Abstract
There is increasing evidence that women’s physiological sexual arousal is facilitated by moderate sympathetic nervous system (SNS) activation. Literature also suggests that the level of SNS activation may play a role in the degree to which SNS activity affects sexual arousal. We provide the first empirical examination of a possible curvilinear relationship between SNS activity and women’s genital arousal using a direct measure of SNS activation in 52 sexually functional women. The relationship between heart rate variability (HRV), a specific and sensitive marker of SNS activation, and vaginal pulse amplitude (VPA), a measure of genital arousal, was analyzed. Moderate increases in SNS activity were associated with higher genital arousal, while very low or very high SNS activation was associated with lower genital arousal. These findings imply that there is an optimal level of SNS activation for women’s physiological sexual arousal.
Keywords: Sympathetic nervous system, female sexual arousal, vaginal pulse amplitude, heart rate variability
There is increasing evidence that sympathetic nervous system (SNS) activation plays a facilitatory role in women’s physiological sexual arousal. Several pharmacological studies have indicated this relationship using biochemical indices. For example, norephinephrine (NE) antagonists, such as clonidine, reduce genital sexual arousal in women (especially in states of heightened SNS (Meston, Gorzalka, & Wright, 1997), while NE agonists, such as ephedrine, appear to increase sexual arousal (Meston & Heiman, 1998). Considering that NE is the dominant neurotransmitter through which the SNS exerts its effects, these studies suggest that SNS activity plays a facilitatory role in female genital arousal. Other studies have found that biochemical markers of SNS activity sampled from women’s blood are increased immediately in anticipation of sex (Ende, Gertner, Hwang, & Kadi, 1989), during sexual arousal (Exton, et al., 2000) and after sexual intercourse (Jovanovic, 1971).
Similarly, activation of the SNS prior to exposure to sexual stimuli appears to boost subsequent sexual arousal. In one study, women who viewed an anxiety-provoking film immediately prior to exposure to a sexual film displayed higher physiological sexual arousal than women who viewed an emotionally neutral film prior to sexual stimuli (Palace & Gorzalka, 1990). Because anxiety is at least partially mediated through activation of the SNS, it is possible that anxiety-induced SNS augmentation triggers higher subsequent sexual arousal. Similarly, exercise - another SNS activator - prior to presentation of sexual stimuli is also associated with heightened genital arousal (Meston & Gorzalka, 1996a, 1996b).
These findings appear to contradict the conventional wisdom that women’s physiological sexual arousal is mediated primarily through the parasympathetic nervous system (PNS; for review, see Giuliano, Rampin, & Allard, 2002). In men, it has been suggested that there is PNS dominance during sexual arousal (Giuliano, Rampin, Benoit, & Jardin, 1995) whereas SNS activation facilitates penile detumescence, especially after sexual activity (Lange, Wincze, Zwick, Feldman, & Hughes, 1981). Moreover, because there is evidence of PNS activation during sexual arousal (e.g., Levin & Macdonagh, 1993), it is reasonable to believe that activating the opposing system (that is, the SNS) would inhibit sexual arousal. However, a likely possibility is that both elements of the autonomic nervous system (ANS) are active during the state of sexual arousal. In concert with localized activity of the PNS in the genital vasculature, generalized SNS activation may increase arousal in other key systems: increasing blood flow, enhancing focus and attention on erotic cues, decreasing food processing in the bowels (which in turn would increase blood availability for genital engorgement), and so on.
In some cases, SNS nerves and neurotransmitter systems (e.g., norephinephrine) act as a vasodilator (Sun, et al., 2002) although the precise mechanisms behind these processes are not well understood. Moreover, several studies have found that SNS outputs act differentially on genital tissue in the presence of sexual stimuli than without such stimulation (Giuliano, et al., 2002; Meston & Gorzalka, 1996b; Wagner & Levin, 1980). It is possible that during physiological sexual arousal, SNS activation of peripheral systems may interact with other processes, such as the relaxation of smooth muscle due to nitric oxide signaling (Kim, Min, Huang, Goldstein, & Traish, 2002), resulting in greater not lesser vaginal engorgement.
Moreover, the level of SNS activation may play an important role in the degree to which SNS activation facilitates physiological sexual arousal in women. Meston & Gorzalka (1996b) examined sexual arousal after three different post-exercise waiting periods (5 minute, 15 minute, 30 minute) and found that, relative to a no exercise baseline, women in the high SNS condition (i.e., 5 minute latency) had decreased genital arousal, while women in the moderate (15 minute latency) and low (30 minute latency) SNS conditions had increased genital arousal. The authors suggested that high SNS activation might inhibit sexual arousal while more moderate levels may play a facilitatory role. Similarly, in another study examining the relationship between level of SNS activation and sexual arousal, women with moderate anxiety demonstrated significantly higher genital arousal than women with either high or low anxiety (Bradford & Meston, 2006).
One of the major criticisms concerning the theorized relationship between moderate SNS activation and women’s physiological sexual arousal is that SNS activity is rarely directly measured in such studies (Meston, 2000), but rather inferred from indirect sources, such as the nature of the experimental manipulation (e.g., using a level of exercise known to increase SNS activity). Consequently, it is difficult to determine to what degree SNS activation is occurring. As such, there is to date no direct evidence for the claim that there is in fact an optimal level of SNS arousal. The present study was designed to address these prior study limitations by using direct measures of SNS activity and female genital arousal.
