva could be concentrated by TCA precipitation and fractionated by non-denaturing PAGE, reproducibly resolving seven major protein bands with molecular sizes ranging from 14 kDa to 68 kDa. When reactivity with total MSP of sera from hospitalized children in a DENV-endemic region was assayed by ELISA, we observed that individuals infected with DENV2 had significantly higher antibody titres to total MSP than non-DENV infected patients. This suggests that greater exposure to arthropod vectors correlates to a higher risk of becoming infected 35, and it seems likely that the lower mean and median ages of NI patients afforded less opportunity for exposure to mosquito bites, and thus lower overall seroreactivity due to decreased mosquito exposure rates.
Almost all serum samples from individual Thai paediatric hospital patients, whether or not they had a DENV infection, were shown by immunoblot assay to contain antibodies that bound to at least one and as many as seven of these proteins. Comparisons between DF, DHF, and NI patient groups showed significant differences (p<0.05) between at least two of the three groups in seroreactivity for p30, p36, and p68. These proteins were individually identified by mass spectrometric analysis as the allergens previously designated Aed a 1 (p68), Aed a 2 (p36), and Aed a 3 (p30) 30, 36. Allergic reactions to mosquito proteins are proposed to occur as a primary immune response to mosquito bites, eliciting production of immunoglobulins IgE and IgG4 37, 38, 39. However, since we did not have sufficient amounts of sera to discriminate between patients’ immunoglobulin isotypes, we do not know if the antibodies detected were allergy related, so this question could be pursued in future studies. Although some individuals experience a gamma interferon-induced DTH response, animal models demonstrate that a mosquito bite generally triggers increases in Th2 cytokines 20, indicative of a polyclonal B cell response 12, 14.
We found these three MSP to be highly immunogenic in humans. Similar findings were presented previously for humans after prolonged exposure to Aedes spp. bites 40 and experimental studies demonstrated that mice exposed to Ae. aegypti salivary proteins reacted to MSP in a similar pattern after only four exposures 24. All three of these MSP have been shown to be involved in disrupting haemostatic and inflammatory responses, generally facilitating blood-feeding 41, 17, 31, 19.
The 36 kDa protein was among the most abundant of Ae aegypti MSP, and a very high proportion of both DF and DHF patients exhibited an antibody response to this protein. We showed that the 36 kDa MSP is a member of the D7 protein family 42. The C-terminal domain of Ae. aegypti D7 (AeD7) protein has been shown to bind biogenic amines such as serotonin, histamine, norepinephrine and epinephrine 18, which facilitate host haemostasis and inflammation, including increasing vasoconstriction, platelet aggregation, and pain induction. Their sequestration by D7 proteins could antagonize these host defence functions and thus promote blood acquisition and pathogen transmission. Recently it has been shown that the N-terminal domain of AeD7 binds with high affinity to cysteinyl leukotrienes, which act as inflammatory mediators 19. D7 or proteins with similar functions are evolutionarily conserved in saliva of haemophagic arthropods 18; nevertheless, our findings suggest that potential inhibition of D7 activity by pre-existing antibody does not have a protective effect against DEN disease.
Our data indicate that a significantly higher proportion of sera from patients with secondary DENV2 infection and no indications of increased vascular permeability, as compared to patients with DHF, had antibodies against the 30 kDa protein in Ae. aegypti saliva. This Ae. aegypti 30 kDa MSP, termed aegyptin, has been shown to bind to collagen (I–V) and interfere with its interaction with major physiological ligands to recruit platelets to sites of an injury such as a bite. This ultimately inhibits platelet aggregation and adhesion, greatly facilitating acquisition of a blood meal 31. The 30 kDa protein is also an allergen; immune response to allergens such as the 30 kDa protein of Ae. aegypti may be diverse in an exposed population such that some individuals can overcome allergic reactions by producing increased levels of IgG antibody against the allergen whereas others might not, instead exhibiting increased production of IgE 43. It is possible that some of the patients who had previously been exposed to aegyptin had IgG antibodies that neutralized the allergenic, haemostatic, and/or immunomodulatory effects of this protein, and this could in part be responsible for a less severe dengue infection. Recently it was reported that aegyptin expression is up-regulated in blood-fed mosquitoes and that IgG antibodies to this protein were found in the pooled sera of both DHF patients and bitten uninfected individuals 44. Unfortunately, as mentioned earlier, we can only speculate about the role of anti-MSP antibody isotype in disease severity since we did not differentiate among Ig isotypes. This may be an important matter to be addressed in further research.
