Abstract
Previous research has demonstrated deficits in pre-response motor activity in schizophrenia, as evidenced by a reduced lateralized readiness potential (LRP). The LRP deficit could be due to increased activation of the incorrect response (e.g., failure to suppress competition) or to reduced activation of the correct response (e.g., a low-level impairment in response preparation). To distinguish these possibilities, we asked whether the LRP impairment is increased under conditions of strong response competition. We manipulated the compatibility of stimulus-response mappings (Experiment 1) and the compatibility of the target with flankers (Experiment 2). In both experiments, the patient LRP was reduced as much under conditions of low response competition as under high competition. These results are incompatible with a failure of patients to suppress competition and are instead consistent with a deficit in activating the correct response.
Previous research using the lateralized readiness potential (LRP)—an event-related potential (ERP) component indexing response preparation processes—has demonstrated an impairment in the ability of patients with schizophrenia to select among competing response alternatives (Karayanidis et al., 2006; Kieffaber, O’Donnell, Shekhar, & Hetrick, 2007; Luck et al., 2009; Mathalon et al., 2002). However, the precise nature of the response preparation deficit in patients with schizophrenia is unknown. Specifically, it is difficult to assess from previous studies whether the reduced LRP in patients with schizophrenia reflects: (1) a failure of top-down attentional processes to suppress competition from the incorrect response or (2) an impairment in the ability to activate response preparation processes more generally. The present study was designed to elucidate the nature of the response preparation impairment in patients with schizophrenia by distinguishing between these possible mechanisms.
The LRP is a widely used tool in the study of response preparation in both healthy subjects and patient groups. The LRP is typically observed when subjects make a left-hand response for one stimulus category and a right-hand response for another stimulus category. The preparation of the response leads to a negative-going potential over the motor cortex contralateral to the responding hand (see review by Smulders & Miller, in press). Activity related to motor preparation is isolated from the rest of the brain’s activity by taking advantage of the contralateral organization of motor processing. That is, by subtracting the activity over the ipsilateral cortex from the activity over the contralateral cortex, the non-lateralized brain activity is subtracted out, leaving just the response-related activity. The LRP has been shown to arise at least in part from motor cortex (Coles, 1989; de Jong, Coles, Logan, & Gratton, 1990), and it typically begins 100–200 ms prior to motor response onset, providing a means of studying the time course of the processes leading up to motor outputs.
A few previous studies have examined the LRP in patients with schizophrenia (Karayanidis et al., 2006; Kieffaber et al., 2007; Luck et al., 2009; Mathalon et al., 2002). These studies reported a reduction in the amplitude of the LRP in patients compared with control subjects (although this difference did not reach statistical significance in all cases). The most recent of these studies (Luck et al., 2009) found a reduction in LRP amplitude of approximately 50% in patients compared with control subjects in a simple letter/digit discrimination task. Thus, the LRP impairment in patients with schizophrenia can be highly robust even in simple task designs. Furthermore, Luck et al. (2009) found no deficit in the ability of patients to perceive and categorize the stimuli, as evidenced by no delay in the timing and no decrease in the amplitude of the P3 rare-minus-frequent difference wave. Therefore, the LRP deficit in patients with schizophrenia does not reflect carry-over from stimulus-related processing deficits and appears to be related to specific deficits in response-related processing.
Determining the cause of the reduced amplitude LRP in patients with schizophrenia is a complicated task. The subtraction procedure used to isolate the LRP from the surrounding and overlapping ERP activity makes it possible to determine the precise time course of differences in response preparation between hemispheres; however, the subtraction of the ipsilateral from the contralateral activity can complicate the interpretation of differences in LRP amplitude. Specifically, the LRP reflects the difference in the amount of activation in the contralateral and ipsilateral hemispheres, which primarily reflect the correct and incorrect response alternatives, respectively. Therefore, the reduced LRP in schizophrenia patients could reflect either an underactivation of the correct response or an overactivation of the incorrect response. These two possibilities have very different implications for the nature of the underlying neural processing abnormality in schizophrenia. An overactivation of the incorrect response alternative would primarily reflect a failure of top-down control to suppress competition from the incorrect response. Alternatively, a decrease in the amount of correct response activation would primarily reflect a low-level motor activation deficit that cannot be attributable to attentional control deficits. That is, if the basic motor circuit involving the primary motor cortex and the striatal-pallidal-thalamic loop were impaired, the LRP would be expected to be reduced even for response commands that are generated in the absence of significant competition from other potential response alternatives.
Both of the proposed explanations for the LRP impairment in patients with schizophrenia are viable alternatives given the nature of the disorder. Patients with schizophrenia demonstrate marked impairments in a variety of high-level executive and attentional control processes (Kerns, Nuechterlein, Braver, & Barch, 2008; Luck & Gold, 2008). However, schizophrenia is also characterized by persistent motor abnormalities, including impairments in facial expressions, eye movement control, dyskinesias, motor stereotypies, parkinsonism, and delayed motor development (Caligiuri, Lohr, & Jeste, 1993; Jones, Rodgers, Murray, & Marmot, 1994; Meehl, 1989; Mittal, Neumann, Saczawa, & Walker, 2008; Puri, Barnes, Chapman, Hutton, & Joyce, 1999; Walker, Lewis, Loewy, & Palyo, 1999). These motor abnormalities can be seen in medication-naïve patients and may be especially important in the diagnosis and pathogenesis of the disorder, given that they manifest much earlier in life (often in early childhood, see Walker, Savoie, & Davis, 1994) compared to other symptoms of the disorder. Because both the cognitive and motor impairments are key aspects of schizophrenia, it is important to determine whether the reduced LRP in this disease reflects cognitive control deficits or a more basic motor abnormality. Both types of impairment may implicate frontal-striatal circuitry, but most likely different components of this circuitry. That is, top-down control failures would suggest a prefrontal focus, whereas failures in activation of motor responses likely implicate different fronto-striatal-thalamic loops (Wiecki & Frank, 2010). Abnormalities in dopaminergic signaling could be implicated in both types of impairment.
The present study was aimed at addressing these two alternative hypotheses by manipulating the degree of competition between the two response alternatives. We compared the LRP under conditions that should produce weak versus strong activation of the incorrect response. If the LRP is reduced in schizophrenia because of a failure to suppress activation of the incorrect response alternative, then the LRP reduction should be observed primarily under conditions that would likely lead to substantial activation of the incorrect response. In contrast, the amount of suppression of the incorrect response should not play a major role in determining LRP amplitude under conditions in which there is little incorrect response activation. If the LRP reduction in schizophrenia reflects a direct failure to activate the correct response because of a more basic motor abnormality, then this reduction in patients relative to controls should be observed whether or not the task condition promotes strong activation of the incorrect response. In other words, if the deficit is the result of basic motor abnormalities, then the impairment should be observed irrespective of the competition from the incorrect response alternative.
Experiment 1
Experiment 1 manipulated the degree of response competition by taking advantage of highly learned stimulus-response mappings (based on Masaki, Wild-Wall, Sangals, & Sommer, 2005). Specifically, participants completed a compatible stimulus-response condition in which the words “Left” and “Right” were mapped onto left- and right-hand buttons, respectively. They also completed an incompatible stimulus-response condition, in which right-hand button presses were made to the stimulus “Left”, and left-hand button presses were made to the stimulus “Right.”
When the word “Left” appears in the compatible condition, there should be very little intrinsic activation of the incorrect right-hand response. A deficit in the ability to suppress the incorrect response should not affect performance or related brain activity in this condition—there is simply not much to suppress. However, when the word “Left” appears in the incompatible condition, this should lead to considerable automatic activation of the incorrect left-hand response because of everyday learning of this association. A deficit in the ability to suppress this incorrect response activation should lead to a substantial reduction in LRP amplitude. Thus, if the response selection impairment in patients with schizophrenia is caused by an overactivation of the incorrect response, the amount of the LRP decrement in the patients should be larger in the incompatible condition compared to the compatible condition (i.e., the patient LRP should be particularly small in the incompatible condition). In contrast, if the patients have a deficit in the activation of the correct response, the patient LRP should be equally reduced when they must activate the left-hand response for the word “Left” in the compatible condition or for the word “Right” in the incompatible condition. Complementary predictions hold for the right-hand responses.