Specifically, we used heart rate variability (HRV), a common measure of SNS activity. When the body is at rest (and the PNS is dominant), the heart beats at slightly irregular intervals due to influences on the heart from respiratory sinus arrhythmia; when there is a fight-or-flight response (and the SNS becomes dominant), influences from respiration are superseded by deeper and faster breathing and the heart begins to beat at a faster, and more regular, rate (Kristal-Boneh, Raifel, Froom, & Ribak, 1995). The degree of variability in the intervals between separate heartbeats represents the relative dominance of the SNS or PNS at that moment. Thus, increases in HRV over time represent an increase in PNS activity, while decreases in HRV represent increases in SNS activity. That is, unlike increases in heart rate (HR), which could be due to either increased SNS activity or decreased PNS activity or some combination, decreases in HRV are thought to reflect the unique contribution of the SNS. Lower HRV is associated with a number of markers of SNS activation, such as muscle sympathetic nerve activity (Kingwell, et al., 1994), norephinephrine activity (Agelink, Ullrich, Baumann, Strum, & Majewski, 2002), and perceived stress (Vrijkotte, van Doornen, & de Geus, 2000). In addition to directly measuring the effects of SNS (and PNS) activation, HRV has the advantage of being a continuous measure, which allows one to observe effects across the full range of data rather than discrete high-medium-low groupings, as has been the case in prior studies of this nature.
In sum, we hypothesized that a moderate increase in SNS activation (that is, slight decreases in HRV) between baseline and presentation of sexual stimuli would be associated with maximal increases of physiological sexual arousal. In contrast, very large increases or decreases in SNS activation would be associated with lower levels of increase (or even decreases) in physiological sexual arousal. In other words, we hypothesized that there would be a curvilinear, or inverted U shaped, relationship between SNS activation and women’s sexual arousal. We also hypothesized that this relationship would be better captured with the use of HRV rather than HR, as HRV is more sensitive to changes in SNS activation (i.e., HRV would predict women’s sexual arousal above and beyond HR).
Method
Participants
Participants were selected from the control conditions of three experiments previously completed and published elsewhere (Hamilton, Fogle, & Meston, 2008; Harte & Meston, 2007; Harte & Meston, 2008). No participant’s data was used more than once. In each study, participants were volunteers recruited from the community using flyers and online advertisements that outlined the sexual nature of the experiments. Potential participants were screened over the phone to ensure they met the inclusion and exclusion criteria. Inclusion criteria were (i) at least 18 years of age, and (ii) currently sexually active. Exclusion criteria were as follows: (i) current self-reported sexual complaints within domains of sexual desire, sexual arousal, and/or sexual pain, and/or a history of treatment for sexual dysfunction; (ii) history of sexual trauma; (iii) use of medication known to affect sexual or vascular functioning, with the exception of hormonal contraceptives1; (iv) untreated Axis I disorders; (v) medical conditions likely to affect sexual arousal (e.g., diabetes); (vi) menopausal status; and (vii) other disqualifying criteria specific to the study. These were as follows. For the Harte and Meston (2008) study, which studied effects of oral nicotine administration, participants could not have a known allergy to nicotine, be a current smoker, be currently taking any medication that would adversely interact with nicotine (e.g., bupropion), have any dental health problems, or any medical condition that would preclude nicotine administration (e.g., cardiovascular disease). For the Hamilton et al. (2008) study, which studied the effects of exercise, participants could not have any injuries that would be exacerbated by exercising or any medical condition that would preclude exercising (e.g., chronic, untreated high blood pressure). In the Harte and Meston (2008) study, approximately 65% of potential participants met study criteria and were enrolled in the study following the initial phone screening; while exact participation rates for the other two studies were not recorded, rates of participation were approximately equal across all studies. In all cases, participants were informed they would be viewing sexually explicit material while their physiological and psychological responses were recorded, and given an option to withdraw at any time. No participant chose this option.
Study Design and Procedure
Although the studies included in this paper had different aims, the studies were all conducted within the same laboratory and study procedures of the sessions included herein were identical. In brief, testing sessions took place in a private, internally locked room with an intercom to allow participants to contact the researcher as needed. Participants were instructed in how to insert a vaginal photoplethysmograph and attach the wires for an electrocardiogram before the session began. After participants inserted the photoplethysmograph, a 5–10 minute adaptation recording was taken to assess baseline genital arousal. Participants then watched a 3-minute neutral film (nonsexual) followed by one of a set of 8.5–10 minute erotic films depicting heterosexual penile-vaginal intercourse. The 8.5-minute films (used in Harte & Meston, 2007; Harte & Meston, 2008) and the 10-minute film (used in Hamilton, et al., 2008)) were standardized in terms of duration of foreplay and oral sex but differed slightly in duration of vaginal intercourse. To standardize time frames across studies, we used the entire 3-min neutral section, but only the last 3 min of the erotic sections of the films; in all cases, this was a vaginal intercourse segment. The two film segments (neutral, erotic) within each film sequence were always presented in the same order. All participants gave informed consent, and were compensated between $15–50 (depending on the number of sessions completed for that study). The Institutional Review Board of the University of Texas approved all the protocols. The specific experimental procedures of the three studies can be found in their respective manuscripts (Hamilton, et al., 2008; Harte & Meston, 2007; Harte & Meston, 2008). Notably, one study (Hamilton, et al., 2008) recruited both women with and without sexual dysfunction; however, data from only women not reporting sexual dysfunction were used in the present sample. Due to randomization of participants into control sessions (included here) and experimental sessions (not included here), some of the participants (n = 20) had previously completed a similar testing session, while others were naïve to the procedures (n = 32).