A significantly higher proportion of patients subsequently diagnosed with severe dengue disease than those with DF or NI produced antibodies to a 68 kDa protein identified as apyrase, a 5′-nucleotidase that inhibits platelet aggregation by destroying adenosine di- and tri-phosphates released from injured cells or by activated platelets 17. This protein is widely conserved within the saliva of blood-sucking arthropods 17, 40, 42, 20 and acts as an anti-coagulant, facilitating the location of capillary vessels during blood feeding 45. It could be expected that encountering antibodies against this protein would make it difficult for mosquitoes to feed; however, it has been reported that mosquitoes acquire a blood meal in the presence of high-titred antibodies against apyrase 46. Furthermore, it has been shown that if apyrase is inhibited, mosquito probing is prolonged 45, 47, suggesting that the presence of anti-apyrase antibodies might actually increase the risk of DENV2 inoculation. In addition, studies indicate that DENV-infected Ae. aegypti exhibit longer probing and feeding times than uninfected mosquitoes 48. The effect of prolonged and multiple probing could increase viral load delivered to the host, leading to a greater number of initially-infected cells and therefore to higher viraemia titres. It has been shown that viral load correlates with disease severity 49.
It is also possible that MSPs have additional functions in vivo to those already determined, such as induction of cytokines that result in lack of endothelial cell adhesion and fluid extravasation, and differential antibody responses could modulate these effects. For example, previous studies indicated that sialokinin-1 cloned from Ae. aegypti, which was initially described as a vasodilator, also had the ability to down-regulate Th1- and up-regulate Th2-type cytokine response when inoculated into mice 14. These effects could be detected in mouse splenocytes harvested 4 to 7 days after mosquito feeding or sialokinin inoculation 14, thus manifesting systemic and long-lasting effects, “the enigma of immunomodulation by rapid-feeding vectors” described by Schneider and Higgs 13.
A previous study suggested that pre-exposure to mosquito salivary proteins may increase the severity of arboviral disease by increasing the availability of susceptible cell types, disrupting immune signalling or a combination of these factors that could potentially enhance the spread of virus 24. We observed significant differences in the proportions of DF and DHF patients with immune responses to certain MSPs, although in no case did all patients in a disease group possess antibodies to a particular protein. In addition, there was no uniform correlation of the magnitude of antibody response (either number of reactive serum bands or intensity of bands) to MSPs with disease severity. Larger studies, including patients who did not require hospitalization, will be necessary to fully understand the role of antibodies and antibody isotypes that react with specific MSP in potential modulation of DENV transmission and DEN pathogenesis. Although lack of an animal model for DENV infection precludes controlled studies testing the role of antibodies to MSP in modulating infection, detailed prospective studies of human responses to MSP in relation to DENV pathogenesis in endemic regions could give further insight into these initial observations.
Acknowledgments
We are grateful to Dr. Jill Troyer, Walter Reed Army Institute of Research, for making the serum samples available at the Arthropod-borne and Infectious Diseases Laboratory. We thank Dr. James zumBrunnen, Department of Statistics, Colorado State University, for his help in the statistical analysis of the data presented in this work. This work was supported in part by a grant from the U.S Medical Research and Materiel Command (MPM and AN) and by National Institutes of Health, USA, contract N01 AI25489 (BJB and CDB).
Footnotes
Disclosures: None of the authors has a potential conflict of interest.