Method
Participants
Twenty-four patients with schizophrenia and 22 control subjects were tested. In our group’s ERP studies of schizophrenia, we always exclude subjects who exhibit artifacts on greater than 50% of trials. Three patients and 1 control subject were eliminated for this reason, yielding a final sample of 21 subjects per group. The following descriptions of the patient and control groups reflect this final sample.
The patients were recruited from outpatient clinics at the Maryland Psychiatric Research Center and were studied during a period of relative clinical stability as indicated by clinical observation and stability of pharmacological treatment. All patients maintained their medication type and dosage for a minimum of 4 weeks prior to study. All patients met DSM-IV (American Psychological Association, 1994) diagnostic criteria for schizophrenia (N=18) or schizoaffective disorder (N=3). A consensus diagnosis was established with a best-estimate approach in which information from a Structured Clinical Interview for DSM Disorders (SCID; First, Spitzer, Miriam, & Williams, 2002) was supplemented with information from past medical records and from clinicians who have had contact with the patient. This information is typically presented at a diagnostic meeting where an additional patient interview is conducted by one of the authors (J.M.G.), with other trained clinicians present.
All of the patients were receiving antipsychotic medications. Clozapine was the antipsychotic used most commonly: alone (N=7), concurrently with risperidone (N=3), and concurrently with quetiapine (N=1). Of the remaining patients, three were receiving risperidone monotherapy, three were receiving olanzapine (two on monotherapy and one in combination with risperidone), one was receiving ziprasidone, and one was receiving quetiapine. One patient was receiving a combination of paliperidone and quetiapine, and one was receiving a combination of ziprasidone, fluphenazine, and quetiapine.
Healthy control subjects were recruited through a combination of random digit dialing, word of mouth, and newspaper advertisements. All controls were screened using the SCID and denied a lifetime history of psychosis, any active Axis I disorder, and recent substance abuse (within 6 months of testing). All participants denied a lifetime history of significant neurological conditions.
The demographic features of the groups are shown in Table 1. The groups were of similar age, race, and gender, but differed in completed years of education (t=3.78, p<0.01), an expectable finding given that the onset of the illness generally occurs in early adulthood. There was no significant difference between groups in parental years of education (t(39)=0.840, p=0.406). The patient group scored significantly lower than the control group on four cognitive ability measures: the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999; t(39)=2.45, p=0.019), the Wechsler Test of Adult Reading (Wechsler, 2001; t(39)=2.09, p=0.04), the MATRICS Consensus Cognitive Battery (Nuechterlein & Green, 2006; t(39)=3.88, p<.001), and the Wide Range Achievement Test: Fourth Edition (Wilkinson and Robertson, 2006; t(39)=2.05, p=0.05). Data for one healthy control was not available for the cognitive ability measures.
Table 1.
Demographic Features of the Final Patient and Control Samples in Experiment 1 (SD in parentheses)
| Patients with schizophrenia | Healthy controls | |
|---|---|---|
| Age | 37.7 (9.9) | 39.3 (9.7) |
| Male/female | 15/6 | 12/9 |
| Years of education | 12.6 (2.3) | 15.1 (1.9) |
| Ethnicity (Caucasian/African American/Other) | 11/10/0 | 8/13/0 |
| Wechsler Test of Adult Reading (WTAR) Standard Score* | 95.5 (15.3) | 105.7 (15.9) |
| Wechsler Abbreviated Scale of Intelligence (WASI) Full Scale- 2 Subtest Estimated IQ* | 97.9 (11.9) | 108.4 (15.4) |
Data missing for one healthy control
Stimuli and task
The stimuli were the words “Left” or “Right” presented at the center of an LCD monitor in black on a medium gray background. The words appeared within a continuously visible rectangle measuring 3.5 × 1.9 degrees of visual angle. The monitor was viewed at a distance of 70 cm. Each stimulus was presented for 200 ms, followed by an intertrial interval of 1200–1400 ms (rectangular distribution). Subjects were instructed to make a button-press response as quickly as possible for each stimulus with the index finger of the left or right hand on a Logitech gamepad. All subjects easily understood the instructions.
Subjects completed 2 blocks of testing in the compatible response mapping condition, in which the words “Left” and “Right” mapped onto the left and right response buttons, respectively. Subjects also completed 2 blocks of testing in the incompatible response mapping condition, in which the words “Left” and “Right” mapped onto the right and left response buttons, respectively. Each block contained 250 trials; a rest break was provided every 50 trials. It should be stressed that compatible and incompatible response mappings were tested in separate blocks. Subjects completed both blocks of one response mapping before switching to the alternate response mapping; the order of testing conditions was counterbalanced across subjects. Subjects completed a practice block of 20 trials immediately prior to each response mapping condition.
Recording and data processing procedures
The EEG was recorded from Ag/AgCl electrodes mounted in an elastic cap using a subset of the International 10/20 System sites (F3, Fz, F4, C3, Cz, C4, P3, Pz, P4, F7, F8, Fp1, Fp2, and left mastoid). The signals were recorded using a right mastoid reference electrode, and the signals were re-referenced offline to the average of the left and right mastoids (Luck, 2005; Nunez, 1981). The horizontal electrooculogram (HEOG) was recorded as the voltage between electrodes placed lateral to the external canthi and was used to measure horizontal eye movements. The vertical EOG was recorded from an electrode beneath the left eye and was used to detect blinks and vertical eye movements. Impedances were kept below 15KΩ. The EEG and EOG were amplified by a Neuroscan Synamps amplifier with a gain of 5,000, a bandpass of 0.05–100 Hz, and a 60-Hz notch filter, and the amplified signals were digitized at 500 Hz and averaged offline. Separate stimulus-locked and response-locked averages were computed, using a baseline of −200 to 0 ms for stimulus-locked averages and −800 to −600 ms for response-locked averages. Because latency measures can be highly sensitive to high-frequency noise, a low-pass filter was applied prior to the latency measures (Gaussian impulse response function, half-amplitude cutoff = 23.2 Hz, full width at half maximum = 18.8 ms). Trials with incorrect behavioral responses or electrophysiological artifacts were excluded prior to averaging using our standard procedures (Woodman & Luck, 2003). All signal processing procedures were performed by an author who was blind to the group membership of the participants (E.S.K.).
To isolate the LRP in each participant, we first created separate waveforms for the hemisphere that was contralateral to the response and the hemisphere that was ipsilateral to the response. We then created a contralateral-minus-ipsilateral difference waveform. This was done separately for each compatibility condition. LRP amplitude and latency were measured from the resulting difference waves. The LRP has a very focused scalp distribution and was therefore scored only at the lateral central sites (C3 and C4).
ERP measurement procedures
LRP amplitudes were measured as the mean amplitude in a given measurement window (see time windows in Table 2) relative to the baseline voltage specified above. The onset latency of the LRP was measured as the time point at which the voltage reached 50% of the peak amplitudei. All measurements were obtained from both stimulus-locked and response-locked averages. To avoid introducing bias that might occur if the measurement windows or electrode sites were chosen on the basis of the observed data, we used the same measurement windows and electrode sites as in our previous study of the LRP in schizophrenia (Luck et al., 2009).
Table 2.
Measurement windows
| Experiment 1 | Experiment 2 | |||
|---|---|---|---|---|
| Measure | Stimulus- Locked (ms) | Response- Locked (ms) | Stimulus- Locked (ms) | Response- Locked (ms) |
| Mean Amplitude | 300 – 600 | −200 – 100 | 200 – 500 | −300 – 0 |
| Onset Latency | 200 – 600 | −300 – 100 | -- | -- |
Statistical analysis procedures
Repeated measures analysis of variance (ANOVA) was used with a 2-tailed alpha level of .05 for all statistical tests, and probability values were adjusted when appropriate with the Greenhouse-Geisser epsilon correction for nonsphericity (Jennings & Wood, 1976). The analyses of behavioral data and the LRP measures included a between-subjects factor of group (patient vs. control) and a within-subjects factor of compatibility (compatible vs. incompatible).