Main Physiological Outcome Measures
Physiological Sexual Arousal
Vaginal pulse amplitude (VPA), an index of genital arousal, was measured throughout the neutral and erotic film stimuli with a vaginal photoplethysmograph (VPP). The VPP signal was sampled at 80 times/sec, low-pass filtered (0.5–30 Hz), digitized (40 Hz), and recorded using AcqKnowledge software (BIOPAC Systems, Inc., Santa Barbara, CA, USA) and a BIOPAC MP100WS hardware unit. Movement and other visually apparent artifacts in the VPP signal were removed manually before reducing VPA data. The standard procedure for removing these artifacts was as follows: In cases where potential artifacts were identified, investigators referred to study session logs regarding participant movement or other reasons to suspect artifact; entire files were visually scanned for any peak larger than the standard display window (−6 mV to +6mV, which captured the majority of true data points); the final list of data points were searched for peak-to-trough measures ±3 SD from the mean. These were then compared to the original file, and any data points that were surrounded by other data that otherwise fit the distribution (indicating randomness of the data point in question) were removed. Approximately 2–4 points per file were removed using these methods, representing a fraction of a percent of the total data considered.
VPA is thought to reflect the heartbeat-to-heartbeat changes in blood flow to the vagina and is specific to sexual arousal and not other forms of arousal such as fear (Laan, Everaerd, & Evers, 1995) or joy (Hamilton & Meston, 2010; Suschinsky, Lalumière, & Chivers, 2009). Larger amplitudes reflect higher vaginal engorgement and thus greater sexual arousal. We reduced the VPA data by computing the differences between peak and trough of each pulse (i.e., the amplitude of the wave for each beat).
Heart Rate Variability
Heart rate was measured throughout the neutral and erotic film stimuli at a rate of 80 samples/sec, using a three-lead electrocardiograph (ECG). The three leads were attached to disposable electrodes placed by the experimenter at the beginning of the session on the participant’s upper right chest, lower left chest, and right ankle2. The signal from the leads was collected in real time with AcqKnowledge software and a BIOPAC Systems ECG100 hardware unit. Movement artifacts were removed manually, and beat-to-beat (NN) intervals were collected using the AcqKnowledge peak finder function.
Different indices of HRV summarize this phenomenon in different ways, but all are fundamentally measures of the magnitude of variability in HR and thus are highly correlated with one another (Kautzner & Hnatkova, 1995). One straight-forward way to analyze HRV is to measure the standard deviation of NN intervals (SDNN) within a specified timeframe. We chose to use the SDNN as our primary index of HRV as it is thought to reflect the unique contribution of the SNS, rather than both increases in SNS mixed with decreases of PNS activity (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). However, to check for convergent validity of the model, we also calculated another measure of HRV, the pNN50. The pNN50 refers to the percent of NN intervals for which successive heartbeat intervals differed by at least 50 milliseconds. Thus, after collecting the NN intervals for each participant, we computed the SDNN and pNN50 for the 3 min neutral section and the last 3 min of the erotic sections using Kubios HRV Analysis Software (Biosignal Analysis and Medical Imagine Group, University of Kuopio, Kuopio, Finland). It should be noted that the two measures of HRV (SDNN and pNN50) were significantly correlated with each other, r = .42, p < 0.01. We also calculated heart rate (HR), which is the inverse of the mean NN interval, separately for the neutral and erotic sections.
Main Self-Report Outcome Measures
Subjective Sexual Arousal
Participant’s perceptions of their sexual arousal were measured before and after the films with the Film Scale (Heiman & Rowland, 1983). This 41-item questionnaire has four subscales: physical sexual arousal (11 items), mental sexual arousal (5 items), positive affect (12 items), and negative affect (13 items). The Film Scale is widely used in sexual health research (Brotto, Basson, & Luria, 2008; Middleton, Kuffel, & Heiman, 2008; van Anders, Brotto, Farrell, & Yule, 2009) and has demonstrated convergent validity with continuous measures of subjectively experienced sexual arousal (Rellini, McCall, Randall, & Meston, 2005). In the present study, only subjective perceptions of physical sexual arousal and mental sexual arousal were considered. The internal consistency of both the full scale and subscales in this sample were excellent (α = .90 – .96).
Sexual Functioning
Individual differences in sexual functioning were assessed with the Female Sexual Functioning Index (FSFI; Rosen, et al., 2000). This 19-item questionnaire assesses sexual functioning in six domains, including desire (2 items), arousal (4 items), vaginal lubrication (4 items), orgasm (3 items), satisfaction (3 items), and sexual pain (3 items). Scores on this measure have been validated to reliably discriminate between women with and without clinically significant sexual problems with a clinical cutoff score of 26.55, and has good divergent validity with measures of marital and relationship satisfaction (Wiegel, Meston, & Rosen, 2005). The internal consistencies of full scale and subscales in this sample were also excellent (α = .91–.93).
Data Reduction
VPA data were analyzed by taking the differences between peak and trough of each pulse wave. These VPA recordings, as well as HR and HRV data, were then averaged across neutral and erotic film sections. Data were standardized across participants by calculating percent change scores (VPA, HR and HRV: change of the average during the neutral film section to the average during the erotic film section; Film Scale: change from pre-film assessment to post-film assessment) for each outcome variable (HRV, VPA, Film Scale scores). This allowed for the direct comparison of participants on variables for which there is no zero point (VPA, HRV), or for which there were multiple measures (pre- and post-Film Scale scores). It should be noted that although percent change is a widely used method for comparing VPA across participants, there is some question as to what this index truly reflects, and if individual variation in tonic levels of vaginal blood flow are exaggerated in this method (see Hatch, 1979, for review).