Disclaimer: The opinions and assertions contained herein are not to be construed as official or as reflecting the views of the U.S government.
Contributor Information
Mammen P Mammen, Jr, Email: mammen.mammen@amedd.army.mil.
Nordin S Zeidner, Email: naz2@cdc.gov.
Barry J Beaty, Email: barry.beaty@colostate.edu.
Jessica E. Prenni, Email: jessica.prenni@colostate.edu.
Ananda Nisalak, Email: anandan@thai.amedd.army.mil.
Carol D Blair, Email: carol.blair@colostate.edu.
References
- 1.Gubler DJ. Dengue and Dengue Hemorrhagic Fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nature Medicine. 2004;10:S98–S109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 3.Monath TP. Dengue: the risk to developed and developing countries. Proc Natl Acad Sci USA. 1994;91:2395–2400. doi: 10.1073/pnas.91.7.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halstead SB, O’Rourke EJ. Antibody-enhanced dengue virus infection in primate leukocytes. Nature. 1977;265:739–741. doi: 10.1038/265739a0. [DOI] [PubMed] [Google Scholar]
- 5.Leitmeyer KC, Vaughn DW, Watts DM, Salas R, Villalobos I, de Chacon, Ramos C, Rico-Hesse R. Dengue Virus Structural Differences That Correlate with Pathogenesis. J Virol. 1999;73:4738–4747. doi: 10.1128/jvi.73.6.4738-4747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rico-Hesse R, Harrison LM, Salas RA, Tovar D, Nisalak A, Ramos C, Boshell J, de Mesa MTR, Nogueira RMR, Travassos da Rosa A. Origins of Dengue Type 2 Viruses Associated with Increased Pathogenicity in the Americas. Virology. 1997;230:244–251. doi: 10.1006/viro.1997.8504. [DOI] [PubMed] [Google Scholar]
- 7.Messer WB, Gubler DJ, Harris E, Sivananthan K, de Silva AM. Emergence and Global Spread of a Dengue Serotype 3, Subtype III Virus. Emerg Infect Dis. 2003;9:800. doi: 10.3201/eid0907.030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards JF, Higgs S, Beaty BJ. Mosquito feeding-induced enhancement of Cache Valley virus (Bunyaviridae) infection in mice. J Med Entomol. 1998;35:261–5. doi: 10.1093/jmedent/35.3.261. [DOI] [PubMed] [Google Scholar]
- 9.Limesand KH, Higgs S, Pearson LD, Beaty BJ. Effect of mosquito salivary gland treatment on vesicular stomatitis New Jersey virus replication and interferon alpha/beta expression in vitro. J Med Entomol. 2003;40:199–205. doi: 10.1603/0022-2585-40.2.199. [DOI] [PubMed] [Google Scholar]
- 10.Styer LM, Bernard KA, Kramer LD. Enhanced Early West Nile Virus Infection in Young Chickens Infected by Mosquito Bite: Effect of Viral Dose. Am J Trop Med Hyg. 2006;75:337–345. [PubMed] [Google Scholar]
- 11.Styer LM, Lim P-Y, Louie KL, Albright RG, Kramer LD, Bernard KA. Mosquito Saliva Causes Enhancement of West Nile Virus Infection in Mice. J Virol. 2011;85:1517–1527. doi: 10.1128/JVI.01112-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider BS, Soong L, Zeidner NS, Higgs S. Aedes aegypti Salivary Gland Extracts Modulate Anti-Viral and TH1/TH2 Cytokine Responses to Sindbis Virus Infection. Viral Immunol. 2004;17:565–573. doi: 10.1089/vim.2004.17.565. [DOI] [PubMed] [Google Scholar]
