Abstract
In 1994, the field of bone biology was significantly advanced by the discovery that activating mutations in the FGFR3 receptor tyrosine kinase account for the common genetic form of dwarfism in humans, achondroplasia. Other conditions soon followed, with the list of human disorders caused by FGFR3 mutations now reaching at least 10. An array of vastly different diagnoses is caused by similar mutations in FGFR3, including syndromes affecting skeletal development (hypochondroplasia, achondroplasia, thanatophoric dysplasia), skin (epidermal nevi, seborrhaeic keratosis, acanthosis nigricans) and cancer (multiple myeloma, prostate and bladder carcinoma, seminoma). Despite many years of research, several aspects of FGFR3 function in disease remain obscure or controversial. As FGFR3-related skeletal dysplasias are caused by growth attenuation of the cartilage, chondrocytes appear to be unique in their response to FGFR3 activation. However, the reasons why FGFR3 inhibits chondrocyte growth while causing excessive cellular proliferation in cancer are not clear. Likewise, the full spectrum of molecular events by which FGFR3 mediates its signaling is just beginning to emerge. This article describes the challenging journey to unravel the mechanisms of FGFR3 function in skeletal dysplasias, the extraordinary cellular manifestations of FGFR3 signaling in chondrocytes, and finally, the progress towards therapy for achondroplasia and cancer.
Keywords: FGFR3, chondrocyte, skeletal dysplasia, MAP kinase, FGF
FGFR3-related skeletal dysplasias
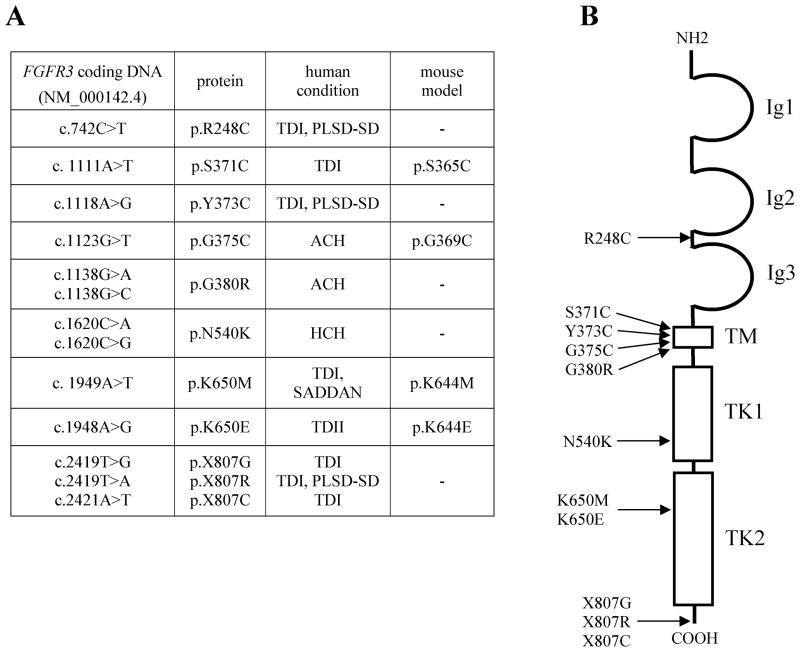
The history of unraveling the FGFR3 (MIM# 134934) function in bone development began in July of 1994 when Shiang et al. reported a mutation in FGFR3 in patients with achondroplasia (Shiang et al., 1994). Other reports followed, identifying mutations in FGFR3 gene in a total of five related skeletal dysplasias, i.e. hypochondroplasia, achondroplasia, SADDAN dysplasia, thanatophoric dysplasia, and platyspondylic lethal skeletal dysplasia, San Diego type (Fig. 1). Among those, achondroplasia, which occurs with an estimated prevalence between 1/16,000 to 1/26,000 live births, represents the most common genetic form of human dwarfism, whereas thanatophoric dysplasia is the most common form of neonatal lethal dwarfism (Brodie et al., 1999; Rousseau et al., 1994; Tavormina at al., 1995; Bellus et al., 1995; Prinos et al., 1995; Tavormina et al., 1999; Oberklaid et al., 1979; Waller et al., 2008).
Figure 1.
A summary of FGFR3 mutations discussed in the text. (A) A summary of human FGFR3 mutations discussed in the text. Mutations in available murine models are also indicated. TD - thanatophoric dysplasia; ACH- achondroplasia; HCH - hypochondroplasia; SADDAN -severe achondroplasia with developmental delay and acanthosis nigricans; PLSD-SD - platys-pondylic lethal skeletal dysplasia, San Diego type. (B) An overview of human FGFR3 protein structure with indicated positions of the mutations discussed in text. Ig1–3 extracellular immunoglobulin-like domains of FGFR3; TM - transmembrane domain; TK1–2 intracellular tyrosine kinase domains.
Achondroplasia
Achondroplasia (ACH; MIM# 100800) is characterized by short stature with disproportionately short arms and legs, a large head and characteristic facial features with frontal bossing and mid-face hypoplasia, exaggerated lumbar lordosis, genu varum and trident hands (Horton et al., 2007). Most individuals with ACH have a heterozygous p.G380R substitution in FGFR3, resulting from either a G>A or G>C point mutation at nucleotide 1138 (Passos-Bueno et al., 1999; W. Wilcox, unpublished). A milder form of achondroplasia is hypochondroplasia (HCH; MIM# 146000) (Hall and Spranger, 1979) caused, in about 70% of cases, by a p.N540K (c.1620C>A or G) substitution in FGFR3 (Rousseau et al., 1996). SADDAN dysplasia (severe achondroplasia with developmental delay and acanthosis nigricans; MIM# 187600) has skeletal abnormalities similar to, but more severe than, those observed in ACH, as well as profound developmental delay, brain structural abnormalities, hearing loss and acanthosis nigricans in the surviving patients (Bellus et al., 2000; Tavormina et al., 1999). SADDAN is due to a p.K650M (c.1949A>T) substitution in FGFR3, also reported in the most severe form of FGFR3-related skeletal dysplasia, thanatophoric dysplasia (Passos-Bueno et al., 1999; Kitoh et al., 1998).
Thanatophoric dysplasia
When a FGFR3 mutation is inherited from two ACH parents, achondroplasia is neonatally lethal with clinical features strongly resembling thanatophoric dyplasia. Thanatophoric dysplasia (TD) is a generally neonatal lethal skeletal dysplasia characterized by micromelia, thoracic hypoplasia and macrocrania (Wilcox et al., 1998). First described in 1967 (Maroteaux et al., 1967), TD has been classified in two clinically defined subtypes. TDI (MIM# 187600) is the most common subtype characterized by a curved femora and occasional cloverleaf skull, whilst cloverleaf skull and short but straight femora are characteristic of TDII (MIM# 187601). TDI originates from several amino acid substitutions in extracellular and intracellular domains of FGFR3 protein, such as p.R248C (c.742C>T), p.Y373C (c.1118A>G) and K650M (Tavormina et al., 1995; Brodie et al., 1998) (Fig. 1). In addition, several stop codon mutations have been described, such as p.X807G (c.2419T>G), p.X807R (c.2419T>A), and p.X807C (c.2421A>T), which result in the elongation of FGFR3 protein at the C-terminus by 141 amino acids (Rousseau et al., 1995). In contrast to TDI, only one mutation (p.K650E; c.1948A>G) has been shown to account for TDII (Wilcox et al., 1998).
Although all FGFR3-related skeletal dyplasias manifest with profound shortening of the long bones, they display a graded spectrum of phenotypic severity, ranging from relatively mild HCH to the neonatal lethal TD. This variance is caused by the relative ‘activating’ potential of a given substitution. First documented by Naski et al. (1996), mutations activate FGFR3, and correspondingly inhibit chondrocyte proliferation to different levels when compared to wild-type (wt) FGFR3, with a relative strength being wt<N540K (HCH)<G380R (ACH)≪R248C (TDI)=Y373C (TDI)<K650M (TDI)≤K650E (TDII) (Krejci et al., 2008a).
Mutations activate FGFR3 by different mechanisms
Extracellular FGF ligands activate FGFR signaling via formation of FGFR dimers, which is assisted by heparin sulphate proteoglycans and mediated by bivalent ligand-receptor interactions (Ibramini et al., 2005). All known mutations activate FGFR3 by facilitating its dimerization, although the exact mechanism varies depending on the location of given mutation (Fig. 1). The G380R (ACH) substitution leads to ligand-independent stabilization of FGFR3 dimers via hydrogen bonds formed between the side-chains of the two arginine residues (Webster and Donoghue, 1996). TDI-associated Y373C and R248C substitutions activate FGFR3 via forming covalently bound dimers, held together by a disulfide bond between the free cysteine residues introduced into the juxtamembrane domain (Y373C) or to the region linking two Ig domains in the extracellular part of FGFR3 (R248C) (Naski et al., 1996; d’Avis et al., 1998). Finally, substitutions in the intracellular tyrosine kinase (TK) domain, such as K650M (TDI) or K650E (TDII), activate FGFR3 by mimicking the conformational changes in the TK domain that are normally induced by ligand-mediated FGFR3 dimerization and autophosphorylation (Webster et al., 1996).