For the purposes of this study, only participants with complete ECG data for both neutral and sexual conditions were considered. Thus, participants were removed from analyses if they had missing ECG data (e.g., due to leads becoming loose during testing) or if their ECG signal was too weak or noisy to provide accurate NN intervals for at least 80% of each condition (n = 2). Data from one other participant was also excluded because her percent change in HR between the neutral and sexual films was more than 3 standard deviations above the mean.
Statistical Analyses
All dependent variables were checked for normality using Shapiro–Wilk tests with p <0.05 denoting a normality violation. Analysis of variance (ANOVA) tests were used to confirm participants from different studies were comparable on major variables. Separate linear regression analyses were conducted for each outcome variable (percent change in VPA, Film Scale mental and physical arousal subscales), with a squared term to model the proposed curvilinear relationship between percent change in HRV and sexual arousal. To test the specificity of HRV as a predictor of women’s genital arousal, we conducted another regression, controlling for HR (and the squared HR term) in the first step and entering HRV (and the squared HRV term) into the second step, with genital arousal as the criterion. In this case, the criterion of interest was the R-squared change from the first to the second step. All analyses were performed using SPSS statistical software version 18.0 (SPSS Inc., Chicago, IL, USA). A two-tailed p < 0.05 was considered statistically significant for all analyses.
Results
Sample Characteristics
The final sample comprised 52 women, aged 18–39 (M = 22.1, SD = 4.3). These women were primarily single (83%) and Caucasian (60%), and all participants were sexually functional as measured by the FSFI (M= 29.4, SD = 3.9; with no scores below clinical cutoff). Characteristics of the participant sample are presented in Table 1. There were no significant differences between studies with respect to any of the demographic or experimental variables. Summary statistics for all experimental variables are presented in Table II.
Table 1.
Participant characteristics (N=52)
| M | SD | |
|---|---|---|
| Age (years) | 22.1 | 4.3 |
| Education (years) | 11.4 | 4.7 |
| FSFI - total score | 29.4 | 3.9 |
|
| ||
| n | % | |
|
| ||
| Ethnicity | ||
| Caucasian | 31 | 60.0 |
| Hispanic | 9 | 17.3 |
| Asian | 5 | 9.6 |
| Other/Missing | 7 | 13.5 |
| Relationship status | ||
| Single/dating | 43 | 82.7 |
| Other | 9 | 17.3 |
| Sexual Identity | ||
| Exclusively heterosexual | 37 | 71.2 |
| Mostly heterosexual | 12 | 23.1 |
| Bisexual | 3 | 5.8 |
| Current oral contraceptive use | ||
| Yes | 27 | 51.9 |
| No | 24 | 46.2 |
| Data missing | 1 | 1.9 |
Abbreviations: FSFI = Female Sexual Function Index.
Table II.
Summary statistics for experimental variables
| Baseline | Erotic | Percent change | ||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| SDNN (ms) | 59.5 | 22.8 | 52.6 | 19.8 | −8.6 | 21.7 |
| pNN50 (%) | 25.7 | 16.3 | 23.5 | 16.2 | −12.5 | 36.7 |
| HR (bpm) | 77.2 | 11.6 | 78.1 | 11.6 | 1.5 | 8.0 |
| VPA (mV) | 1.6 | 0.4 | 2 | 0.7 | 22.6 | 20.2 |
|
| ||||||
| Difference scores | ||||||
| M | SD | |||||
|
| ||||||
| Film Scale | ||||||
| Perceived physical arousal | 1.6 | 1.3 | ||||
| Mental sexual arousal | 1.6 | 1.4 | ||||
Abbreviations: HR = heart rate; VPA = vaginal pulse amplitude; pNN50 = percent of NN intervals for which successive heartbeat intervals differed by at least 50 milliseconds; SDNN = standard deviation of NN intervals.
HRV Model of Sexual Arousal
The model that included percent change in SDNN (and the squared SDNN term, which is necessary to model the single-bended curve) as predictors was significant in predicting percent change in VPA (i.e., genital arousal), R2 = .18, f2 = .22, F(2, 51) = 5.01, p < 0.05. The model was not significant, however, in predicting percent change in subjectively perceived physical arousal scores, R2 =.01, f2 = . 01, F(2, 49) = .19, p = ns, or mental sexual arousal scores, R2 =.04, f2 = . 04, F(2, 47) = .78, p = ns. Follow-up analyses indicated that there was low concordance between participant’s genital arousal and ratings of their arousal: that is, the correlations between change in VPA and change in perceived physiological arousal (r = .03, p = ns) and between change in VPA and change in mental sexual arousal (r = −.01, p = ns) were both non-significant.