- 13.Schneider BS, Higgs S. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans of the R Soc Trop Med Hyg. 2008;102:400–408. doi: 10.1016/j.trstmh.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeidner NS, Higgs S, Happ CM, Beaty BJ, Miller BR. Mosquito feeding modulates Th1 and Th2 cytokines in flavivirus susceptible mice: an effect mimicked by injection of sialokinins, but not demonstrated in flavivirus resistant mice. Parasite Immunol. 1999;21:35–44. doi: 10.1046/j.1365-3024.1999.00199.x. [DOI] [PubMed] [Google Scholar]
- 15.Wasserman HA, Singh S, Champagne DE. Saliva of the yellow fever mosquito, Aedes aegypti, modulates murine lymphocyte function. Parasite Immunol. 2004;26:295–306. doi: 10.1111/j.0141-9838.2004.00712.x. [DOI] [PubMed] [Google Scholar]
- 16.Champagne DE, Ribeiro JM. Sialokinin I and II: vasodilatory tachykinins from the yellow fever mosquito Aedes aegypti. Proc Natl Acad Sci USA. 1994;91:138–142. doi: 10.1073/pnas.91.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Champagne DE, Smartt CT, Ribeiro JM, James AA. The salivary gland-specific apyrase of the mosquito Aedes aegypti is a member of the 5′-nucleotidase family. Proc Natl Acad Sci USA. 1995;92:694–698. doi: 10.1073/pnas.92.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calvo E, Mans BJ, Andersen JF, Ribeiro JMC. Function and Evolution of a Mosquito Salivary Protein Family. J Biol Chem. 2006;281:1935–1942. doi: 10.1074/jbc.M510359200. [DOI] [PubMed] [Google Scholar]
- 19.Calvo E, Mans BJ, Ribeiro JMC, Andersen JF. Multifunctionality and mechanism of ligand binding in a mosquito antiinflammatory protein. Proc Natl Acad Sci USA. 2009;106:3728–3733. doi: 10.1073/pnas.0813190106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen YL, Simons FE, Peng Z. A mouse model of mosquito allergy for study of antigen-specific IgE and IgG subclass responses, lymphocyte proliferation, and IL-4 and IFN-gamma production. Int Arch Allergy Immunol. 1998;116:269–277. doi: 10.1159/000023955. [DOI] [PubMed] [Google Scholar]
- 21.Belkaid Y, Kamhawi S, Modi G, Valenzuela J, Noben-Trauth N, Rowton E, Ribeiro J, Sacks DL. Development of a Natural Model of Cutaneous Leishmaniasis: Powerful Effects of Vector Saliva and Saliva Preexposure on the Long-Term Outcome of Leishmania major Infection in the Mouse Ear Dermis. J Exp Med. 1998;188:1941–1953. doi: 10.1084/jem.188.10.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamhawi S, Belkaid Y, Modi G, Rowton E, Sacks D. Protection Against Cutaneous Leishmaniasis Resulting from Bites of Uninfected Sand Flies. Science. 2000;290:1351–1354. doi: 10.1126/science.290.5495.1351. [DOI] [PubMed] [Google Scholar]
- 23.Donovan MJ, Messmore AS, Scrafford DA, Sacks DL, Kamhawi S, McDowell MA. Uninfected Mosquito Bites Confer Protection against Infection with Malaria Parasites. Infect Immun. 2007;75:2523–2530. doi: 10.1128/IAI.01928-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider BS, McGee CE, Jordan JM, Stevenson HL, Soong L, Higgs S. Prior Exposure to Uninfected Mosquitoes Enhances Mortality in Naturally-Transmitted West Nile Virus Infection PLoS One. Vol. 2. 2007. p. e1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Innis BL, Nisalak A, Nimmannitya S, Kusalerdchariya S, Chongswasdi V, Suntayakorn S, Puttisri P, Hoke CH. An Enzyme-Linked Immunosorbent Assay to Characterize Dengue Infections Where Dengue and Japanese Encephalitis Co-Circulate. Am J Trop Med Hyg. 1989;40:418–427. doi: 10.4269/ajtmh.1989.40.418. [DOI] [PubMed] [Google Scholar]