Both K650M and K650E-FGFR3 mutants show abnormal intracellular localization, as they mature poorly after synthesis and accumulate in the endoplasmic reticulum (ER) (Raffioni et al., 1998), possibly via increased interaction with shisa protein within the ER (Yamamoto et al., 2005). In their ER-based signaling, K650M and K650E-FGFR3 appear to use non-canonical ways to activate the downstream signaling intermediates such as ERK MAP kinase (Lievens and Liboi, 2003, Lievens et al., 2006). This feature, however, does not appear to be essential for the development of skeletal dysplasias, since other FGFR3 mutants such as R248C and Y373C mature normally and signal from the cell membrane, yet cause a more severe skeletal phenotype (Krejci et al., 2008a; Passos-Bueno, 1999).
Is the FGF ligand important in the context of a FGFR3 activating mutation?
Compared to the clear role of FGFR3 in inhibition of bone growth, the nature and role of its cognate ligand in this process remains somewhat unclear. Experimental studies often demonstrate that mutations activate FGFR3 in a constitutive, ligand-independent fashion (Su et al., 1997; Naski et al., 1996; Krejci et al., 2010b). When expressed at low levels, however, the activity of mutated FGFR3 depends on FGF ligand, as demonstrated for ACH (G380R) or TD (R248C, K650E)-FGFR3 (Monsonego-Ornan et al., 2000; Naski et al., 1996). Finally, both human TD chondrocytes as well as those isolated from K644M-Fgfr3 (corresponding to human TDI mutation K650M) mice require ligand for FGFR3 activation (Iwata et al., 2001; Legeai-Mallet et al., 1998). The simplest explanation for the discrepant data is that in the overexpressed state, the chance for spontaneous FGFR3 dimerization is high, which in general facilitates ligand-independent activation of FGFR3. In contrast, the chance of spontaneous dimerization is low at physiological levels of expression, and thus ligand-mediated recruitment of FGFR3 dimers may facilitate activation even in case of highly activating R248C and Y373C mutations.
Several FGF ligands can activate FGFR3, including FGF1, 2, 4, 8, 9, 17–20 (Zhang et al., 2006). In mice, deletion of Fgf18 leads to increased chondrocyte proliferation and differentiation (Liu et al., 2002), similar to that observed in Fgfr3 null mice (Deng et al., 1996), implying that FGF18 acts as a physiological ligand for FGFR3 in mice. As FGF18 is not expressed by cartilage but only in the adjacent perichondrium (Liu et al., 2002), it appears that perichondrium regulates the FGFR3 activity in growth plate via FGF18 secretion in mice. In humans, the overall size of the growing long bones makes perichondrial-borne FGFs unlikely to efficiently penetrate into the entire growth plate cartilage. In addition, FGF18 was not found to be expressed in human perichondrium or in the underlying cartilage, suggesting that other FGFs are involved in FGFR3 activation in humans. These may be FGF1, 2 and 17 that are all expressed in the human growth plate and experimentally capable of inhibiting chondrocyte proliferation (Krejci et al., 2007a).
FGFR3 is a physiological negative regulator of bone growth
FGF growth factors are one of the major systems for cellular communication throughout development, life, and disease. The extracellular signals delivered by at least 18 different FGF ligands are transmitted by four receptor tyrosine kinases, FGFR1–4, which exert different physiological functions due to the differences in their temporal and spatial distribution of expression (Johnson and Williams, 1993). Although FGFR3 is expressed in brain, lung and spinal cord in addition to cartilage (Avivi et al., 1991; Peters et al., 1993), its major domain of normal physiological function appears to be the regulation of cartilage growth. Fgfr3 null mice have long bone overgrowth due to expanded zones of epiphyseal growth plate cartilage caused by increased chondrocyte proliferation (Colvin et al., 1996; Deng et al., 1996). Similar overgrowth of the skeleton can be observed in the human CATSHL syndrome (camptodactyly, tall stature, scoliosis, and hearing loss; MIM# 610474) or spider lamb syndrome in sheep, which are both caused by loss of FGFR3 function (Toydemir et al., 2006; Beever et al., 2006). Such data clearly indentify FGFR3 as a physiological negative regulator of skeletal growth, which restricts the length of long bones via inhibition of chondrocyte proliferation. It is important to note, that the profound dwarfing phenotypes in individuals carrying gain-of-function mutations in FGFR3, as discussed in this review, are not due to novel FGFR3 functions in cartilage, but rather exaggeration of its physiological roles.
Mitogenic FGFR3 signaling in skin disorders and cancer
Skeletal dysplasias are not the only disorders with pathological FGFR3 signaling. In fact, at least six other conditions unrelated to bone are caused by somatic FGFR3 mutations similar or identical to those associated with TD. These include conditions caused by skin overgrowth such as epidermal nevi (MIM# 162900), seborrheic keratosis (MIM# 182000) and acanthosis nigricans, as well as various types of cancer (multiple myeloma, bladder and cervical carcinoma, seminoma) (Cappellen et al., 1999; Hafner et al., 2006; Logie et al., 2005; Goriely et al., 2009; Chesi et al., 1997). The occurrence of FGFR3 mutations in the abovementioned syndromes, which are all caused by excessive cell proliferation, suggests a pro-mitogenic FGFR3 effect on a diverse spectrum of cell types including keratinocytes, melanocytes, epithelial cells, lymphocytes and spermatocytes. This assumption was evaluated extensively in multiple myeloma, where FGFR3 carrying a TD mutation acts as a bona fide oncogene, providing advantage to the tumor cells by increasing their proliferation and survival, and decreasing their growth dependence on external mitogenic stimuli (Li et al., 2001; Zhu et al., 2005; Plowright et al., 2000; Chesi et al., 2001).
Altogether, the growth-inhibitory role of FGFR3 in cartilage appears unique when compared to the effects of aberrant FGFR3 signaling in other tissues and contrary to the general view of the physiological roles of FGF growth factors, which are regarded as powerful mitogens for cells of mesenchymal origin. In the following sections, we attempt to describe the majority of known mechanisms mediating FGFR3 signaling in chondrocytes. It is important to note that the fundamental cellular processes underlying the extraordinary growth-inhibitory function of FGFR3 in cartilage are poorly characterized to date, and still await discovery.
Cellular phenotypes regulated by FGFR3 in cartilage
Vertebrate long bones elongate via endochondral ossification where a cartilage template is replaced by a bony matrix (Karsenty et al., 1999). This highly regulated process occurs in the epiphyseal growth plate cartilage, found at the ends of growing long bones. Chondrocytes arise from mesenchymal condensations in the growing limb buds around E12.5 in mice or embryonal week 5 in humans, to establish the skeletal elements. As the elements grow, chondrocytes in the center enlarge, transitioning from a mitotically active, type II collagen expressing stage to post-mitotic, type X collagen expressing hypertrophic chondrocytes, which eventually die and are replaced by trabecular bone (Kosher et al., 1986). This process includes apoptosis of hypertrophic chondrocytes, accompanied by vascular invasion of the growth plate, resorption of the cartilaginous matrix and recruitment of osteoprogenitor cells (Erlebacher et al., 1995).
On histological examination, growth plate cartilage has a high level of organization with three major morphologically distinct regions. The foremost part of the growth plate is represented by a reserve zone characterized by disordered chondrocytes surrounded by an abundant extracellular matrix. A band of proliferating cartilage lies underneath the reserve zone, easily distinguished by columns of proliferating cells. Later on, the chondrocytes cease cell division to undergo progressive hypertrophy during which they alter the extracellular matrix, depositing collagen type X and calcified mineral. Overall, the rate of growth depends on several factors such as the rate of chondrocyte proliferation and terminal differentiation, extracellular matrix production, the size of hypertrophic cells, and the rate of vascular invasion and chondrocyte apoptosis.