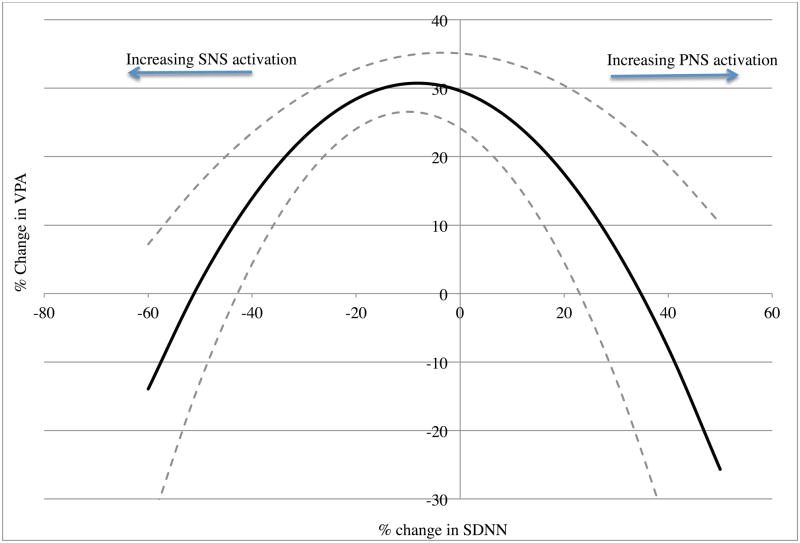
The model that included percent change in pNN50 (and the squared pNN50 term) as predictors was also significant in predicting percent change in VPA, R2 = .12, f2 = .14, F(2, 49) = 3.22, p <.05. In both cases, visual inspection of the predicted values confirmed that moderate increases in SNS (i.e., small positive percent change in HRV) were associated with the highest levels of VPA, while large changes in SNS (either large positive or large negative changes in HRV) were associated with the lowest levels of VPA (see Figure 1).
Figure 1.
Relationship between HRV and genital arousal. Note: Predicted model is in bold; dashed lines represent one standard error from the mean.
HRV vs. HR in Predicting Sexual Arousal
The model which included percent change in HR (and its squared term) in the first step and percent change in SDNN in the second step was again significant in predicting percent change in VPA, R2 = .22, f2 = .22, F(4, 51) = 3.19, p < 0.05. Moreover, the R-squared change between the first and second step was significant, R2 = .22, f2 = .14, F(2, 47) = 6.37, p < 0.01, indicating that the HRV terms predicted VPA above and beyond the effects of HR alone.
Discussion
This study was the first to report a curvilinear relationship between SNS activity and women’s sexual arousal using a direct measure of SNS activation. Results indicated that moderate increases in SNS activity were associated with higher physiological sexual arousal responses, while both very low and very high SNS activation were associated with lower physiological sexual arousal. Women’s perceptions of their physiological sexual arousal, as well as their mental sexual arousal, did not display the same curvilinear pattern.
It is important to note the non-linear nature of the relationship between SNS activation and women’s genital arousal. It is perhaps not surprising that the optimal level of SNS activation for facilitating sexual arousal is moderate: the most obvious analogy is that of the well-known Yerkes-Dodson curve (Yerkes & Dodson, 1908), later revised by Hebb (1955), in which maximal performance is achieved at moderate levels of stress or arousal. In both cases, low levels of SNS activation do not adequately prime the body for activity, while very high levels of SNS activation elicit protective systems (e.g., release of cortisol) and extinguish positive affect and motivation for the activity. Moreover, the curvilinear relationship may help explain why previous work using direct stimulation of SNS pathways to female genitals found that SNS activation is associated with inhibited genital arousal (S. Sato, Hayashi, & Garfield, 1989; Y. Sato, Hotta, Nakayama, & Suzuki, 1996): SNS activation that was too high would be just as detrimental as activation that is lower or inhibited. Blood flow to the vagina in these cases would be reduced, as blood would be diverted to major muscle groups to prepare for action (fight-or-flight). It should be noted that the changes in HRV (and thus, SNS activity) observed in this sample were relatively subtle, and thus replication at states of higher SNS activation (that is, greater variance of HRV) are necessary to confirm the model holds in other ranges of arousal.
Heart rate variability predicted levels of physiological sexual arousal above and beyond the effects of heart rate. These findings underscore the fact that while the two measures are similar, there are important differences; namely, HRV is more sensitive to changes in autonomic nervous system activity than HR (Mangin, et al., 1998). This may be because while HRV is largely dependent on the two components of the autonomic nervous system, HR may be under the influence of multiple physiological control systems such as the endocrine system (Malik & Camm, 1993).
Not all measures of sexual arousal, namely women’s perceptions of their physiological arousal and mental sexual arousal, followed a curvilinear relationship. This is in line with previous studies demonstrating low concordance between women’s subjective ratings and laboratory measures of sexual arousal. Low concordance is not unusual in women, and may reflect a wide variety of factors, above and beyond physical arousal, which influence women’s mental sexual arousal (for review, see Chivers, Seto, Lalumière, Laan, & Grimbos, 2010). While the orientation to the experimental sessions included in this analysis were virtually identical, it is possible that different orientation to the experiments as a whole may have introduced error variance to our measure of subjective arousal. For example, Chivers et al., (2010) noted that in studies recruiting women with and without sexual dysfunction, such as one of the studies in our sample, concordance among women without dysfunction is notably low. Another possible explanation is that our measures of subjectively perceived sexual arousal were not as sensitive as the measures of HRV and genital arousal, and thus we did not have enough power to detect an effect. Specifically, our measure of subjective arousal was pre-post and not continuously collected as were HRV or genital arousal measures. If so, presumably such effects would be smaller than those that were detected. Based on these findings, then, it should not be assumed that moderate increases in SNS activation would result in increases in subjectively experienced sexual arousal.