Results
Behavior
The means of the median RTs and mean accuracy (percent correct) are shown in Table 3, along with a summary of the statistical analyses for these variables (F and p values will be given in the text only for tests not listed in the tables). Both groups were faster in the compatible condition than in the incompatible condition, leading to a significant main effect of compatibility. Patients were slower than control subjects in both conditions, leading to a significant group effect. Patients were somewhat more affected by compatibility than were controls, but the interaction between group and compatibility was not significant. Accuracy in both groups was highest in the compatible condition and lowest in the incompatible condition, leading to a significant main effect of compatibility. In addition, the patients were less accurate overall than the controls, leading to a significant group effect but no interaction between group and compatibility.
Table 3.
Experiment 1 behavioral results and stimulus- and response-locked LRP measures at C3/4 (standard errors in parentheses), along with F and p values for the statistical analyses.
| Patients | Controls | Statistics | ||||||
|---|---|---|---|---|---|---|---|---|
| Dependent Variable | Compatible | Incompatible | Compatible | Incompatible | Group df = 1,40 |
Compatibility df = 1,40 |
Group × Compatibility df = 1,40 |
|
| Accuracy (%) | 94.2 (.010) | 90.3 (.014) | 96.7 (.005) | 94.0 (.007) |
F = 6.1 p = .018 |
F = 31.9 p < .001 |
F = 1.47 p = .23 |
|
| Median RT (ms) | 475.2 (17.74) | 584.1 (30.93) | 419.5 (10.34) | 490.3 (17.92) |
F = 8.1 p = .007 |
F = 50.5 p < .001 |
F = 2.3 p = .14 |
|
| Stimulus- Locked | Amplitude (μV) | −.92 (.221) | −1.15 (.206) | −1.90 (.246) | −1.74 (.259) |
F = 84.8 p = .016 |
F = .08 p = .779 |
F = 2.8 p = .102 |
| Onset Latency (ms) | 303.8 (12.20) | 370.3 (23.51 | 272.0 (6.23) | 313.5 (9.60) |
F = 7.2 p = .01 |
F = 20.1 p < .001 |
F = 1.1 p = .31 |
|
| Response- Locked | Amplitude (μV) | −1.32 (.203) | −1.30 (.217) | −2.20 (.242) | −2.11 (.282) |
F = 7.0 p = .012 |
F = .31 p = .58 |
F = .11 p = .74 |
| Onset Latency (ms) | −156.0 (12.59) | −179.1 (16.07) | −149.4 (6.95) | −151.7 (6.28) |
F = 1.3 p = .269 |
F = 6.8 p = .013 |
F = 4.6 p = .039 |
|
ERP waveforms
Contralateral-minus-ipsilateral grand average difference waves are shown in Figures 1 and 2. Figure 1 shows the ERPs for each condition separately, with patient and control averages overlaid to facilitate comparison across groups. The same data are depicted in Figure 2, with the compatible and incompatible conditions overlaid separately for the patient and control groups to facilitate evaluation of the compatibility effect in each group. LRP measures and statistics are summarized in Table 3. As in previous studies (Karayanidis et al., 2006; Kieffaber et al., 2007; Luck et al., 2009; Mathalon et al., 2002), LRP amplitude was substantially reduced in patients compared to control subjects for both the stimulus-locked and response-locked averages.
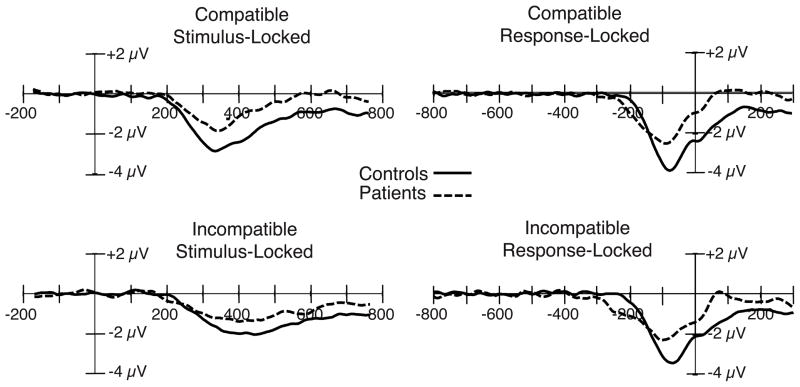
Figure 1.
Stimulus-locked (left) and response-locked (right) grand average ERP difference waveforms (contralateral-minus-ipsilateral) for the compatible and incompatible stimulus categories, collapsed across the C3 and C4 electrode sites, with patient and control waveforms overlaid. A digital low-pass filter was applied offline before plotting the waveforms shown here and in the subsequent figures (Gaussian impulse response function, half-amplitude cutoff = 23.2 Hz, full width at half maximum = 18.8 ms).
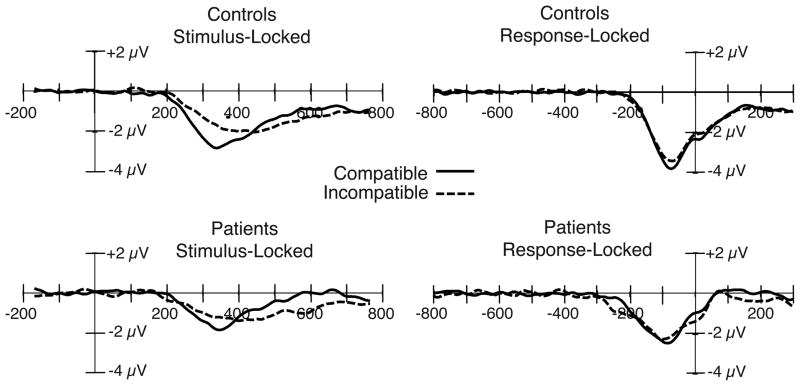
Figure 2.
Stimulus-locked (right) and response-locked (left) grand average ERP difference waveforms (contralateral-minus-ipsilateral) from the patient and control groups, collapsed across the C3 and C4 electrode sites, with compatible and incompatible stimulus categories overlaid.
Stimulus-locked LRP amplitude
In both compatibility conditions, LRP amplitude was substantially reduced in the patient group compared to the control group (see Figure 1, left), leading to a highly significant group main effect. The patient reduction in LRP amplitude was somewhat larger for the compatible condition (approximately 50%) than for the incompatible condition (approximately 30%), but neither the main effect of compatibility nor the compatibility × group interaction reached significance (see Figure 2, left). Note that the direction of this nonsignificant trend is opposite to what would be expected if the patient LRP impairment reflected a failure to inhibit the incorrect response (this would have produced a larger impairment on incompatible trials, in which inhibition of the incorrect response plays a larger role). To provide positive evidence of a patient LRP reduction in the compatible condition, a separate analysis was conducted just for this condition; the main effect of group was again significant (t(40) = 2.96, p = .005).ii
If the size of the compatibility effect is proportional to the overall size of the LRP, then a larger compatibility effect in patients might be masked by the smaller overall size of the LRP in this group. We therefore examined the proportional change in LRP amplitude across conditions. Specifically, for each participant, we computed the difference between the LRP amplitude on compatible trials and incompatible trials and divided that difference score by the sum of the LRP amplitude in the two conditions. This measure allowed us to assess the change in LRP amplitude across conditions, unconfounded by differences in the absolute size of the LRP. This contrast measure revealed no significant difference between patients and controls (t(40) = 1.28, p = .21), providing further evidence that the reduction in LRP amplitude in the patients is not modulated by compatibility.
Response-locked LRP amplitude
For the response-locked averages, LRP amplitude was again reduced in patients compared to controls (see Figure 1, right), leading to a highly significant group effect. The LRP amplitude did not differ significantly as a function of compatibility (see Figure 2, right), resulting in no main effect of compatibility. Furthermore, the reduction in patient LRP amplitude was similar across compatibility conditions (approximately 50%), leading to no compatibility × group interaction. The patient reduction was significant even when only the data from the compatible condition were considered (t(40) = −2.79, p = .008).iii Similarly, our proportional measure of LRP amplitude yielded no significant difference between patients and controls in the relative change in LRP amplitude across conditions (t(40) = 1.27, p = .21). These results are incompatible with the hypothesis that the reduction in the patient LRP amplitude is the result of difficulty overcoming the incorrect response alternative. That is, the reduction in LRP amplitude did not differ on the basis of the amount of competition from the incorrect response, despite the significant decrement in the LRP amplitude overall. Together, these results suggest that patients have a deficit in correct response activation.