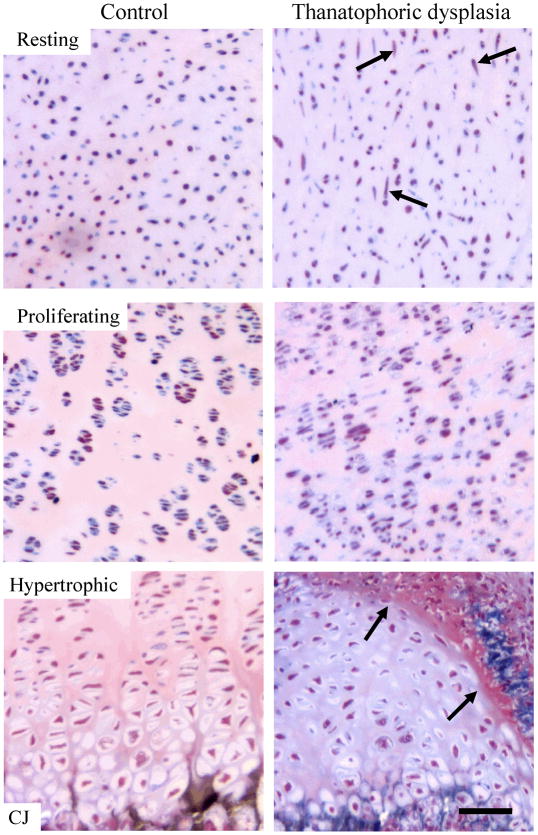
One of the striking features of TD is the profound disorganization of the entire growth plate architecture, easily detected in the histological sections of TD growth plates (Wilcox et al., 1998). Both proliferating and hypertrophic zones are disrupted, with much smaller than normal zones of proliferating chondrocytes and small or non-existing columns of hypertrophic chondrocytes (Fig. 2). Thus, simple histological analysis of TD growth plates suggests at least four cellular phenotypes are regulated by aberrant FGFR3 signaling. First, TD growth plate cartilage shows marked shortening and disorganization of the proliferative zone, due to the inhibitory effect of FGFR3 signaling on chondrocyte proliferation. Second, an increased cell-to-extracellular matrix ratio appears in both the proliferative and hypertrophic zone of the TD growth plate when compared to control, suggesting a negative influence of FGFR3 on the amount of the chondrocyte extracellular matrix. Third, the zone of hypertrophic cartilage is markedly shortened due to impaired chondrocyte differentiation. Finally, an alteration of chondrocyte cellular shape can be observed in TD cartilage, manifested as an increased number of spindle-shaped, fibroblast-like chondrocytes in the resting zone (Wilcox et al., 1998) (Fig. 2).
Figure 2.
Histologic appearance of human control and thanatophoric dysplasia (TD) growth plate cartilage. Histologic appearance of resting, proliferative, and hypertrophic zone of the femoral growth plate of a control 23 weeks of gestation fetus (left panel) compared to a 24 weeks of gestation TD fetus (R248C-FGFR3; right panel). Note the increased amount of spindle-shaped cells in the TD resting cartilage (arrows), loss of proliferating cells in the TD proliferative zone, and an increased cell-to-matrix ratio in both the TD proliferative and hypertrophic zones. Also note the overall disorganization of both the proliferative and hypertrophic zones as well as the mesenchymal tissue invading the hypertrophic zone in TD (arrows). Sections were stained with Goldner’s trichrome. CJ - chondro-osseous junction. Scale bar: 100 μm.
FGFR3-mediated inhibition of chondrocyte proliferation
To date, profound inhibition of chondrocyte proliferation is the best documented feature of FGFR3 signaling in cartilage, being experimentally confirmed in human TD and in the growth plates of G380R, S365C (corresponding to human TDI mutation p.S371C) and K644E-Fgfr3 mice (Segev et al., 2000; Li et al., 1999; Chen et al., 2001) (Fig. 2). Yet the cellular mechanisms underlying the growth-arrest phenotype remain unclear. Some believe that FGFR3 induces chondrocyte apoptosis (L’Hote and Knowles, 2005), but very little evidence supports this conclusion. First, apoptosis was not detected within the growth plate cartilage of G380R-Fgfr3 mice (Naski et al., 1998), nor do the growth plates of G380R, S365C and K644E-Fgfr3 mice resemble those with a cartilage-specific deletion of Vegfa, which suffer from significant chondrocyte apoptosis (Segev et al., 2000; Li et al., 1999; Chen et al., 2001; Zelzer et al., 2004). Second, apoptotic chondrocytes are not found in the histological preparations of human TD cartilage as compared, for instance, to individuals suffering from chondrodysplasia punctata Conradi-Hünermann type (MIM# 302960) or hydropsectopic calcification-moth-eaten dysplasia (MIM# 215140), where apoptotic chondrocytes can be easily indentified within the growth plate (W. Wilcox, unpublished). Moreover, only a tiny increase in apoptosis (1–2% versus control) was reported in primary cultures of TD chondrocytes (Legeai-Mallet et al., 1998), but we failed to confirm this observation using the same model (P. Krejci, unpublished). In CFK2 chondrocytic cells, expression of G380R-FGFR3 protected cells from apoptosis caused by serum-starvation while still inhibiting cell growth (Henderson et al., 2000). Although an apoptotic response can be induced by expression of G380R or K650E-FGFR3 in ATDC5 (chondrogenic cell line) or RCS cells (proliferating chondrocytes derived from rat chondrosarcoma) (Yamanaka et al., 2003; Krejci et al., 2010a), this is likely an artifact of FGFR3 overexpression. As we have demonstrated in RCS chondrocytes, activation of endogenous FGFR3 results in a potent growth-arrest phenotype with features of premature cellular senescence instead of apoptosis, which is triggered only when activating FGFR3 mutants are highly overexpressed (Krejci et al., 2010a).
While the cellular events underlying growth inhibition by FGFR3 signaling in cartilage remain poorly characterized, the molecular mechanism of the initial growth-arrest has been partially characterized. Several inhibitors of G1 phase of a cell cycle belonging to cip/kip (p21Waf1) or INK4 family (p16, p18, p19) accumulate in ACH and TD growth plates or their mouse counterparts (Legeai-Mallet et al., 2004; Li et al., 1999; Chen et al., 1999). The molecular events accompanying FGFR3-mediated chondrocyte growth arrest have been intensively studied using RCS chondrocytes (Krejci et al., 2004; Aikawa et al., 2001; Dailey et al., 2003; Priore et al., 2006; Kolupaeva et al., 2008). Although they replicate many features of FGFR3 signaling in cartilage in vivo, RCS chondrocytes represent an immortalized cell line and thus the mechanism of the cell cycle regulation might partially differ in these cells when compared to in vivo chondrocytes. In addition, several signaling systems coordinate chondrocyte proliferation in a complex three-dimensional cartilage environment, which is impossible to replicate in cell culture in vitro.
In RCS chondrocytes, FGFR3 activation leads to profound growth-arrest in the G1 phase of the cell cycle, caused by disintegration of cyclin D3-cyclin dependent kinase 6 (cdk6) complex followed by increased association of p21Waf1 and p27Kip1 with cyclin-cdk2 and cyclin-cdk4 complexes leading to inhibition of their kinase activities. This results in underphosphorylation of the p107 and p130 pocket proteins, causing their activation and subsequent inhibition of progress through the cell cycle. Both p21Waf1 and p27Kip1 accumulate upon FGFR3 activation by protein stabilization due to interaction with transcriptionally induced cyclin D1 (Aikawa et al., 2001; Krejci et al., 2004) (Fig. 3).
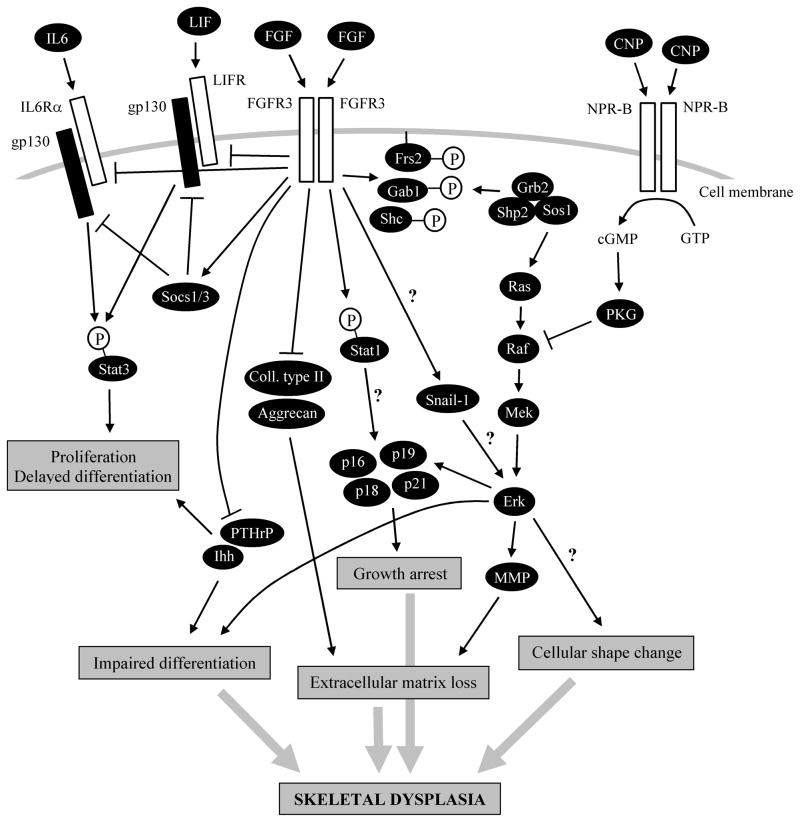
Figure 3.