Our results are somewhat limited in their generalizability, as our sample did not include women who were post-menopausal. To that end, at least one study has indicated that SNS facilitation of sexual arousal may be limited to pre-menopausal women (Brotto & Gorzalka, 2002). As with all studies on sexuality, it is possible that participants uncomfortable with study procedures self-selected out of our sample. Women who decline participation in sexual psychophysiology studies tend to have less sexual experience and more sexual inhibition than those who volunteer (Morokoff, 1986; but see also Woo, Brotto, & Yule, 2010 for contrary results). It is thus possible that our findings would not generalize to women who would not volunteer for sex research. It is also worthy to note that, because the post-hoc nature of the analyses, we were not able to control for many potential third variables (e.g., hormonal changes), and thus we cannot rule out the possibility that SNS facilitation of sexual arousal is itself mediated by other factors. It is also possible that there were practice effects in those participants for whom this was not the first testing session. While it has been shown that women’s genital arousal is not susceptible to habituation effects with the number of erotic presentations used in the current study (Laan & Everaerd, 1995), it is possible that habituation played some small role in the heart rate variability measures. Finally, because of the between-subjects nature of the data, we do not know that the same relationship between SNS activity and genital arousal would be found in the same woman. That is, based solely on these data, we do not know if increasing SNS would improve sexual arousal; we can only conclude that a higher measured SNS level is associated with higher sexual arousal. To that end, individual differences in the time to maximum arousal may have introduced variance in the level of arousal sampled in the last three minutes of the sexual film; that is, some women may have been at maximum arousal while others were not. Presumably, however, women not at their maximum level of genital arousal would also be those who demonstrated non-optimal levels of SNS activity. Despite these limitations, these findings indicate that SNS activation may play a relatively more important role for female physiological sexual arousal than has been previously assumed.
Acknowledgments
The first, second and fourth authors are supported by award numbers F31MH085416, F31 DAO26276, and R01 HD51676 respectively, from the National Institute of Health (NIH). The third author was supported by a doctoral fellowship from the Natural Sciences and Engineering Research Council of Canada. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the NSERC.
Footnotes
The use of oral contraceptives has been implicated in decreased sexual desire functioning (Sanders, Graham, Bass, & Bancroft, 2001). However, it does not appear that oral contraceptive use significantly impacts measures of vaginal sexual arousal (Laan, Everaerd, van Bellen, & Hanewald, 1994) nor heart rate variability (Schueller, Feuring, Sharkova, Grimm, & Christ, 2006).
The setup for the ECG leads was as recommended by the hardware manufacturer to prevent electrical interference with the other data collection units connected to the same system (e.g., the VPP) and to avoid putting electrodes on moving limbs (e.g., arms, which move to fill out surveys). However, the ECG signal was robust (Optimal ground placement, n.d.).
Contributor Information
Tierney Ahrold Lorenz, Email: tierney.lorenz@gmail.com, Department of Psychology, University of Texas at Austin, Austin, TX, USA.
Christopher B. Harte, Email: charte@mail.utexas.edu, Department of Psychology, University of Texas at Austin, Austin, TX, USA.
Lisa Dawn Hamilton, Email: ldhamilton@mta.ca, Department of Psychology, Mount Allison University, Sackville, New Brunswick, Canada.
Cindy M. Meston, Email: meston@psy.utexas.edu, Department of Psychology, University of Texas at Austin, Austin, TX, USA.
References
- Agelink M, Ullrich H, Baumann B, Strum S, Majewski T. Effects of reboxetine, a selective norepinephrine reuptake inhibitor, on sympathetic and parasympathetic outflow to the heart: Preliminary data. Psychopharmacologia. 2002;163:151–156. doi: 10.1007/s00213-002-1146-7. [DOI] [PubMed] [Google Scholar]
- Bradford A, Meston C. The impact of anxiety on sexual arousal in women. Behaviour Research and Therapy. 2006;44:1067–1077. doi: 10.1016/j.brat.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotto L, Basson R, Luria M. A mindfulness based group psychoeducational intervention targeting sexual arousal disorder in women. Journal of Sexual Medicine. 2008;5:1646–1659. doi: 10.1111/j.1743-6109.2008.00850.x. [DOI] [PubMed] [Google Scholar]
- Brotto L, Gorzalka B. Genital and subjective sexual arousal in postmenopausal women: Influence of laboratory-induced hyperventilation. Journal of Sex and Marital Therapy. 2002;28:39–53. doi: 10.1080/00926230252851186. [DOI] [PubMed] [Google Scholar]
- Chivers ML, Seto MC, Lalumière ML, Laan E, Grimbos T. Agreement of sef-reported and genital measures of sexual arousal in men and women: A meta-analysis. Archives of Sexual Behavior. 2010;39:5 – 56. doi: 10.1007/s10508-009-9556-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ende N, Gertner S, Hwang S, Kadi R. Measurements of postcoital sympathetic activity in females by means of vanillylmandelic acid. Hormones and Behavior. 1989;23:150–156. doi: 10.1016/0018-506x(89)90081-0. [DOI] [PubMed] [Google Scholar]