Stimulus-locked LRP latency
In the stimulus-locked averages, LRP onset latency was earlier for the compatible condition than for the incompatible condition in both groups, leading to a significant main effect of compatibility on LRP onset latency. LRP onset latency was significantly delayed for the patient group relative to the control group, indicating that the patients required more time after the presentation of the stimulus to choose the appropriate response. The difference between patients and controls was larger for the incompatible condition (M = 57 ms) compared to the compatible condition (M = 32 ms), but the group × compatibility interaction did not reach significance. This is the same pattern that was observed for RT, for which the difference between patients and controls was greater in the incompatible condition (M = 94 ms) than in the compatible condition (M = 56 ms), but did not lead to a significant group × compatibility interaction.
Response-locked LRP latency
The response-locked averages shown in the right panels of Figures 1 and 2 make it possible to assess the amount of time that passed between the onset of the LRP (reflecting the onset of response preparation) and the execution of the button-press response. An earlier onset in the response-locked waveform reflects a greater amount of time between the onset of response preparation and the execution of the response. For the controls, LRP onset latency was nearly identical for the compatible and incompatible conditions (Figure 2, top right). However, the patients exhibited an earlier LRP onset latency (i.e., a longer time between the onset of the LRP and the execution of the response) for the incompatible condition compared to the compatible condition (Figure 2, bottom right), leading to a significant main effect of compatibility and a significant group × compatibility interaction. Although there was a tendency for patients to take more time than controls from the onset of the LRP to the execution of the button press (Figure 1, right), the overall group effect did not approach significance. Thus, the main difference in response-locked onset latency between patients and controls was a greater period between LRP onset and the response in the incompatible condition in patients. Although this might appear to be evidence that compatibility has a larger effect on response activation in patients than in controls, such a conclusion would be unwarranted. Multiple processing stages occur between the onset of response activation and the execution of the response, and the observed latency effect could reflect a slowing of any of these processes. The finding that LRP amplitude for patients was equally reduced on compatible and incompatible trials suggests that the latency effect reflects a later stage of processing and not the response activation process reflected by the LRP.
Discussion
This experiment replicated several key results from previous research. First, both RT and LRP onset latency were substantially slowed in the incompatible condition compared to the compatible conditions. This pattern was previously observed in young adults with a nearly identical task by Masaki et al. (2005). This effect was quite large in the present study for both RT (Cohen’s d = 1.30 for controls and 1.38 for patients) and stimulus-locked LRP onset latency (Cohen’s d = 0.94 for controls and 0.73 for patients), demonstrating that our manipulation of response competition was effective. Second, LRP amplitude was reduced by approximately 50% in patients relative to controls, accompanied by a significant slowing of stimulus-locked LRP onset latency. This replicates the pattern observed by Luck et al. (2009) using a similar task.
The main new contribution of the present experiment was the comparison of LRP amplitude for compatible and incompatible conditions. If the LRP reduction in patients reflects a failure to suppress activation of the incorrect response, then this LRP reduction should be apparent primarily in task conditions that lead to stronger activation of the incorrect response (i.e., in the incompatible condition). However, the patient LRP reduction was no larger in the incompatible condition than in the compatible condition, and the patient LRP amplitude was strongly reduced even in the compatible condition. In the compatible condition, it is unlikely that the presentation of the word “Left” created much activation of the right-hand response or that the word “Right” created much activation of the left-hand response, so the presence of a large and robust patient LRP reduction in the compatible condition provides positive evidence that the observed LRP reduction in patients does not require strong competition from the incorrect response. Moreover, even when the overall decrease in LRP amplitude in the patients was taken into account, the patients still did not show a significant effect of compatibility on the amplitude of the LRP.
One possible explanation for the presence of a patient LRP reduction in the compatible condition is that patients who completed the incompatible condition prior to the compatible condition had learned the “Left”-right and “Right”-left mapping, which then carried over into the compatible condition and activated the incorrect response. To assess this possibility, we examined the amplitude of the compatible LRP separately for the patients and controls who completed the compatible condition first. The patients showed a significant reduction in the amplitude of the compatible LRP compared to controls even in this subset of subjects (t(24)=2.44, p=.023), ruling out the possibility that the reduced LRP amplitude in the compatible condition was a consequence of carryover from the incompatible condition. Instead, it appears to reflect a relatively pure impairment in the ability to activate the correct response.
The conclusion that the patient LRP amplitude reduction is approximately equal for the compatible and incompatible conditions is based on the lack of an interaction between group and compatibility, and it is always important to carefully consider the strength of a conclusion that is based on a null result. Several factors make this conclusion quite strong in the present study. First, our manipulation of compatibility was quite effective, leading to a large effect size and highly significant RT, accuracy, and LRP effects in both patients and control subjects. Second, we obtained highly significant group differences in LRP amplitude and onset latency, closely replicating previous research. This indicates that we did not have unusual patient or control samples. Third, we observed a very large and significant patient reduction in LRP amplitude in the compatible condition. This provides positive evidence that patient LRPs are reduced even under conditions of minimal response competition. Finally, we observed a significant interaction between group and compatibility for response-locked LRP onset latency, demonstrating that we have the power to detect such interactions when they are present. The present results provide strong evidence against the hypothesis that the patient LRP reduction is caused by an impairment in the ability to suppress incorrect response activation. Instead, they are consistent with a failure to fully activate the correct response. As will be discussed later, this could reflect dysfunction of the LRP generator in primary motor cortex or dysfunction in the processes that feed into this generator.
It should be noted that we are not concluding that patients and controls exhibit equal sensitivity to response competition. Indeed, we observed nonsignificant trends toward larger effects of compatibility on RT and accuracy in patients than in controls, and response-locked LRP onset latency was significantly more influenced by compatibility in patients than in control subjects. Instead, we are drawing the more focused conclusion that the reduced LRP amplitude in patients does not reflect a failure to suppress incorrect response activation. Response competition may influence many other aspects of processing, either before or after the stage at which the LRP is generated (as evidenced by the increase in response-locked onset latency for the patients in the incompatible condition).
Experiment 2
The goal of Experiment 2 was to provide converging evidence from a different manipulation of response competition. We had previously conducted a modified version of the flanker task (Eriksen & Eriksen, 1974) to examine the error-related negativity (Morris, Holroyd, Mann-Wrobel, & Gold, submitted). The data from this experiment were re-analyzed in a manner that would test the current hypothesis. In this experiment, subjects pressed a left- or right-hand button to indicate whether a central triangle pointed leftward or rightward, and this central triangle was accompanied by flanking stimuli that were compatible (triangles pointing in the same direction as the target), incompatible (triangles pointing in the opposite direction to the target), or neutral (squares).
Previous research has demonstrated that incompatible flankers strongly activate the incorrect response, which may even lead to an initial period in which the incorrect response is more strongly activated than the correct response (Gratton, Coles, Sirevaag, Eriksen, & Donchin, 1988). This is demonstrated by an LRP that is initially positive in polarity (i.e., negative contralateral to the incorrect response hand), followed by a reversal to the typical negative polarity (i.e., negative contralateral to the correct response hand) prior to execution of the correct response. In other words, subjects initially activate the incorrect response, but this incorrect response activation is ultimately outweighed by activation of the correct response. The initial positivity is often termed the Gratton dip (because positive was plotted downward in the original study by Gratton et al., 1988).
If schizophrenia patients are impaired at suppressing activation of the incorrect response, such an impairment should be especially evident on incompatible trials, leading to a reduced LRP or a larger Gratton dip. However, if the reduced LRP in schizophrenia patients reflects a more general failure of response activation, the LRP should be reduced equivalently on compatible and incompatible trials.
Method
Participants
Twenty patients with schizophrenia and 15 control subjects were tested. Six patients and one control subject were eliminated from the sample because more than 50% of their trials contained artifacts, yielding a final sample of 14 subjects per group. The following descriptions of the patient and control groups reflect this final sample.