Molecular mechanisms of FGFR3 signaling in cartilage. Aberrant activation of FGFR3 alters chondrocyte behaviour by inducing premature growth arrest, loss of extracellular matrix, altered differentiation and changes in cell shape. Together, these cellular phenotypes (grey arrows) contribute to profound disruption of the growth plate cartilage resulting in skeletal dysplasia. At the molecular level, the growth arrest phenotype is mediated by induction of several inhibitors of the cell cycle, belonging to cip/kip family (p21) or INK4 family (p16, p18, p19), whereas the loss of the extracellular matrix originates from both inhibition of production of major matrix components (collagen type II and aggrecan), and active matrix degradation, mediated by several members of matrix metalloproteinase family (MMP). Expression of two important physiological regulators of chondrocyte differentiation, Indian hedgehog (Ihh) and parathyroid hormone related protein (PTHrP), is inhibited by FGFR3 in cartilage, likely contributing to impaired chondrocyte hypertrophic differentiation. ERK MAP kinase is a major pathway for growth arrest, extracellular matrix loss and impaired chondrocyte differentiation. FGFR3 causes prolonged activation of the ERK signaling module (RAS-RAF-MEK-ERK), mediated by adapter (GAB1, FRS2 and SHC)-driven recruitment of SHP2-GRB2-SOS1 complexes to the cell membrane, where they activate RAS. In addition, SNAIL1 transcription factor is involved in regulation of FGFR3-mediated ERK activity, although the exact nature of this regulation is not presently clear (question marks). The FGFR3-mediated activation of the ERK pathway is antagonized by CNP signaling, which inhibits ERK pathway by inactivation of RAF kinase, via inhibitory phosphorylation mediated by cGMP-activated protein kinase (PKG). Some FGFR3 mutants also activate STAT1, possibly via direct phosphorylation at Y701. It is, however, currently unclear whether activated STAT1 or other STATs induce cell cycle inhibitor expression in cartilage, thereby contributing to the FGFR3-mediated growth arrest (question mark). Finally, chronic activation of FGFR3 leads to inhibition of canonical cytokine-STAT signaling in chondrocytes, via both induction of SOCS inhibitors of cytokine signaling and inhibition of expression of receptors for IL6 (IL6Rα) or LIF (LIFR). As the latter cytokines represent positive regulators of chondrocyte proliferation, their inhibition might contribute to the pathological effects of FGFR3. NPR-B - natriuretic peptide receptor B/guanylyl cyclase B; GTP - guanosine-5′-triphosphate; cGMP - cyclic guanosine monophosphate; gp130 - glycoprotein 130.
FGFR3-mediated regulation of the chondrocyte extracellular matrix
In TD or mouse models for human ACH, an increased cell to matrix ratio can be observed within the entire growth plate, suggesting decreased matrix production or increased turnover (Yasoda et al., 2004) (Fig. 2). In vitro, RCS chondrocytes lose most of their abundant, cartilage-like extracellular matrix within the first 72 hours of FGFR3 activation. This process is partially mediated by FGFR3-mediated inhibition of expression of the basic components of the matrix such as Aggrecan, Chondroitin sulfate proteoglycan 4, and Collagen type II (Krejci et al., 2004; unpublished). In addition, FGFR3 activation induces active matrix degradation, via up-regulated expression, release and activation of several matrix metalloproteinases (MMP) including MMP3, 9, 10 and 13 (Krejci et al., 2005). Thus, FGFR3 signaling decreases the chondrocyte extracellular matrix by both inhibiting its synthesis and promoting its degradation (Fig. 3).
Molecular mechanisms of FGFR3 signaling in cartilage
The signaling pathways used by FGFR’s have received significant attention over the past 20 years. Many downstream mediators involved in FGFR signal transduction have been characterized, including MAP kinases, phospholipase Cγ, protein kinase C, Src, phosphatidylinositol 3-kinase and AKT and others (reviewed in Klint and Claesson-Welsh, 1999). The following section addresses the pathways of FGFR3 signal transduction involved specifically in the skeletal dysplasias.
FGFR3 interactions with Indian hedgehog (Ihh)/parathyroid hormone related protein (PTHrP)
The process of chondrocyte transition from proliferation to hypertrophy depends on the intricate cross-talk between the Ihh and PTHrP signaling pathways. PTHrP is produced by the perichondrium or resting and proliferating chondrocytes in the embryonic or postnatal growth plates, respectively, and increases chondrocyte proliferation by delaying hypertrophic differentiation. Ihh, on the other hand, is produced by early hypertrophic chondrocytes and induces PTHrP expression to increase chondrocyte proliferation (Lanske et al., 1996; Karaplis et al., 1994; Vortkamp et al., 1996; Chau et al., 2011; Chen et al., 2008). Although Ihh can also increase chondrocyte proliferation independent of PTHrP (Long et al., 2001), the fact that the mitogenic action of PTHrP depends on Ihh synthesized by post-mitotic chondrocytes constitutes a feedback loop that represents one of the major regulators of growth plate length (reviewed in Kronenberg, 2006).
First noted in the mouse model expressing G380R-FGFR3 under the control of the Col2a1 promoter, aberrant FGFR3 activation in cartilage caused inhibition of expression of both Ihh and its receptor patched (Naski et al., 1998). Similar phenotypes were found in the growth plates of S365C, G369C (corresponding to human TDI mutation p.G370C) or K644E-Fgfr3 murine models for TD, involving, in addition to Ihh, the PTHrP-receptor (Chen et al., 1999; 2001; Li et al., 1999). Although these findings are at variance with the normal expression of Ihh and PTHrP, reported by others in human ACH and TD growth plates or those of the K644E-Fgfr3 mouse (Cormier et al., 2002; Iwata et al., 2000), they open the possibility that inhibition of Ihh/PTHrP signaling contributes to disrupted chondrocyte differentiation, observed in TD.
The effect of FGFR3 on other signaling pathways is not surprising given that growth plate cartilage development requires a complex spatiotemporal interaction of several different signaling systems, i.e. Ihh, bone morphogenetic protein (BMP), PTHrP, FGFR3, Wnt and others (Pogue and Lyons, 2006; Macsai et al., 2008; Kronenberg 2006). It is, however, not clear whether FGFR3-mediated downregulation of Ihh/PTHrP in the growth plate contributes directly to the pathology of skeletal dysplasias or simply represents a consequence of the inhibition of chondrocyte proliferation, which would lead to fewer Ihh-generating prehypertrophic chondrocytes. In addition, FGFR3 may alter chondrocyte differentiation unrelated to Ihh/PTHrP. In our opinion, several lines of evidence suggest that inhibition of PTHrP/Ihh signaling indeed contributes to the pathology of the FGFR3-related skeletal dysplasias (Fig. 3). First, FGFR3 activation inhibits PTHrP expression not only in the growth plate but also in the cultured chondrocytes (Yamanaka et al., 2003). Second, downregulation of PTHrP-receptor and Ihh precedes the appearance of bone abnormalities mediated by FGF signaling (Chen et al., 2001). Finally, administration of exogenous PTHrP or its related parathyroid hormone rescues the growth-inhibitory effects of FGFR3 in both cultured ATDC chondrocytes and mouse limb explant cultures (Yamanaka et al., 2003; Ueda et al., 2007).
STAT
As early as 1997, it was shown that the TDII mutant FGFR3 (K650E) activates the STAT1 (signal transducer and activator of transcription) transcription factor, evidenced by its increased nuclear accumulation and DNA binding activity. This correlated with the accumulation of the p21Waf1 cell cycle inhibitor, and growth arrest in 293T cells (Su et al., 1997). Many studies soon followed, detecting activating STAT1 phosphorylation at Y701 by K650M or K650E-FGFR3 in PC12 pheochromocytoma cells, HeLa cervical cancer cells, and ATDC5 chondrogenic cells (Hart et al., 2001; Ronchetti et al., 2001; Lievens et al., 2004; Nowroozi et al., 2005; Harada et al., 2007). Moreover, in the cartilage of ACH- and TD-affected human fetuses as well as in mice carrying the TD mutationsin FGFR3, STAT1 and STAT5 accumulated and showed nuclear localization, suggesting their activation (Legeai-Mallet et al., 1998; 2004; Chen et al., 1999; Li et al., 1999). Finally, in two experimental studies, the loss of STAT1 partially rescued the growth-inhibitory action of FGF signaling in both in vitro and in vivo chondrocyte environments (Sahni et al., 1999; 2001).
Being a major effector of interferon γ signaling, STAT1 inhibits cell proliferation via p21Waf1, as a part of an antiviral cell response (Chin et al., 1996). It was suggested that a similar mechanism operates in chondrocytes bearing mutated FGFR3, although no experimental proof has shown that K650E-FGFR3 uses STAT1 to induce p21Waf1 and thereby inhibit cell growth (Su et al., 1997). To date, the activation of STAT1 by FGFR3 is believed by many to be the major mechanism of pathological FGFR3-medited inhibition of cartilage growth (reviewed in Ornitz 2005; L’Hote and Knowles, 2005; Dailey et al., 2005; Baujat et al., 2008; Harada et al., 2009; Martinez-Frias et al., 2010), regardless of the limited evidence experimentally confirming this hypothesis.
In our opinion, there are many concerns regarding the validity of the STAT hypothesis. The loss of FGFR3 leads to significant skeletal overgrowth byincreased chondrocyte proliferation, which is not phenocopied in Stat1−/− mice (Durbin et al., 1996; Deng et al., 1996). In primary chondrocytes isolated from Stat1−/− mice, RCS chondrocytes and PC12 cells, ERK and p38 MAP kinases but not STAT1 increase p21Waf1 and cause growth inhibition (Murakami et al., 2004; Raucci et al., 2004; Krejci et al.,2004; 2008b; Nowroozi et al., 2005). Crossing Stat1−/− mice with those carrying the ACH mutation in Fgfr3 did not rescue the ACH phenotype although increased chondrocyte proliferation was observed (Murakami et al., 2004). Finally, in TD or its mouse models, premature synchondrosis and fusion of ossification centers is triggered by aberrant FGFR3 activation. This phenotype stems from the loss of proliferating chondrocytes in the synchondroses, independent of STAT1 but mediated by ERK MAP kinase (Matsushita et al., 2009).