- Exton N, Chau Truong T, Exton M, Wingenfeld S, Leygraf N, Saller B, et al. Neuroendocrine response to film-induced sexual arousal in men and women. Psychoneuroendocrinology. 2000;25:187–199. doi: 10.1016/s0306-4530(99)00049-9. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Rampin O, Allard J. Neurophysiology and pharmacology of female genital sexual response. Journal of Sex and Marital Therapy. 2002;28:101–121. doi: 10.1080/00926230252851230. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Rampin O, Benoit G, Jardin A. Neural control of penile erection. Urologic Clinics of North America. 1995;22:747 – 766. [PubMed] [Google Scholar]
- Hamilton LD, Fogle EA, Meston CM. The roles of testosterone and alpha-amylase in exercise-induced sexual arousal in women. Journal of Sexual Medicine. 2008;5:845 – 853. doi: 10.1111/j.1743-6109.2007.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton LD, Meston CM. The role of salivary cortisol and DHEA-S in response to sexual, humorous, and anxiety-inducing stimulis. Hormones and Behavior. 2010;59:765 –771. doi: 10.1016/j.yhbeh.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte C, Meston C. Gender comparisons in the concordance between physiological and subjective sexual arousal. Paper presented at the International Society for the Study of Women’s Sexual Health; Orlando, FL. 2007. [Google Scholar]
- Harte C, Meston C. The inhibitory effects of nicotine on physiological sexual arousal in nonsmoking women: Results from a randomized, double blind, placebo controlled, cross over trial. Journal of Sexual Medicine. 2008;5:1184–1197. doi: 10.1111/j.1743-6109.2008.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch JP. Vaginal photoplethysmography: Methodological considerations. Archives of Sexual Behavior. 1979;8:357–374. doi: 10.1007/BF01541879. [DOI] [PubMed] [Google Scholar]
- Hebb D. Drives and the CNS (conceptual nervous system) Psychological Review. 1955;62:243 – 254. doi: 10.1037/h0041823. [DOI] [PubMed] [Google Scholar]
- Heiman J, Rowland D. Affective and physiological sexual response patterns: The effects of instructions on sexually functional and dysfunctional men. Journal of Psychosomatic Research. 1983;27:105–116. doi: 10.1016/0022-3999(83)90086-7. [DOI] [PubMed] [Google Scholar]
- Jovanovic U. The recording of physiological evidence of genital arousal in human males and females. Archives of Sexual Behavior. 1971;1:309–320. doi: 10.1007/BF01638059. [DOI] [PubMed] [Google Scholar]
- Kautzner J, Hnatkova K. Correspondence of different methods for heart rate variability measurement. In: Malik M, Camm AJ, editors. Heart Rate Variability. Armonk, NY: Futura; 1995. pp. 119–126. [Google Scholar]
- Kim N, Min K, Huang Y, Goldstein I, Traish A. Biochemical and functional characterization of alpha-adrenergic receptors in the rabbit vagina. Life Sciences. 2002;71:2909–2920. doi: 10.1016/s0024-3205(02)02162-8. [DOI] [PubMed] [Google Scholar]
- Kingwell B, Thompson J, Kaye D, McPherson G, Jennings G, Esler M. Heart rate spectral analysis, cardiac norepinephrine spillover, and muscle sympathetic nerve activity during human sympathetic nervous activation and failure. Circulation. 1994;90:234 –240. doi: 10.1161/01.cir.90.1.234. [DOI] [PubMed] [Google Scholar]
- Kristal-Boneh E, Raifel M, Froom P, Ribak J. Heart rate variability in health and disease. Scandinavian Journal of Work, Environment and Health. 1995;21:85–95. doi: 10.5271/sjweh.15. [DOI] [PubMed] [Google Scholar]
- Laan E, Everaerd W. Habituation of female sexual arousal to slides and film. Archives of Sexual Behavior. 1995;24:517 – 541. doi: 10.1007/BF01541832. [DOI] [PubMed] [Google Scholar]
- Laan E, Everaerd W, Evers A. Assessment of female sexual arousal: Response specificity and construct validity. Psychophysiology. 1995;32:476 – 485. doi: 10.1111/j.1469-8986.1995.tb02099.x. [DOI] [PubMed] [Google Scholar]
- Laan E, Everaerd W, van Bellen G, Hanewald G. Women’s sexual and emotional responses to male- and female-produced erotica. Archives of Sexual Behavior. 1994;23:153–169. doi: 10.1007/BF01542096. [DOI] [PubMed] [Google Scholar]
- Lange JD, Wincze JP, Zwick W, Feldman S, Hughes K. Effects of demand for performance, self-monitoring of arousal, and increased sympathetic nervous system activity on male erectile response. Archives of Sexual Behavior. 1981;10:433 – 464. doi: 10.1007/BF01541436. [DOI] [PubMed] [Google Scholar]
- Levin R, Macdonagh R. Increased vaginal blood flow induced by implant electrical stimulation of sacral anterior roots in the conscious woman: A case study. Archives of Sexual Behavior. 1993;22:471–475. doi: 10.1007/BF01542560. [DOI] [PubMed] [Google Scholar]
- Malik M, Camm A. Components of heart rate variability: What they really mean and what we really measure. The American Journal of Cardiology. 1993;72:821 – 822. doi: 10.1016/0002-9149(93)91070-x. [DOI] [PubMed] [Google Scholar]
- Mangin L, Swynghedauw B, Benis A, Thibault N, Lerebours G, Carrè F. Relationships between heart rate and heart rate variability: study in conscious rats. Journal of Cardiovascular Pharmacology. 1998;32:601 – 607. doi: 10.1097/00005344-199810000-00012. [DOI] [PubMed] [Google Scholar]