Patients and control subjects were recruited and assessed using the same methods as in Experiment 1. All patients met diagnostic criteria for schizophrenia (N=11) or schizoaffective disorder (N=3). All patients were taking antipsychotic medication. Risperidone was the most frequently prescribed antipsychotic, either alone (N=4) or in conjunction with aripiprizole (N=1). Fluphenazine was prescribed to 4 patients. Of the remaining patients, two were receiving olanzapine; aripiprazole, ziprasidone, and quetiapine were each used by a single patient. The demographic features of the groups are shown in Table 4. The groups were of similar age, race, gender, and education level. Both the patients and controls were older, on average, than the subjects tested in Experiment 1, which allows us to further assess the generality of the pattern of results observed in Experiment 1.
Table 4.
Demographic Features of the Final Patient and Control Samples in Experiment 2 (SD in parentheses)
| Patients with schizophrenia | Healthy controls | |
|---|---|---|
| Age | 50.0 (4.5) | 46.0 (11.3) |
| Male/female | 11/3 | 13/1 |
| Years of education | 13.5 (1.5) | 15.0 (2.4) |
| Ethnicity (Caucasian/African American/Other) | 8/5/1 | 6/8/0 |
Stimuli and task
All stimuli were presented on an LCD monitor in white on a black background. The monitor was viewed at a distance of 100 cm. The target stimuli were leftward or rightward pointing triangles, measuring .22 × .18 degrees of visual angle and presented in the center of the monitor. The flanker stimuli consisted of two items, one above and one below the target. These items were either triangles (all pointing rightward or all pointing leftward) or squares (.20 × .20 degrees). The flankers and targets were aligned vertically and spaced .29 degrees apart (center to center). To maximize their effect, the flankers were presented for 100 ms prior to target onset. The target was then presented for 70 ms, during which the flankers remained visible. This was followed by a blank response interval of 2000 ms. Feedback about task performance was then displayed for 1000 ms, followed by an intertrial interval of 1950 ms. Subjects were instructed to respond as quickly and accurately as possible by pressing a button on a hand-held response box with the hand that corresponded to the direction in which the target was pointing. To encourage fast and accurate responses, participants were penalized 2 cents for incorrect responses, rewarded 2 cents for correct responses, and penalized 5 cents for responses slower than 1100 ms (regardless of accuracy). All subjects easily understood the instructions.
Flankers either pointed in the same direction as the target stimulus (congruent trials), pointed in the opposite direction (incongruent trials), or were squares (neutral trials). Subjects completed 6 blocks of testing in which congruent, incongruent, and neutral trials were presented in a random order with equal frequency, with the constraint that no trial type was repeated on more than three consecutive trials. Each block contained 54 trials. Subjects completed a practice block of 24 trials before beginning the testing.
Recording, data processing, and analysis procedures
The recording, data processing, and analysis procedures were identical to those for Experiment 1, except as stated here. EEG was recorded from Ag/AgCl electrodes mounted in an elastic cap using a subset of the International 10/20 System sites (F3, Fz, F4, C3, Cz, C4, P3, Pz, P4, F7, F8, Fp1, Fp2, FC3, FCz, FC4, FT7, FT8, O1, Oz, O2, CP3, CPz, CP4, P7, P8, T7, T8, TP7, TP8, and left earlobe). The signals were recorded using a right earlobe reference electrode, and the signals were re-referenced offline to the average of the left and right earlobes (Luck, 2005; Nunez, 1981). The EEG and EOG were amplified by a Neuroscan Synamps amplifier with a gain of 500 and a bandpass of 0.1–100 Hz, and the amplified signals were digitized at 500 Hz and averaged offline.
The LRP was isolated identically to the method described for Experiment 1, time-locked to the target onset or to the response, separately for the compatible, neutral, and incompatible trials.
Results and Discussion
Behavior
The means of the median RTs and mean accuracy (percent correct) are shown in Table 5, along with a summary of the statistical analyses for these variables. RTs for both groups were fastest for the compatible trials, slower for the neutral trials, and slowest for the incompatible trials, leading to a significant main effect of compatibility. Patients were slower than control subjects in all three conditions, leading to a significant group main effect. The patient slowing was increased in the incompatible condition relative to the compatible and neutral conditions, leading to a significant group × compatibility interaction. This significant interaction is behavioral evidence that the patient group had difficulty resolving response competition. Accuracy in both groups was highest for the compatible condition, intermediate for the neutral condition, and lowest for the incompatible condition, leading to a significant main effect of compatibility. The patients were less accurate than control subjects in all three conditions, leading to a significant group effect. The effect of compatibility on accuracy was somewhat greater for patients than for controls, but the group × compatibility interaction did not reach significance.
Table 5.
Experiment 2 behavioral results and stimulus- and response-locked LRP measures at C3/4 (standard errors in parentheses), along with F, p, and epsilon (ε) values for the statistical analyses.
| Patients | Controls | Statistics | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dependent Variable | Time Window (ms) | Compatible | Neutral | Incompatible | Compatible | Neutral | Incompatible | Group df = 1,26 |
Compatibility df = 2,52 |
Group × Compatibility df = 2,52 |
| Accuracy (%) | -- | 97.9 (.007) | 96.5 (.011) | 83.0 (.022) | 99.2 (.004) | 98.7 (.006) | 89.6 (.023) |
F = 5.4 p = .029 |
F = 62.2 p < .001 ε = .57 |
F = 2.6 p = .112 ε = .57 |
| Median RT (ms) | -- | 449.7 (9.19) | 497.0 (10.96) | 568.4 (13.12) | 399.1 (12.47) | 444.4 (12.45) | 496.1 (15.05) |
F = 12.2 p = .002 |
F = 328.1 p < .001 ε = .68 |
F = 4.0 p = .024 ε = .68 |
| Stimulus- Locked Amplitude (μV) | 200 to 300 | −.41 (.206) | .15 (.272) | .53 (.351) | −.90 (.273) | .28 (.203) | 1.26 (.253) |
F = 2.16 p = .154 |
F = .382 p = .672 ε = .95 |
F = .537 p = .577 ε = .95 |
| 300 to 400 | −1.13 (.287) | −.49 (.272) | −.43 (.440) | −1.66 (.277) | −1.61 (.276) | −.71 (.384) | ||||
| 400 to 500 | −.41 (.269) | −.74 (.317) | −2.01 (.423) | −.42 (.323) | −1.31 (.358) | −2.43 (.353) | ||||
| Response- Locked Amplitude (μV) | −200 to −100 | −.83 (.404) | −1.32 (.433) | −1.65 (.469) | −1.41 (.307) | −.59 (.190) | −.61 (.316) |
F = .132 p = .719 |
F = .041 p = .960 ε = .97 |
F = 2.2 p = .121 ε = .97 |
| −100 to 0 | −1.07 (.385) | −1.87 (.342) | −2.63 (.475) | −2.45 (.266) | −2.57 (.214) | −3.11 (.326) | ||||
| 0 to 100 | .20 (.272) | −.10 (.548) | −.24 (.573) | −.49 (.250) | −.37 (.225) | −.54 (.373) | ||||
ERP waveforms
Contralateral-minus-ipsilateral grand average difference waves are shown in Figures 3 and 4. These figures follow the same organization as Figures 1 and 2, with Figure 3 presenting overlaid patient and control waveforms and Figure 4 presenting overlaid compatible, incompatible, and neutral waveforms within each group. LRP measures and statistics are summarized in Table 5.
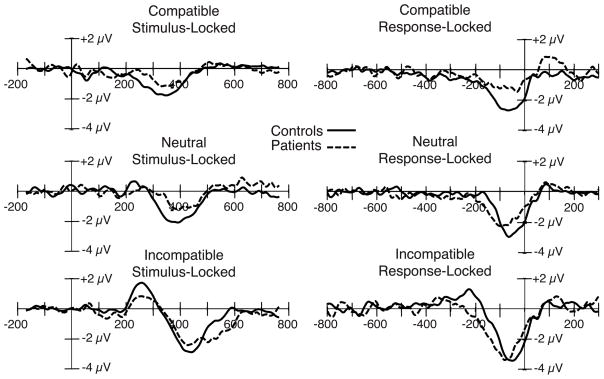
Figure 3.