STAT1 activation by FGFR3: A major mechanism or just an unusual signaling feature restricted to K650 mutants?
The majority of evidence supporting the role of STAT1 in FGFR3 signaling in cartilage was obtained using the K650M or K650E-FGFR3 mutants. Many studies report that other FGFR3 activating mutants associated with skeletal dysplasias or cancer do not cause STAT1 activation or only activate STAT1 several-fold less than K650M or K650E-FGFR3. In addition, FGFR3 mutations only exaggerate the normal physiological function of FGFR3 in cartilage, but no activation of STAT1 occurs by wt FGFR3 (Su et al., 1997; Legeai-Mallet et al., 1998; Plowright et al., 2000; Ronchetti et al., 2001; Nowroozi et al., 2005; Harada et al., 2007; Krejci et al., 2008a; Hart et al., 2000; Lievens and Liboi, 2003; Lievens et al., 2004).
We addressed this issue directly, comparing the ability to activate STAT1 among wt FGFR3 and six mutants associated with skeletal dysplasias including N540K (HCH), G380R (ACH), R248C, Y373C (TDI), K650M (SADDAN, TDI) and K650E (TDII). When expressed in different cellular environments, only K650M or K650E-FGFR3 significantly activated STAT1 or STAT5. Similar results were obtained in cell-free kinase assays utilizing FGFR3 as kinase and STAT1 as a substrate, demonstrating that, at the basic biochemical level, only K650M or K650E-FGFR3 mutants are capable of STAT1 activation, via direct phosphorylation at Y701 (Krejci et al., 2008a). It is important to note that mutations involving K650 represent less than 5% of documented cases of FGFR3-related skeletal dysplasias (Passos-Bueno et al, 1999), thus making STAT1 activation unlikely to represent the major mechanism of pathological FGFR3 signaling in cartilage (Fig. 3).
FGFR3 interferes with canonical cytokine-STAT signaling in chondrocytes
The abovementioned evidence argues that STAT1 or STAT5 activation, via FGFR3-mediated phosphorylation at Y701 or Y694, is likely not the major intermediate of FGFR3 signaling in cartilage. Nevertheless, STATs may play an unexpected role in the pathology of FGFR3-related skeletal dysplasias. It is firmly established that, regardless of their phosphorylation status, STAT1 and STAT5 accumulate in ACH and TD growth plates as well as in their mouse models (Legeai-Mallet et al., 2004; Chen et al., 1999; Li et al., 1999). In ACH and TD, high amounts of STATs found in pre-hypertrophic and hypertrophic chondrocytes correlate directly with the severity of the disease. STAT accumulation is paralleled by that of p21Waf1 leading to conclusion, in agreement with the STAT hypothesis, that FGFR3 utilizes STATs to induce p21Waf1 (Legeai-Mallet et al., 2004).
We found similar accumulation of multiple STAT proteins in RCS chondrocytes or mouse limb explant cultures, induced by chronic FGFR3 activation (Krejci et al., 2009). RCS cells also accumulate p21Waf1 and growth arrest upon FGFR3 activation, but both latter events are independent of STATs, being mediated by the ERK MAP kinase pathway instead (Krejci et al., 2004; 2008b; Raucci et al., 2004). A detailed experimental analysis of the mechanisms of STAT accumulation in RCS chondrocytes revealed that FGFR3 inhibits canonical cytokine-STAT signaling by interferon γ, interleukin 6 (IL6), IL11 and leukemia inhibitory factor (LIF), via induction of SOCS1 and SOCS3 inhibitors of cytokine signaling, inhibition of expression of the LIF- and IL6-receptors, and possibly other mechanisms (Krejci et al., 2009; Ben-Zvi et al., 2006). Moreover, it appears that RCS chondrocytes upregulate STAT expression in an attempt to compensate for FGFR3-mediated inhibition of cytokine-STAT signaling activity.
The IL6-family (IL6, IL11, LIF) cytokines represent important positive regulators of cartilage development, as demonstrated by profound cartilage disruption and dwarfism in Stüve-Wiedemann syndrome (MIM# 601559), which stems from loss-of-function of the LIF receptor (Dagoneau et al., 2004), or by the phenotype of knock-in mice expressing gp130 unable to signal via STAT1/3 (Sims et al., 2004). The inhibitory effect of FGFR3 signaling on cytokine-STAT signaling, described in RCS chondrocytes (Krejci et al., 2009), provides an alternate hypothesis for the role of STATs in FGFR3-related skeletal dysplasias (Fig. 3).
ERK MAP kinase pathway
The RAS-RAF-MEK-ERK signaling module represents a major pathway utilized by FGFR3. Sustained ERK activation is observed in the presence of ACH and TD mutations, and is associated with decreased chondrocyte proliferation, decreased matrix production, increased matrix degradation, and altered cell shape and differentiation, whereas inhibition of MEK can increase hypertrophic cell size and matrix production (Krejci et al., 2004; 2005, 2008b; Raucci et al., 2004; Murakami et al., 2004; Nowroozi et al., 2005; Raffioni et al., 1998; Yasoda et al., 2004). Mice expressing constitutively-active (ca) MEK1 under the control of the Col2a1 promoter display an ACH-like dwarfism, characterized by shortened appendicular skeleton due to incomplete chondrocyte hypertrophy, but without an effect on proliferation. Crossing caMek1 mice with Fgfr3−/− mice rescued the skeletal overgrowth caused by deletion of Fgfr3 (Murakami et al., 2004). Recently, ERK was found to be responsible for another effect of aberrant FGFR3 signaling on cartilage, namely premature fusion of the synchondroses (two opposing cartilage growth plates in the developing vertebrae and skull). This fusion leads to narrowing of the spinal canal at the foramen magnum and may contribute to spinal stenosis, both significant neurological complications of ACH (Matsushita et al., 2009; Sebastian et al., 2011).
In chondrocytes, unlike most other cell types, FGFR3 activation elicits prolonged ERK activation lasting for up to 24 hours (Krejci et al., 2004). This correlates with the known cellular responses to ERK, where transient activation appears to be crucial for mitogenic signaling of growth factors but sustained activity frequently leads to growth arrest (Roovers and Assoian, 2000). We believe that the unique growth-inhibitory outcome of FGFR3 activation observed in chondrocytes lies in the ability to maintain prolonged ERK activation. The molecular mechanism underlying this feature is unknown.
Modulation of FGFR3-ERK signaling by C-type natriuretic peptide (CNP)
Natriuretic peptides comprise a family of three structurally related peptides: atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and CNP, which mediate their function via binding and activation of transmembrane guanylyl cyclase receptors: guanylyl cyclase A (NPRA) and B (NPRB) (Garbers, 1990). In contrast to ANP and BNP, which are produced in the heart and act as renal hormones (Nakao et al., 1992), CNP is expressed by chondrocytes and acts as a physiological regulator of cartilage. Genetic manipulations in mice have shown the essential role of CNP signaling in regulation of cartilage growth. Nppc (CNP) null mice are dwarfed due to the size reduction of both proliferative and hypetrophic zone of the epiphyseal growth plates (Chusho et al., 2001), in contrast to transgenic mice overexpressing CNP under the control of the Col2a1 promoter, which show a general skeletal overgrowth due to expansion of proliferating and hypertrophic chondrocytes within the growth plate (Yasoda et al., 2004). Targeting other components of CNP signaling system produces similar phenotypes, with profound dwarfism observed in mice with impaired function of CNP receptor NPRB, or protein kinase G-II (PKGII) which represents major signaling intermediate of the CNP pathway (Tsuji and Kunieda, 2005; Pfeifer et al., 1996). Furthermore, a form of human dwarfism, acromesomelic dysplasia Maroteaux-type (MIM# 602875), is caused by loss-of-function of NPRB (Bartels et al., 2004), altogether implicating CNP as an important physiological positive regulator of cartilage growth.
In their groundbreaking study, Yasoda at al. showed that CNP overexpression partially rescues the dwarfism caused by the G380R (ACH)-FGFR3 mutation in mice (Yasoda et al., 2004). In RCS chondrocytes, CNP rescued many phenotypes of FGFR3 activation, including the inhibition of proliferation, loss of the extracellular matrix, induction of MMP activity and others. CNP interfered with FGFR3-mediated activation of the RAS-ERK pathway, likely via PKGII-dependent inhibitory phosphorylation of RAF1 kinase at S43, resulting in the uncoupling of the RAS/RAF1 interaction, and decreased activation of ERK (Krejci et al., 2005; Yasoda et al., 2004; Suhasini et al., 1998). These data demonstrate that CNP executes its positive regulation of cartilage growth, in part, via inhibition of the FGFR3 signaling system (reviewed in Pejchalova et al., 2007) (Fig. 3). Moreover, since CNP and FGFR3 represent physiological positive and negative regulators of cartilage growth, the interaction between the two pathways constitutes one of the major mechanisms of regulation of the dimensions of the skeleton.