- Meston C. Sympathetic nervous system activity and female sexual arousal. American Journal of Cardiology. 2000;86:30 – 34. doi: 10.1016/s0002-9149(00)00889-4. [DOI] [PubMed] [Google Scholar]
- Meston C, Gorzalka B. Differential effects of sympathetic activation on sexual arousal in sexually dysfunctional and functional women. Journal of Abnormal Psychology. 1996a;105:582–591. doi: 10.1037//0021-843x.105.4.582. [DOI] [PubMed] [Google Scholar]
- Meston C, Gorzalka B. The effects of immediate, delayed, and residual sympathetic activation on sexual arousal in women. Behaviour Research and Therapy. 1996b;34:143–148. doi: 10.1016/0005-7967(95)00050-x. [DOI] [PubMed] [Google Scholar]
- Meston C, Gorzalka B, Wright J. Inhibition of subjective and physiological sexual arousal in women by clonidine. Psychosomatic Medicine. 1997;59:399 – 407. doi: 10.1097/00006842-199707000-00010. [DOI] [PubMed] [Google Scholar]
- Meston C, Heiman J. Ephedrine-activated physiological sexual arousal in women. Archives of General Psychiatry. 1998;55:652 – 656. doi: 10.1001/archpsyc.55.7.652. [DOI] [PubMed] [Google Scholar]
- Middleton L, Kuffel S, Heiman J. Effects of experimentally adopted sexual schemas on vaginal response and subjective sexual arousal: A comparison between women with sexual arousal disorder and sexually healthy women. Archives of Sexual Behavior. 2008;37:950–961. doi: 10.1007/s10508-007-9310-0. [DOI] [PubMed] [Google Scholar]
- Morokoff PJ. Volunteer bias in the psychophysiological study of female sexuality. Journal of Sex Research. 1986;22:35–51. [Google Scholar]
- Palace E, Gorzalka B. The enhancing effects of anxiety on arousal in sexually dysfunctional and functional women. Journal of Abnormal Psychology. 1990;99:403–411. doi: 10.1037//0021-843x.99.4.403. [DOI] [PubMed] [Google Scholar]
- Rellini A, McCall K, Randall P, Meston C. The relationship between women’s subjective and physiological sexual arousal. Psychophysiology. 2005;42:116–124. doi: 10.1111/j.1469-8986.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, et al. The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. Journal of Sex and Marital Therapy. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- Sanders SA, Graham CA, Bass JL, Bancroft J. A prospective study of the effects of oral contraceptives on sexuality and well-being and their relationship to discontinuation. Contraception. 2001;64:51–58. doi: 10.1016/s0010-7824(01)00218-9. [DOI] [PubMed] [Google Scholar]
- Sato S, Hayashi RH, Garfield RE. Mechanical responses of the rat uterus, cervix, and bladder to stimulation of hypogastric and pelvic nerves in vivo. Biology of Reproduction. 1989;40:209 – 219. doi: 10.1095/biolreprod40.2.209. [DOI] [PubMed] [Google Scholar]
- Sato Y, Hotta H, Nakayama H, Suzuki H. Sympathetic and parasympathetic regulation of the uterine blood flow and contraction in the rat. Journal of the Autonomic Nervous System. 1996;59:151–158. doi: 10.1016/0165-1838(96)00019-7. [DOI] [PubMed] [Google Scholar]
- Schueller PO, Feuring M, Sharkova Y, Grimm W, Christ M. Effects of synthetic progestagens on autonomic tone, neurohormones and C-reactive protein levels in young healthy females in reproductive age. International Journal of Cardiology. 2006;111:42–48. doi: 10.1016/j.ijcard.2005.06.052. [DOI] [PubMed] [Google Scholar]
- Sun D, Huang A, Mital S, Kichuk M, Marboe C, Addonizio L, et al. Norepinephrine elicits beta 2-receptor-mediated dilation of isolated human coronary arterioles. Circulation. 2002;106:550 – 555. doi: 10.1161/01.cir.0000023896.70583.9f. [DOI] [PubMed] [Google Scholar]
- Suschinsky KD, Lalumière ML, Chivers ML. Sex differences in patterns of genital sexual arousal: measurement artifacts or true phenomena? Archives of Sexual Behavior. 2009;38:559–573. doi: 10.1007/s10508-008-9339-8. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Van Anders S, Brotto L, Farrell J, Yule M. Associations among physiological and subjective sexual response, sexual desire, and salivary steroid hormones in healthy premenopausal women. Journal of Sexual Medicine. 2009;6:739–751. doi: 10.1111/j.1743-6109.2008.01123.x. [DOI] [PubMed] [Google Scholar]
- Vrijkotte T, van Doornen L, de Geus E. Effects of work stress on ambulatory blood pressure, heart rate, and heart rate variability. Hypertension. 2000;35:880 – 886. doi: 10.1161/01.hyp.35.4.880. [DOI] [PubMed] [Google Scholar]
- Wagner G, Levin R. Effect of atropine and methylatropine on human vaginal blood flow, sexual arousal and climax. Acta Pharmacologica et Toxicologica. 1980;46:321–325. doi: 10.1111/j.1600-0773.1980.tb02461.x. [DOI] [PubMed] [Google Scholar]
- Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): Cross-validation and development of clinical cutoff scores. Journal of Sex and Marital Therapy. 2005;31:1–20. doi: 10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- Woo JST, Brotto LA, Yule MA. Do East Asian and Euro-Canadian Women differ in sexual psychophysiology research participation? Journal of Sex Research. 2010;47:345–354. doi: 10.1080/00224490902999294. [DOI] [PubMed] [Google Scholar]
- Yerkes R, Dodson J. The relation of strength of stimulus to rapidity of habit formation. Journal of Comparative Neurology and Psychology. 1908;18:459–482. [Google Scholar]