Stimulus-locked (left) and response-locked (right) grand average ERP difference waveforms (contralateral-minus-ipsilateral) for the compatible, neutral, and incompatible stimulus categories, collapsed across the C3 and C4 electrode sites, with patient and control waveforms overlaid. Stimulus-locked waveforms were time locked to the onset of the target stimulus.
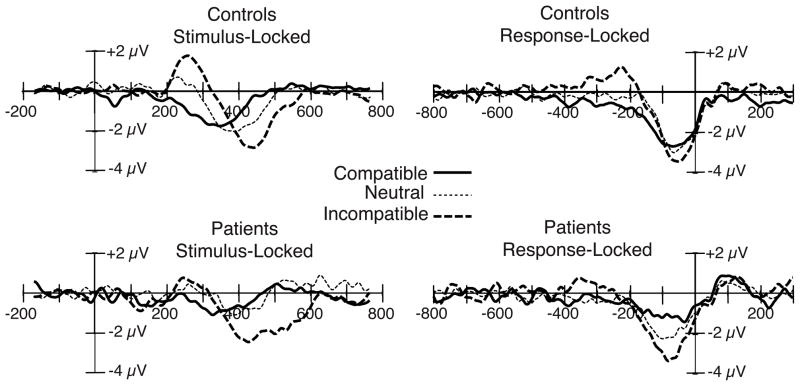
Figure 4.
Stimulus-locked (left) and response-locked (right) grand average ERP difference waveforms (contralateral-minus-ipsilateral) from the patient and control groups, collapsed across the C3 and C4 electrode sites, with compatible, neutral, and incompatible stimulus categories overlaid. Stimulus-locked waveforms were time locked to the onset of the target stimulus.
Stimulus-locked LRP amplitude
Because the flankers appeared 100 ms before the presentation of the target stimulus, the LRP in some conditions began earlier than in Experiment 1. Therefore, the time windows used to measure the components were shifted by 100 ms (see Table 2) relative to Experiment 1. Furthermore, the presence of the opposite-polarity Gratton dip in some conditions made it difficult to characterize the LRP with a measurement window lasting 300 ms. Therefore, we measured the patient and control waveform amplitudes in consecutive 100-ms periods within the measurement windows listed in Table 2, and we included time period as a factor in the ANOVA.
Indeed, we observed both a significant main effect of time (F(2,52) = 49.36, p < .001) and a significant time × compatibility interaction (F(4,104) = 33.48, p < .001). Additionally, the modulation of the LRP across time was somewhat different for the patients and controls, as evidenced by a marginally significant time × group interaction (F(2,52) = 3.33, p = .055). No other main effects or interactions reached significance (see Table 5).
To separately analyze the Gratton dip period and the subsequent periods, we computed separate ANOVAs for each of the 100-ms time periods. The initial portion of the waveform (from 200 to 300 ms) was positive in polarity for the incompatible condition (the Gratton dip) and negative in polarity for the compatible condition, resulting in a significant effect of compatibility (F(2,52) = 14.63, p < .001) but no effect of group (F(1,26) = .480, p = .494) and no group × compatibility interaction (F(2,52) = 2.24, p = .117). The LRP was most evident in the 300–400 ms time period, in which all conditions showed a negative polarity LRP (reflecting greater activation of the correct response than the incorrect response). In this time range, the effect of compatibility was only marginally significant (F(2,52) = 2.96, p = .076), likely due to the polarity change of the incompatible LRP; however, the patient LRP amplitude was reduced in all three conditions, resulting in a significant effect of group (F(1,26) = 6.61, p = .016) but no group × compatibility interaction (F(2,52) = .785, p = .433). For the subsequent portion of the waveform (400 to 500 ms), the compatibility effect re-emerged (F(2,52) = 17.49, p < .001); however, neither the effect of group (F(1,26) = 1.01, p = .325) nor the group × compatibility interaction (F(2,52) = .431, p = .633) reached significance. Thus, stimulus-locked LRP amplitude was reduced in patients for both compatible and incompatible trials, as in Experiment 1, but only during some time periods.
We also computed a proportional measure of the difference in LRP amplitude across conditions, as in Experiment 1. No significant difference between patients and controls was found for this proportional measure (t(26) = 1.73, p = .10), providing further evidence that the patient LRP amplitude reduction is not driven by an inability to overcome activation of the incorrect response.
Response-locked LRP amplitude
We completed a similar analysis for the response-locked data, measuring the LRP amplitude in consecutive 100-ms time periods and including a factor of time period in the ANOVA. Again, this analysis led to both a significant main effect of time (F(2,52) = 86.64, p < .001) and a significant time × compatibility interaction (F(4,104) = 16.75, p < .001). Additionally, the modulation of the LRP across time was different for the patients and controls, as evidenced by a significant time × group interaction (F(2,52) = 7.87, p = .002). No other main effects or interactions reached significance (see Table 5).
Separate ANOVAs were again conducted for each of the 100-ms time periods. A Gratton dip (positive voltage) was visible initially in the incompatible waveform for both groups of subjects; however, the Gratton dip was much smaller and somewhat earlier in the patients compared to the control subjects. This led to a significant main effect of condition (F(2,52) = 3.95, p = .029) in the −300 to −200 ms time period and a marginally significant group × compatibility interaction (F(2,52) = 3.19, p = .052) in the −200 to −100 ms time range. No other main effects or interactions were significant in this time range. After the Gratton dip, both groups exhibited a negativity for the −100 to 0 ms interval, consistent with activation of the correct response. The LRP amplitude in this range was largest for the incompatible condition, intermediate for the neutral condition, and smallest for the compatible condition for both groups, leading to a significant main effect of compatibility (F(2,52) = 6.80, p = .004). Furthermore, the patient LRP appeared smaller than the control LRP in all three conditions, leading to a significant main effect of group (F(1,26) = 6.22, p = .019) but no group × compatibility interaction (F(2,52) = 1.22, p = .299) for the −100 to 0 ms time period. Thus, as with the stimulus-locked LRP, the response-locked LRP was significantly reduced in patients on both compatible and incompatible trials, but only during some time periods. Moreover, an analysis of the proportional difference in LRP amplitude across conditions revealed no significant difference between patients and controls (t(26) = 1.43, p = .17).
As in Experiment 1, we found no evidence of a greater patient reduction in LRP amplitude on incompatible trials. If anything, the patient reduction was most prominent on compatible trials (although this interaction did not approach significance). The failure to find a greater decrement in patient LRP amplitude in the incompatible condition is contrary to the idea that the patients overactivated the incorrect response alternative. Moreover, the Gratton dip—which directly reflects activation of the incorrect response—tended to be smaller rather than larger in patients compared to control subjects. If anything, there was a trend toward a smaller patient deficit for incompatible trials than for compatible trials. This is the pattern that would be expected if patients have difficulty activating a given response, whether it is the correct response or the incorrect response. This provides converging evidence with Experiment 1 that the LRP decrement in patients reflects a failure of response activation rather than a failure to suppress competition.
Stimulus-locked and response-locked LRP latency
The presence of the Gratton dip made it difficult to measure the onset latency of the LRP. That is, the initial positivity in the incompatible condition made it difficult to determine whether a group difference in the onset latency of the LRP reflected an actual difference in onset latency of the LRP or rather reflected a difference in the size of the Gratton dip (which was significantly different between groups, see amplitude analysis above). Therefore, to avoid misinterpretations of the results, latencies will not be reported for this experiment.
General Discussion
This study sought to determine whether the reduced LRP amplitude exhibited by schizophrenia patients is related to a difficulty in overcoming competition from the incorrect response or whether it instead reflects impaired response activation. In other words, the present study was designed to determine whether the LRP impairment in schizophrenia is related to cognitive control deficits or to more basic motor abnormalities. Both in a task that manipulated highly learned stimulus-response mappings (Experiment 1) and a task that manipulated the compatibility of flanker stimuli with a central target (Experiment 2), patients with schizophrenia showed a significant attenuation of LRP amplitude compared to controls. These results are consistent with previous reports of reduced LRPs in schizophrenia (Karayanidis et al., 2006; Kieffaber et al., 2007; Luck et al., 2009; Mathalon et al., 2002).