Phosphoinositol-3-kinase (PI3K)/AKT pathway
When activated by FGFRs, PI3K phosphorylates membrane phosphatidylinositol to create the phospholipids phosphatidylinositol 3,4,5-triphosphate and phosphatidylinositol 3,4-biphosphate (Brazil and Hemmings, 2001), which provide docking sites for the PH domain within AKT kinase causing its translocation and activation at the cell membrane (Franke et al., 1997). Several lines of evidence implicate PI3K/AKT signaling in cartilage development. Akt1/2 null mice exhibit severe dwarfism and delayed bone development (Peng et al., 2003), similar to the mice lacking IGF-1 receptor (Liu et al., 1993), suggesting that IGF-1 signaling in cartilage occurs primarily via the PI3K/AKT pathway. IGF-1 mediates the action of growth hormone on cartilage and bone (Daughaday and Rotwein, 1989), and is capable of rescue, in a PI3K-dependent manner, the proliferation of ATDC5 chondrogenic cells expressing activated FGFR3 (Koike et al., 2003). Interestingly, FGFR3 activation in RCS chondrocytes results in weak initial AKT activation that is later inhibited below the basal levels of activity (Krejci et al., 2004; Raucci et al., 2004). Moreover, the expression of a constitutively active AKT in the RCS cells partially rescues the growth-inhibitory effects of FGFR3 signaling, possibly via direct targeting of cyclin E/cdk2 activity, which is inhibited by FGFR3 (Priore et al., 2006).
The evidence mentioned above suggests that the PI3K/AKT pathway counteracts the effects of FGFR3 signaling in chondrocytes, and therefore the lack of AKT activation by FGFR3 may directly contribute to the unique effects of FGFR3 signaling in chondrocytes. The mechanism of this feature is unclear and open for future investigation. One intriguing possibility lies in the competition of the PI3K/AKT and ERK pathways for active FGFR3, mediated through interactions with the adapter protein GAB1. Phosphorylated by FGFR3, GAB1 binds both the p85 subunit of PI3K (to activate PI3K/AKT pathway) and SHP2 phosphatase (to activate ERK). SHP2 activates the ERK pathway via recruiting GRB2-SOS1 dimers to the cell membrane, but also dephosphorylates p85 binding sites on GAB1, thus compromising p85 recruitment (Yu et al., 2002; Hadari et al., 1998; Ong et al., 2001). The strong SHP2 recruitment by GAB1 (and corresponding massive ERK activation), both triggered by FGFR3 in chondrocytes (Krejci et al., 2004; 2007b), may thus account for the lack of PI3K/AKT activity as a result of diminished p85 binding to GAB1.
Activating mutations stabilize FGFR3
Although the pattern of FGFR3 expression in ACH or TD growth plates resembles that of wt FGFR3, mutations appear to upregulate FGFR3. First noted by Delezoide et al. in 1997, this phenomenon was since observed in human ACH growth plates as well as in G369C and K644E-Fgfr3 (TD) mice (Delezoide et al., 1997; Monsonego-Ornan et al., 2000; Legeai-Mallet et al., 2004; Li et al., 1999; Chen et al., 1999). When compared to wt FGFR3, mutant FGFR3 accumulates to an extent depending on the level of activity (Cho et al., 2004; Guo et al., 2008). It is now clear that a quantitative increase in activation of canonical pathways rather than activation of novel pathways by FGFR3 accounts for skeletal dysplasias. In this context, accumulation of mutated FGFR3 in chondrocytes may be critical to the development of skeletal dysplasias, yet little is known about the mechanisms regulating this phenomenon.
One explanation for FGFR3 stabilization might lie in decreased degradation due to defective lysosomal targeting of mutated FGFR3. For many protein kinases, this process depends on ubiquitination by c-Cbl ubiquitin ligase, which earmarks the client protein for degradation in the proteasome or lysosome (Sanjay et al., 2001). When expressed in Cos7 cells, K644E-FGFR3 accumulated 30% more than wt FGFR3, which correlated with decreased ubiquitination mediated by c-Cbl, and lysosomal localization of FGFR3. Interestingly, c-Cbl was found to be phosphorylated by FGFR3 at levels corresponding to FGFR3 activity, implying that this phosphorylation may account for defective FGFR3 degradation (Cho et al., 2004). Alternatively, c-Cbl may be sequestered from FGFR3 via its interaction with sprouty2. As demonstrated by Guo et al., sprouty2 is induced by aberrant FGFR3 signaling both in vivo and in vitro at levels positively correlated with the level of FGFR3 activation. Moreover, FGFR3 phosphorylates sprouty2 on Y55, which appears to be critical for its interaction with c-Cbl (Guo et al., 2008).
Although the abovementioned evidence clearly implicates c-Cbl-mediated ubiquitination in FGFR3 degradation, these data are in contrast with studies reporting strong ubiquitination of G380R, K650E, R248C, Y373C and X807R-FGFR3, correlating with FGFR3 activity, that had no effect on FGFR3 stability, and was independent of c-Cbl (Monsonego-Ornan et al., 2002; Bonaventure et al., 2007). Future research should answer whether c-Cbl mediates poly-ubiquitination of FGFR3, which signals for degradation, or mono-ubiquitination which serves a regulatory rather then degradative function. Moreover, a detailed analysis of c-Cbl recruitment reveals a complex interaction between c-Cbl and members of FGFR-proximal signaling complexes such as FRS2, GAB1, GRB2 and SHP2 (Wong et al., 2002), suggesting that cell type-specific differences in the dynamics of such signaling complexes may influence c-Cbl’s effect on FGFR3 stability.
Areas for future research
In this article, we attempted to describe the known molecular mechanisms of FGFR3-related skeletal dysplasias. It is safe to conclude that, despite 16 years of research, we still remain ignorant of many features of FGFR3 signaling in cartilage. Important questions covering virtually all aspects of FGFR3 function at both the molecular and cell/tissue level await answers. A detailed knowledge of FGFR3 signal transduction in chondrocytes is not only essential for our understanding the function of FGFR signaling system in general, but also for designing a treatment for ACH or FGFR3-driven tumors. Even for the most investigated feature of FGFR3’s effects, growth arrest, much remains unknown. ERK is clearly part of the story, as demonstrated by its targeting via chemical or CNP-mediated inhibition, over-expression of a dominant-negative RAS mutant or siRNA-mediated knock-down (Krejci et al., 2004, 2005, 2008b; Raucci et al., 2004). Yet even complete inhibition of ERK activity results only in approximately 50% rescue of the growth arrest (Krejci et al., 2005; 2007c), clearly suggesting that there are other pathways that contribute significantly. This might be the p38 MAP kinase pathway or the STAT1 pathway as suggested by others (Raucci et al., 2004), but our experiments exclude both options (Krejci et al., 2004; 2008b). Therefore, there are as yet unknown mechanisms accounting for half of the growth-inhibitory effect of FGFR3 signaling in chondrocytes. Some areas for future research are briefly discussed below.
FGFR3-specific adapterome and its role in prolonged ERK activation in chondrocytes
At present, little is known about FGFR3’s interaction with its proximal signaling complexes, particularly those involved in ERK activation. Activated FGFRs recruit the ERK pathway via adapter-mediated translocation of GRB2-SOS1 dimers to the cell membrane where they activate the small GTPase RAS, and subsequently the RAF-MEK-ERK signaling module. FRS2 is a major adapter involved in this process. FGFRs phosphorylate FRS2 on at least six tyrosine residues that then serve as binding sites for GRB2-SOS1 (Y196, Y306, Y349, Y392) and SHP2-GRB2-SOS1 (Y436, Y471) complexes, which direct a significant amount of SOS1 (son of sevenless) RAS guanine nucleotide exchange factor to the cell membrane (Kouhara et al., 1997; Hadari et al., 1998). In addition to its positive role in ERK activation, FRS2 also represents a site of potent negative feedback. ERK phosphorylates FRS2 at multiple threonine residues that cluster into three sites adjacent to the phosphorylated tyrosines (T132/T135/T138, T376 and T452/T455/T458/T463), leading to diminished GRB2-SOS1 recruitment, and downregulation of ERK activity (Lax et al., 2002).
We found that FRS2 negative-feedback is preserved in FGFR3-ERK signaling in chondrocytes, but without a corresponding down-regulation of ERK activity (Krejci et al., 2007b). In addition to FRS2, FGFR3 employs additional adapters such as SHC and GAB1 to activate ERK in chondrocytes, implying a functional compensation for the FRS2-negative feedback. We argue that utilization of multiple adapters to recruit GRB2-SOS1 may render FGFR3-mediated ERK activation insensitive to FRS2-mediated negative feedback, thus allowing for its prolonged activation. In our opinion, the extraordinary ability of FGFR3 to induce sustained ERK activation in chondrocytes is the key to many unique features of FGFR3 signaling. A detailed elucidation of FGFR3 interactions with its adapter proteins may thus shed light on many aspects of FGFR3-related skeletal dysplasias that are poorly characterized to date. Future research should illuminate the entire composition of the FGFR3-specific adapterome (a small proteome consisted of adapter molecules used by FGFR3 to engage its downstream signaling), as well as the specific roles each adapter plays in FGFR3 signaling.