- 26.Vaughn D, Nisalak A, Solomon T, Kalayanarooj S, Nguyen M, Kneen R, Cuzzubbo A, Devine P. Rapid serologic diagnosis of dengue virus infection using a commercial capture ELISA that distinguishes primary and secondary infections. Am J Trop Med Hyg. 1999;60:693–698. doi: 10.4269/ajtmh.1999.60.693. [DOI] [PubMed] [Google Scholar]
- 27.Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO. Guidelines for Treatment of Dengue Fever/Dengue Haemorrhagic Fever in Small Hospitals. WHO Regional Office for South-East Asia; New Delhi, India: 1999. [Google Scholar]
- 29.Owhashi M, Harada M, Suguri S, Ohmae H, Ishii A. The role of saliva of Anopheles stephensi in inflammatory response: identification of a high molecular weight neutrophil chemotactic factor. Parasitol Research. 2001;87:376–382. doi: 10.1007/s004360000355. [DOI] [PubMed] [Google Scholar]
- 30.Simons FE, Peng Z. Mosquito allergy: recombinant mosquito salivary antigens for new diagnostic tests. Int Arch Allergy Immunol. 2001;124:403–405. doi: 10.1159/000053771. [DOI] [PubMed] [Google Scholar]
- 31.Calvo E, Tokumasu F, Marinotti O, Villeval J-L, Ribeiro JMC, Francischetti IMB. Aegyptin, a Novel Mosquito Salivary Gland Protein, Specifically Binds to Collagen and Prevents Its Interaction with Platelet Glycoprotein VI, Integrin alpha-2-beta-1, and von Willebrand Factor. J Biol Chem. 2007;282:26928–26938. doi: 10.1074/jbc.M705669200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calvo E, Tokumasu F, Mizurini DM, McPhie P, Narum DL, Ribeiro JMC, Monteiro RQ, Francischetti IMB. Aegyptin displays high-affinity for the von Willebrand factor binding site (RGQOGVMGF) in collagen and inhibits carotid thrombus formation in vivo. FEBS J. 2010;277:413–427. doi: 10.1111/j.1742-4658.2009.07494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mans BJ, Calvo E, Ribeiro JMC, Andersen JF. The Crystal Structure of D7r4, a Salivary Biogenic Amine-binding Protein from the Malaria Mosquito Anopheles gambiae. J Biol Chem. 2007;282:36626–36633. doi: 10.1074/jbc.M706410200. [DOI] [PubMed] [Google Scholar]
- 34.Sun D, McNicol A, James AA, Peng Z. Expression of functional recombinant mosquito salivary apyrase: A potential therapeutic platelet aggregation inhibitor. Platelets. 2006;17:178–184. doi: 10.1080/09537100500460234. [DOI] [PubMed] [Google Scholar]
- 35.Lane R, Moss R, Hsu Y, Wei T, Mesirow M, Kuo M. Anti-arthropod saliva antibodies among residents of a community at high risk for Lyme disease in California. Am J Trop Med Hyg. 1999;61:850–859. doi: 10.4269/ajtmh.1999.61.850. [DOI] [PubMed] [Google Scholar]
- 36.Peng Z, Yang J, Wang H, Simons FER. Production and characterization of monoclonal antibodies to two new mosquito Aedes aegypti salivary proteins. Insect Biochem Mol Biol. 1999;29:909–914. doi: 10.1016/s0965-1748(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 37.Reunala T, Brummer-Korvenkontio H, Palosuo K, Miyanij M, Ruiz-Maldonado R, Love A, Francois G, Palosuo T. Frequent occurrence of IgE and IgG4 antibodies against saliva of Aedes communis and Aedes aegypti mosquitoes in children. Int Arch Allergy Immunol. 1994;104:366–71. doi: 10.1159/000236693. [DOI] [PubMed] [Google Scholar]