The key new result of the present study is that the patient LRP reduction was no greater under conditions of high than low response competition, indicating that the LRP reduction in schizophrenia is not driven by a failure to overcome competition from the incorrect response. A substantial deficit was observed even when, for example, a left-hand response was given in response to the word “Left” or a right-hand response was given in response to a set of right-pointing arrows. It is implausible that the amount of response competition was as great on these compatible trials as it was on the incompatible trials. Moreover, even when the overall decrease in the size of the LRP in the patients was taken into account, the patients and controls were similarly affected by the compatibility manipulation. The finding of equivalent patient LRP reductions on compatible and incompatible trials provides strong evidence against the hypothesis that these reductions are a consequence of an impaired ability to suppress activation of the incorrect response. Instead, these results indicate that patients have an overall deficit in the activation of the correct response.
What might be causing the reduction in response activation in the patients? There are two primary mechanisms that could lead to this result. First, the cognitive processes responsible for sending the motor command for the selected response to motor cortex might be impaired, independent of the processes that precede the selection of this response, leading to decreased input to the motor cortex. Second, the neural circuit that generates the LRP in primary motor cortex might be impaired, which would lead directly to reduced LRP amplitude. This is plausible given that motor cortex—where the LRP is largely generated—operates through a dopamine-mediated loop with the basal ganglia. Either of these possibilities would be consistent with the observed reduction in response activation in patients with schizophrenia.
Although little is known about the specific motor abnormalities that occur in schizophrenia, we can look to other disorders that affect the motor system for insight into the proposed mechanisms of LRP impairment in schizophrenia. Specifically, the LRP has been measured in two disorders of the basal ganglia that result in significant disruptions of the motor system—Parkinson’s Disease and Huntington’s Disease. Surprisingly, this research has shown that despite significant disruption of the motor system in these disorders, resulting in the generation of abnormal movements (e.g., tremor, choreiform movements, etc.), no reduction in LRP amplitude is found in patients with Parkinson’s Disease (Praamstra, Plat, Meyer, & Horstink, 1999) or patients with Huntington’s Disease (Beste, Saft, Andrich, Gold, & Falkenstein, 2008) compared with healthy controls. Neither the disruption of GABAergic inhibition within the basal ganglia that characterizes Huntington’s Disease nor the disruption of dopaminergic inputs to the basal ganglia present in Parkinson’s Disease produces the pattern of decreased LRP amplitude that is exhibited in schizophrenia. This suggests that the LRP reduction observed in schizophrenia is not a result of a disruption of the basal ganglia portion of the motor loop. A similar dissociation between schizophrenia and Parkinson’s Disease has been observed in the context of sequence learning (Sullivan et al., 2001). However, we cannot rule out the possibility that some other form of disruption in the basal ganglia—such as the prefrontal-striatal disruption implicated in reward learning studies (Gold, Waltz, Prentice, Morris, & Heerey, 2008)—is responsible for the LRP reduction in schizophrenia.
It is worth considering whether the LRP abnormalities exhibited by the patients could be a consequence of either medication or nicotine use in the patients. Although we cannot conclusively exclude the possibility that antipsychotic medications are contributing to the LRP effect, the pervasive response-related abnormalities (such as RT slowing) in schizophrenia patients do not seem to be caused by antipsychotic medications (Medalia, Gold, & Merriam, 1988; Zahn, Pickar, & Haier, 1994). In addition, motor abnormalities were well documented in the preneuroleptic era (Reiter, 1926), and have been reliably observed in medication-naïve patients (Peralta & Cuesta, 2001). Such evidence is indirect, and it is important for future research to examine the LRP in unmedicated, first-episode, and prodromal patients to fully assess the possible relationship between antipsychotic medications and LRP abnormalities. It also seems unlikely that the increased use of nicotine in patients with schizophrenia is contributing to the LRP deficit, because nicotine increases the amplitude and decreases the onset latency of the LRP (Houlihan, Pritchard, Guy, & Robinson, 2002), contrary to the pattern shown by the patients in the present study. Thus, the reduced LRP in schizophrenia likely reflects a response activation impairment from the disease itself rather than pharmacological confounds.
It is important to note that the present study manipulated stimulus-response compatibility by disrupting highly learned stimulus-response mappings, such as the correspondence between a left-pointing arrow and a left-hand response. This allowed us to examine response preparation processes while minimizing other processes that may differ between patients with schizophrenia and controls, such as learning rate, working memory, etc. The LRP has also been examined in patients with schizophrenia in the context of more arbitrary stimulus-response mappings (Karayanidis, et al., 2006; Kieffaber, et al., 2007; Luck, et al., 2009), and these studies have found a similar reduction in LRP amplitude in patients. Therefore, the LRP reduction observed here generalizes to contexts in which arbitrarily defined stimulus-response mappings are used. However, it is also possible that deficits in the high-level cognitive processes that lead to selection of a response contribute to the patient LRP deficit in contexts with complex stimulus-response mappings that rely heavily on cognitive processes. Therefore, it is possible that a larger deficit would be observed on incompatible trials in these contexts. This remains an important issue for future research.
It is also worth asking whether the response-related effects in the present study may be driven by sensory processing deficits in the patients. This question was directly addressed in a previous study (Luck et al., 2009), in which both response-related processing and stimulus categorization were examined in patients with schizophrenia. As in the present study, a substantial reduction in LRP amplitude was found in the patients; however, the timing and amplitude of the rare-minus-frequent P3 difference wave was virtually identical in patients and controls, indicating that both groups were equally able to classify the stimuli as belonging to the rare or the frequency category. These results indicate that patients with schizophrenia were unimpaired in stimulus evaluation (for simple alphanumeric visual stimuli) but differed in response-related processing. Therefore, response-related deficits in patients with schizophrenia can exist independent of early sensory processing deficits.
Acknowledgments
This study was made possible by a grant from the National Institute of Mental Health (R01MH065034) and by University of Maryland General Clinical Research Center Grant Number M01- RR-16500. E.S.K. was supported by an NSF graduate research fellowship.
Footnotes
It should be noted that the absolute onset time of a component cannot be measured. That is, it is impossible to determine the precise moment that the component began, relative to the ongoing EEG. This is especially true for averaged waveforms, where the onset latency will reflect the trials with the earliest onsets rather than the average of the single-trial onset latencies (see Chapter 2 in Luck, 2005). Thus, the 50% peak latency measure used here provides a relative measure of onset latency rather than the absolute onset time. However, this is a particularly accurate and sensitive measure of onset time (see Kiesel, Miller, Jolicoeur, & Brisson, 2008; Luck et al., 2006; Miller, Patterson, & Ulrich, 1998).
To provide a finer analysis of the time course of LRP amplitude across conditions, we deconstructed the LRP amplitude measurement window into consecutive 100 ms time windows (300 to 400 ms, 400 to 500 ms, and 500 to 600 ms). Neither the compatibility × group nor the time × compatibility × group interactions reached significance (F(1,40) = 2.81, p = .102; and F(2,80) = .120, p = .840, respectively). Note that the trend in the compatibility × group interaction reflects a tendency for the group difference to be larger on the compatible trials, not on the incompatible trials.
As with stimulus-locked LRP amplitude, we deconstructed the measurement window for the response-locked amplitude into consecutive 100 ms time windows (−200 to −100 ms, −100 to 0 ms, and 0 to 100 ms). Neither the compatibility × group nor the time × compatibility × group interactions reached significance (F(1,40) = .123, p = .728; F(2,80) = 1.70, p = .196, respectively). As was observed in the stimulus-locked averages, the trend in the compatibility × group interaction reflects a tendency for the group difference to be larger on the compatible trials, not on the incompatible trials.