The cell/tissue phenotypes regulated by FGFR3 in cartilage
Another important area of future research lies in the exact nature of cellular processes underlying aberrant FGFR3 activation in cartilage. Profoundly disturbed chondrocyte proliferation and hypertrophic differentiation can be easily observed in the growth plate sections of individuals suffering from TD (Fig. 2), but we know very little about cellular processes that mediate such changes. In vivo evidence supporting the induction of apoptosis is weak. As discussed in detail above, we argue that induction of premature chondrocyte senescence might represent a part of FGFR3 effect on chondrocyte cell function (Krejci et al., 2010b). Directly related to this question is emerging evidence that aberrant FGFR3 activation causes profound changes in the cellular fate of chondrocytes, causing their de-differentiation or perhaps partial trans-differentiation into a bone-like cell, manifested by induction of several genes expressed in mineralized tissues, such as Osteomodulin, Bone sialoprotein, CTGF, Osteonectin, Osteocalcin, Osteoglycin, Osteoactivin and Carbonic anhydrase II (Weizman et al., 2005; Chen et al., 1999; Dailey et al., 2003; P. Krejci, unpublished). However, evidence supporting this hypothesis is limited mostly to in vitro observations, leaving this question open for future investigation. Due to the lack of suitable in vitro cellular models for FGFR3-mediated chondrocyte differentiation, careful analyses of existing mouse models for FGFR3-related skeletal dysplasias might provide answers to these intriguing questions.
Intracellular effectors of FGFR3 signaling in chondrocytes
Finally, the spectrum of FGFR3 substrates is likely much broader than currently appreciated. Experiments targeted at identification of protein motifs phosphorylated by FGFR3, such as substrate profiling analysis, may yield many novel molecules involved in FGFR3 signaling. Moreover, it is likely that the substrate-specificity differs significantly between wt and mutated FGFR3, as we recently demonstrated for FGFR3-mediated phosphorylation of STAT1 at Y701, which is efficiently phosphorylated by K650M or K650E-FGFR3 but not by the other activating mutants (Krejci et al., 2008b).
Some of the FGFR3 substrates may act as intracellular effectors mediating its signaling in cartilage. A search for such molecules may uncover unexpected players in the mechanism of FGFR3 signaling, as recently demonstrated by the discovery of participation of the SNAIL transcription factor in FGFR3 signaling in skeletal dysplasias (de Frutos et al., 2007). SNAIL functions as a transcriptional repressor of the E-cadherin gene, through which it mediates epithelial-to-mesenchymal transition in a variety of developmental and pathological processes (Batlle et al., 2000). Overexpression of SNAIL1 in developing bones causes an ACH-like phenotype in mice, while abolition of SNAIL1 abrogates FGFR3-mediated ERK activation in cultured chondrocytes, clearly defining SNAIL1 as a novel mediator of pathological FGFR3 signaling in cartilage (de Frutos et al., 2007).
Therapeutic targeting of FGFR3
The varied nature of different FGFR3 disorders necessitates development of disease-specific therapies. In multiple myeloma (MM), FGFR3 exists in the over-expressed state in plasma cells residing in the bone marrow, thus making them potentially accessible to treatment delivered via the circulation. This contrasts to the situation in skeletal dysplasias, where FGFR3 is expressed at physiological levels in cartilage with its poor blood supply and dense matrix that is generally regarded as impenetrable for larger molecules. Although the suppression of FGFR3 function represents the general therapeutic goal, clinical objectives may vary substantially, depending on the disease. For instance, while the elimination of the FGFR3-expressing cells is desired in MM, restoration of normal chondrocyte growth is the major treatment objective in skeletal dysplasias. While an aggressive, partially toxic treatment might be used in adults suffering from life-threatening MM or other cancers, such a therapy administered to growing children to increase height would not be acceptable.
The mechanics of FGFR3 signaling offers targeting opportunities for different approaches, aimed at FGFR3 production and maturation, suppression of its activation or activation of downstream signaling mediators (reviewed in Aviezer et al., 2003). Several of such possibilities have been explored experimentally and are briefly discussed below. To date, no FGFR3 targeting via locked nucleic acid (LNA) or adeno-associated virus (AAV)-mediated expression of truncated, soluble FGFR3 variant have been reported, although these approaches have been used successfully for receptor tyrosine kinases (Davidoff et al., 2002; Zhang et al., 2011), and thus represent a viable option to inhibit FGFR3 signaling in vivo. In an approach to target biogenesis of FGFR3, the Golgi transport inhibitor nordihydroguaiaretic acid (NDGA) was recently reported to suppress FGFR3 signaling in MM cells, causing their apoptosis (Meyer et al, 2008). NDGA thus offers a possibility to target FGFR3 via inhibition of a specific cellular compartment, although this activity may be limited to K650M or K650E-FGFR3 mutants, known to signal preferentially from the Golgi apparatus (Lievens and Liboi, 2003). Recently, FGFR3 was found to be a client of the heat-shock protein 90 (HSP90) molecular chaperone, and thus the disruption of FGFR3/HSP90 interaction may inhibit FGFR3 signaling in both MM cells and chondrocytes via interference with its proper folding and maturation (Nakashima et al., 2010; Laederich et al., 2011).
With the original invention of protein tyrosine kinase inhibitors based on an oxindole core (Mohammadi et al., 1997), it was possible to design a small chemical molecule that binds FGFR3 directly and inhibits its kinase activity, either via a competition with ATP for the ATP-binding site, or through an ATP-independent mechanism. Today, several such inhibitors exist, including compounds named SU5402, PD173074, SU6668, PD161570, PKC412, CHIR-258, NF449 and AZD1480, which display variable inhibitory activity towards FGFRs and corresponding activity against FGFR signaling in experimental cell or animal models in vitro (Mohammadi et al., 1998; Nakashima et al., 2010; Chen et al., 2005; Krejci et al., 2010a; Trudel et al., 2005; Xin et al., 2006; Scuto et al., 2011). A central issue of chemical inhibitors is their target specificity, as known compounds typically inhibit other FGFR kinases in addition to FGFR3, as well as other kinases such as VEGFR, PDGFR, JAK and IGF1R (Krejci et al., 2010a; Scuto et al., 2011). Although the slight lack of specificity of a given inhibitor might not represent a problem, or even be advantageous for anti-cancer therapy, it could certainly present an important problem when used for treatment in FGFR3-related skeletal dysplasias. In fact, PD173074 (Mohammadi et al., 1998), which is to our opinion the one of the most specific FGFR3 inhibitors known to date, inhibits both FGFR3 and FGFR1 with the same efficiency, and thus might interfere with physiological functions of FGFR1 when used for treatment of ACH.
Another conceptually different approach to target FGFR3 directly is represented by an anti-FGFR3 antibody, specifically designed to bind the extracellular part of FGFR3 and interfere with ligand-mediated FGFR3 dimerization and activation. To date, two such antibodies were developed, PRO-001 and R3Mab, both showing potent anti-tumor activity in mice xenograft models to FGFR3-driven MM and bladder carcinoma (Trudel et al., 2006; Qing et al., 2009). Anti-FGFR3 antibodies represent an excellent alternative to chemical FGFR3 inhibitors, due to their greater target specificity. When used in skeletal dysplasias, however, FGFR3 targeting via antibody however carries an inherent risk of an antibody-dependent cell cytotoxic reaction in cartilage (Qing et al., 2009).
Compared to other means of FGFR3 inhibition, CNP is a physiological negative regulator of FGFR3 in cartilage. As discussed in detail elsewhere in this article, the predominant localization of the CNP pathway in cartilage, its relative straightforward manipulation, as well as an existing practical demonstration of CNP’s potential to rescue pathological FGFR3 signaling in murine models makes CNP the foremost candidate for a therapy aimed at improving cartilage growth in FGFR3-related skeletal dysplasias. According to a recent public announcement, a stable CNP analogue developed by BioMarin Pharmaceuticals (http://www.bmrn.com) is progressing well towards treatment for ACH, with human clinical trials expected to begin in 2012.
Acknowledgments
This work was supported by NIH 5P01HD022657; NIH GCRC Grant M01-RR00425; Yang Sheng Tang USA Company; Ministry of Education, Youth and Sports of the Czech Republic (MSM0021622430); Grant Agency of the Czech Republic (301/09/0587, 305/11/0752) and a Winnick Family Research Scholars award (WRW).