- 38.Peng Z, Beckett AN, Engler RJ, Hoffman DR, Ott NL, Simons FER. Immune responses to mosquito saliva in 14 individuals with acute systemic allergic reactions to mosquito bites. J Allergy Clin Immunol. 2004;114:1189–1194. doi: 10.1016/j.jaci.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 39.Brummer-Korvenkontio H, Lappalainen P, Reunala T, Palosuo T. Detection of mosquito saliva-specific IgE and IgG4 antibodies by immunoblotting. J Allergy Clin Immunol. 1994;93:551–555. doi: 10.1016/s0091-6749(94)70066-4. [DOI] [PubMed] [Google Scholar]
- 40.Brummer-Korvenkontio H, Palosuo T, Francois G, Reunala T. Characterization of Aedes communis, Aedes aegypti and Anopheles stephensi mosquito saliva antigens by immunoblotting. Int Arch Allergy Immunol. 1997;112:169–74. doi: 10.1159/000237450. [DOI] [PubMed] [Google Scholar]
- 41.Ribeiro JMC, Francischetti IMB. Role of Arthropod Saliva in Blood Feeding: Sialome and Post-Sialome Perspectives. Annu Rev Entomol. 2003;48:73–88. doi: 10.1146/annurev.ento.48.060402.102812. [DOI] [PubMed] [Google Scholar]
- 42.Valenzuela JG, Pham VM, Garfield MK, Francischetti IMB, Ribeiro JMC. Toward a description of the sialome of the adult female mosquito Aedes aegypti. Insect Biochem Mol Biol. 2002;32:1101–1122. doi: 10.1016/s0965-1748(02)00047-4. [DOI] [PubMed] [Google Scholar]
- 43.Ozcan E, Notarangelo LD, Geha RS. Primary immune deficiencies with aberrant IgE production. J Allergy Clin Immunol. 2008;122:1054–1062. doi: 10.1016/j.jaci.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 44.Wasinpiyamongkol L, Patramool S, Luplertlop N, Surasombatpattana P, Doucoure S, Mouchet F, Séveno M, Remoue F, Demettre E, Brizard J-P, Jouin P, Biron DG, Thomas F, Missé D. Blood-feeding and immunogenic Aedes aegypti saliva proteins. Proteomics. 2010;10:1906–1916. doi: 10.1002/pmic.200900626. [DOI] [PubMed] [Google Scholar]
- 45.Ribeiro JM, Rossignol PA, Spielman A. Role of mosquito saliva in blood vessel location. J Exp Biol. 1984;108:1–7. doi: 10.1242/jeb.108.1.1. [DOI] [PubMed] [Google Scholar]
- 46.Mathews GV, Sidjanski S, Vanderberg JP. Inhibition of Mosquito Salivary Gland Apyrase Activity by Antibodies Produced in Mice Immunized by Bites of Anopheles stephensi Mosquitoes. Am J Trop Med Hyg. 1996;55:417–423. doi: 10.4269/ajtmh.1996.55.417. [DOI] [PubMed] [Google Scholar]
- 47.Rossignol PA, Ribeiro JMC, Spielman A. Increased Intradermal Probing Time in Sporozoite-Infected Mosquitoes. Am J Trop Med Hyg. 1984;33:17–20. doi: 10.4269/ajtmh.1984.33.17. [DOI] [PubMed] [Google Scholar]
- 48.Platt KB, Linthicum KJ, Myint KSA, Innis BL, Lerdthusnee K, Vaughn DW. Impact of Dengue Virus Infection on Feeding Behavior of Aedes aegypti. Am J Trop Med Hyg. 1997;57:119–125. doi: 10.4269/ajtmh.1997.57.119. [DOI] [PubMed] [Google Scholar]
- 49.Vaughn David W, Green S, Kalayanarooj S, Innis Bruce L, Nimmannitya S, Suntayakorn S, Endy Timothy P, Raengsakulrach B, Rothman Alan L, Ennis Francis A, Nisalak A. Dengue Viremia Titer, Antibody Response Pattern, and Virus Serotype Correlate with Disease Severity. J Infect Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]