References
- American Psychological Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: 1994. [Google Scholar]
- Beste C, Saft C, Andrich J, Gold R, Falkenstein M. Stimulus-response compatibility in Huntington’s disease: A cognitive-neurophysiological analysis. J Neurophysiol. 2008;99:1213–1223. doi: 10.1152/jn.01152.2007. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Lohr JB, Jeste DV. Parkinsonism in neuroleptic-naive schizophrenic patients. Am J Psychiatry. 1993;150 doi: 10.1176/ajp.150.9.1343. [DOI] [PubMed] [Google Scholar]
- Coles MGH. Modern mind-brain reading: Psychophysiology, physiology and cognition. Psychophysiology. 1989;26:251–269. doi: 10.1111/j.1469–8986.1989.tb01916.x. [DOI] [PubMed] [Google Scholar]
- de Jong R, Coles MG, Logan GD, Gratton G. In search of the point of no return: The control of response processes. Journal of Experimental Psychology: Human Perception & Performance. 1990;16:164–182. doi: 10.1037/0096-1523.16.1.164. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16:143–149. doi: 10.3758/BF03203267. [DOI] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition with Psychotic Screen. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Gold J, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: A deficit in the representation of value. Schizophr Bull. 2008;34:835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Sirevaag EJ, Eriksen CW, Donchin E. Pre- and post-stimulus activation of response channels: A psychophysiological analysis. Journal of Experimental Psychology: Human Perception and Performance. 1988;14:331–344. doi: 10.1037/0096-1523.14.3.331. [DOI] [PubMed] [Google Scholar]
- Houlihan ME, Pritchard WS, Guy TD, Robinson JH. Smoking/nicotine affects the magnitude and onset of lateralized readiness potentials. Journal of Psychophysiology. 2002;16:37–45. doi: 10.1027//0269-8803.16.1.37. [DOI] [Google Scholar]
- Jennings JR, Wood CC. The e-adjustment procedure for repeated-measures analyses of variance. Psychophysiology. 1976;13:277–278. doi: 10.1111/j.1469-8986.1976.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344:1398–1402. doi: 10.1016/S0140-6736(94)90569-X. [DOI] [PubMed] [Google Scholar]
- Karayanidis F, Nicholson R, Schall U, Meem L, Fulham R, Michie PT. Switching between univalent task-sets in schizophrenia: ERP evidence of an anticipatory task-set reconfiguration deficit. Clin Neurophysiol. 2006;117:2172–2190. doi: 10.1016/j.clinph.2006.06.716. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Nuechterlein KH, Braver TS, Barch DM. Executive functioning component mechanisms and schizophrenia. Biol Psychiatry. 2008;64:26–33. doi: 10.1016/j.biopsych.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Kieffaber PD, O’Donnell BF, Shekhar A, Hetrick WP. Event related brain potential evidence for preserved attentional set switching in schizophrenia. Schizophrenia Research. 2007;93:355–365. doi: 10.1016/j.schres.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesel A, Miller J, Jolicoeur P, Brisson B. Measurement of ERP latency differences: A comparison of single-participant and jackknife-based scoring methods. Psychophysiology. 2008;45:250–274. doi: 10.1111/j.1469-8986.2007.00618.x. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An Introduction to the Event-Related Potential Technique. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Luck SJ, Fuller RL, Braun EL, Robinson B, Summerfelt A, Gold JM. The speed of visual attention in schizophrenia: Electrophysiological and behavioral evidence. Schizophrenia Research. 2006;85:174–195. doi: 10.1016/j.schres.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Gold JM. The construct of attention in schizophrenia. Biological Psychiatry. 2008;64:34–39. doi: 10.1016/j.biopsych.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Kappenman ES, Fuller RL, Robinson B, Summerfelt A, Gold JM. Impaired response selection in schizophrenia: Evidence from the P3 wave and the lateralized readiness potential. Psychophysiology. 2009;46:776–786. doi: 10.1111/j.1469-8986.2009.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki H, Wild-Wall N, Sangals J, Sommer W. The functional locus of the lateralized readiness potential. Psychophysiology. 2005;41:220–230. doi: 10.1111/j.1469-8986.2004.00150.x. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Fedor M, Faustman WO, Gray M, Askari N, Ford JM. Response-monitoring dysfunction in schizophrenia: an event-related brain potential study. Journal of Abnormal Psychology. 2002;111:22–41. doi: 10.1037/0021-843X.111.1.22. [DOI] [PubMed] [Google Scholar]
- Medalia A, Gold JM, Merriam A. The effects of neuroleptics on neuropsychological test results of schizophrenics. Archives of Clinical Neuropsychology. 1988;3:249–271. [PubMed] [Google Scholar]
- Meehl P. Schizotaxia revisited. Arch Gen Psychiatry. 1989;46:935–944. doi: 10.1001/archpsyc.1989.01810100077015. [DOI] [PubMed] [Google Scholar]
- Miller J, Patterson T, Ulrich R. Jackknife-based method for measuring LRP onset latency differences. Psychophysiology. 1998;35:99–115. doi: 10.1111/1469-8986.3510099. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Neumann C, Saczawa M, Walker E. Longitudinal progression of movement abnormalities in relation to psychotic symptoms in adolescents at high risk for schizophrenia. Arch Gen Psychiatry. 2008;65:165–171. doi: 10.1001/archgenpsychiatry.2007.23. [DOI] [PubMed] [Google Scholar]
- Morris S, Holroyd CB, Mann-Wrobel M, Gold J. Dissociation of response and feedback negativity in schizophrenia: Electrophysiological and computational evidence for a deficit in the representation of value. doi: 10.3389/fnhum.2011.00123. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez PL. Electric Fields of the Brain. New York: Oxford University Press; 1981. [Google Scholar]
- Peralta V, Cuesta MJ. Neuromotor abnormalities in neuroleptic-naive psychotic patients: Antecedents, clinical correlates, and prediction of treatment response. Comprehensive Psychiatry. 2001;52:139–145. doi: 10.1016/j.comppsych.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Plat EM, Meyer AS, Horstink MW. Motor cortex activation in Parkinson’s disease: Dissociation of electrocortical and peripheral measures of response generation. Mov Disord. 1999;14:790–799. doi: 10.1002/1531-8257(199909)14:5<790::AID-MDS1011>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Puri BK, Barnes TR, Chapman MJ, Hutton SB, Joyce EM. Spontaneous dyskinesia in first episode schizophrenia. J Neurol Neurosurg Psychiatry. 1999;66:76–78. doi: 10.1136/jnnp.66.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter PJ. Extrapyramidal motor-disturbances in dementia praecox. Acta Psychiatrica et Neurologica Scandinavica. 1926;1:287–309. doi: 10.1111/j.1600-0447.1926.tb11031.x. [DOI] [Google Scholar]
- Smulders FTY, Miller JO. Lateralized Readiness Potential. In: Luck SJ, Kappenman ES, editors. Oxford Handbook of Event-Related Potential Components. New York: Oxford University Press; in press. [Google Scholar]
- Sullivan EV, Fama R, Shear PK, Cahn-Weiner DA, Stein M, Zipursky RB, Pfefferbaum A. Motor sequencing deficits in schizophrenia: A comparison with Parkinson’s disease. Neuropsychology. 2001;15:342–350. doi: 10.1037/0894-4105.15.3.342. [DOI] [PubMed] [Google Scholar]
- Walker E, Lewis N, Loewy R, Palyo S. Motor dysfunction and risk for schizophrenia. Development and Psychopathology. 1999;11:509–523. doi: 10.1017/S0954579499002187. [DOI] [PubMed] [Google Scholar]
- Walker E, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophr Bull. 1994;20:441–451. doi: 10.1093/schbul/20.3.441. [DOI] [PubMed] [Google Scholar]
- Wiecki TV, Frank MJ. Neurocomputational models of motor and cognitive deficits in Parkinson’s disease. Progress in Brain Research. 2010;183:275–298. doi: 10.1016/S0079-6123(10)83014-6. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Serial deployment of attention during visual search. Journal of Experimental Psychology: Human Perception and Performance. 2003;29:121–138. doi: 10.1037/0096-1523.29.1.121. [DOI] [PubMed] [Google Scholar]
- Zahn TP, Pickar D, Haier RJ. Effects of clozapine, fluphenazine, and placebo on reaction time measures of attention and sensory dominance in schizophrenia. Schizophrenia Research. 1994;13:133–144. doi: 10.1016/0920-9964(94)90094-9. [DOI] [PubMed] [Google Scholar]