References
- Aikawa T, Segre GV, Lee K. Fibroblast growth factor inhibits chondrocytic growth through induction of p21 and subsequent inactivation of cyclin E-Cdk2. J Biol Chem. 2001;276:29347–52. doi: 10.1074/jbc.M101859200. [DOI] [PubMed] [Google Scholar]
- Aviezer D, Golembo M, Yayon A. Fibroblast growth factor receptor-3 as a therapeutic target for achondroplasia-genetic short limbed dwarfism. Curr Drug Targets. 2003;4:353–65. doi: 10.2174/1389450033490993. [DOI] [PubMed] [Google Scholar]
- Avivi A, Zimmer Y, Yayon A, Yarden Y, Givol D. Flg-2, a new member of the family of fibroblast growth factor receptors. Oncogene. 1991;6:1089–92. [PubMed] [Google Scholar]
- Bartels CF, Bukulmez H, Padayatti P, Rhee DK, van Ravenswaaij-Arts C, Pauli RM, Mundlos S, Chitayat D, Shih LY, Al-Gazali LI, et al. Mutations in the transmembrane natriuretic peptide receptor NPR-B impair skeletal growth and cause acromesomelic dysplasia, type Maroteaux. Am J Hum Genet. 2004;75:27–34. doi: 10.1086/422013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–9. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- Baujat G, Legeai-Mallet L, Finidori G, Cormier-Daire V, Le Merrer M. Achondroplasia. Best Pract Res Clin Rheumatol. 2008;22:3–18. doi: 10.1016/j.berh.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Beever JE, Smit MA, Meyers SN, Hadfield TS, Bottema C, Albretsen J, Cockett NE. A single-base change in the tyrosine kinase II domain of ovine FGFR3 causes hereditary chondrodysplasia in sheep. Anim Genet. 2006;37:66–71. doi: 10.1111/j.1365-2052.2005.01398.x. [DOI] [PubMed] [Google Scholar]
- Bellus GA, Hefferon TW, Ortiz de Luna RI, Hecht JT, Horton WA, Machado M, Kaitila I, McIntosh I, Francomano CA. Achondroplasia is defined by recurrent G380R mutations of FGFR3. Am J Hum Genet. 1995;56:368–73. [PMC free article] [PubMed] [Google Scholar]
- Bellus GA, Spector EB, Speiser PW, Weaver CA, Garber AT, Bryke CR, Israel J, Rosengren SS, Webster MK, Donoghue DJ, et al. Distinct missense mutations of the FGFR3 lys650 codon modulate receptor kinase activation and the severity of the skeletal dysplasia phenotype. Am J Hum Genet. 2000;67:1411–21. doi: 10.1086/316892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi T, Yayon A, Gertler A, Monsonego-Ornan E. Suppressors of cytokine signaling (SOCS) 1 and SOCS3 interact with and modulate fibroblast growth factor receptor signaling. J Cell Sci. 2006;119:380–7. doi: 10.1242/jcs.02740. [DOI] [PubMed] [Google Scholar]
- Bonaventure J, Horne WC, Baron R. The localization of FGFR3 mutations causing thanatophoric dysplasia type I differentially affects phosphorylation, processing and ubiquitylation of the receptor. Febs J. 2007;274:3078–93. doi: 10.1111/j.1742-4658.2007.05835.x. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–64. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- Brodie SG, Kitoh H, Lachman RS, Nolasco LM, Mekikian PB, Wilcox WR. Platyspondylic lethal skeletal dysplasia, San Diego type, is caused by FGFR3 mutations. Am J Med Genet. 1999;84:476–80. [PubMed] [Google Scholar]
- Brodie SG, Kitoh H, Lipson M, Sifry-Platt M, Wilcox WR. Thanatophoric dysplasia type I with syndactyly. Am J Med Genet. 1998;80:260–2. doi: 10.1002/(sici)1096-8628(19981116)80:3<260::aid-ajmg15>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Cappellen D, De Oliveira C, Ricol D, de Medina S, Bourdin J, Sastre-Garau X, Chopin D, Thiery JP, Radvanyi F. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet. 1999;23:18–20. doi: 10.1038/12615. [DOI] [PubMed] [Google Scholar]
- Chau M, Forcinito P, Andrade AC, Hegde A, Ahn S, Lui JC, Baron J, Nilsson O. Organization of the indian hedgehog - parathyroid hormone-related protein system in the postnatal growth plate. J Mol Endocrinol. 2011;47:99–107. doi: 10.1530/JME-10-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lee BH, Williams IR, Kutok JL, Mitsiades CS, Duclos N, Cohen S, Adelsperger J, Okabe R, Coburn A, et al. FGFR3 as a therapeutic target of the small molecule inhibitor PKC412 in hematopoietic malignancies. Oncogene. 2005;24:8259–67. doi: 10.1038/sj.onc.1208989. [DOI] [PubMed] [Google Scholar]
- Chen L, Adar R, Yang X, Monsonego EO, Li C, Hauschka PV, Yayon A, Deng CX. Gly369Cys mutation in mouse FGFR3 causes achondroplasia by affecting both chondrogenesis and osteogenesis. J Clin Invest. 1999;104:1517–25. doi: 10.1172/JCI6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Li C, Qiao W, Xu X, Deng C. A Ser(365)-->Cys mutation of fibroblast growth factor receptor 3 in mouse downregulates Ihh/PTHrP signals and causes severe achondroplasia. Hum Mol Genet. 2001;10:457–65. doi: 10.1093/hmg/10.5.457. [DOI] [PubMed] [Google Scholar]
- Chen X, Macica C, Nasiri A, Broadus AE. Regulation of articular chondrocyte proliferation and differentiation by indian hedgehog and parathyroid hormone-related protein. Arthritis Rheum. 2008;58:3788–97. doi: 10.1002/art.23985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesi M, Brents LA, Ely SA, Bais C, Robbiani DF, Mesri EA, Kuehl WM, Bergsagel PL. Activated fibroblast growth factor receptor 3 is an oncogene that contributes to tumor progression in multiple myeloma. Blood. 2001;97:729–36. doi: 10.1182/blood.v97.3.729. [DOI] [PubMed] [Google Scholar]
- Chesi M, Nardini E, Brents LA, Schrock E, Ried T, Kuehl WM, Bergsagel PL. Frequent translocation t(4;14)(p16.3; q32.3) in multiple myeloma is associated with increased expression and activating mutations of fibroblast growth factor receptor 3. Nat Genet. 1997;16:260–4. doi: 10.1038/ng0797-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin YE, Kitagawa M, Su WC, You ZH, Iwamoto Y, Fu XY. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science. 1996;272:719–22. doi: 10.1126/science.272.5262.719. [DOI] [PubMed] [Google Scholar]
- Cho JY, Guo C, Torello M, Lunstrum GP, Iwata T, Deng C, Horton WA. Defective lysosomal targeting of activated fibroblast growth factor receptor 3 in achondroplasia. Proc Natl Acad Sci U S A. 2004;101:609–14. doi: 10.1073/pnas.2237184100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chusho H, Tamura N, Ogawa Y, Yasoda A, Suda M, Miyazawa T, Nakamura K, Nakao K, Kurihara T, Komatsu Y, et al. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci U S A. 2001;98:4016–21. doi: 10.1073/pnas.071389098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12:390–7. doi: 10.1038/ng0496-390. [DOI] [PubMed] [Google Scholar]
- Cormier S, Delezoide AL, Benoist-Lasselin C, Legeai-Mallet L, Bonaventure J, Silve C. Parathyroid hormone receptor type 1/Indian hedgehog expression is preserved in the growth plate of human fetuses affected with fibroblast growth factor receptor type 3 activating mutations. Am J Pathol. 2002;161:1325–35. doi: 10.1016/S0002-9440(10)64409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Avis PY, Robertson SC, Meyer AN, Bardwell WM, Webster MK, Donoghue DJ. Constitutive activation of fibroblast growth factor receptor 3 by mutations responsible for the lethal skeletal dysplasia thanatophoric dysplasia type I. Cell Growth Differ. 1998;9:71–8. [PubMed] [Google Scholar]
- Dagoneau N, Scheffer D, Huber C, Al-Gazali LI, Di Rocco M, Godard A, Martinovic J, Raas-Rothschild A, Sigaudy S, Unger S, et al. Null leukemia inhibitory factor receptor (LIFR) mutations in Stuve-Wiedemann/Schwartz-Jampel type 2 syndrome. Am J Hum Genet. 2004;74:298–305. doi: 10.1086/381715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey L, Ambrosetti D, Mansukhani A, Basilico C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev. 2005;16:233–47. doi: 10.1016/j.cytogfr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Dailey L, Laplantine E, Priore R, Basilico C. A network of transcriptional and signaling events is activated by FGF to induce chondrocyte growth arrest and differentiation. J Cell Biol. 2003;161:1053–66. doi: 10.1083/jcb.200302075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughaday WH, Rotwein P. Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev. 1989;10:68–91. doi: 10.1210/edrv-10-1-68. [DOI] [PubMed] [Google Scholar]
- Davidoff AM, Nathwani AC, Spurbeck WW, Ng CY, Zhou J, Vanin EF. rAAV-mediated long-term liver-generated expression of an angiogenesis inhibitor can restrict renal tumor growth in mice. Cancer Res. 2002;62:3077–83. [PubMed] [Google Scholar]
- de Frutos CA, Vega S, Manzanares M, Flores JM, Huertas H, Martinez-Frias ML, Nieto MA. Snail1 is a transcriptional effector of FGFR3 signaling during chondrogenesis and achondroplasias. Dev Cell. 2007;13:872–83. doi: 10.1016/j.devcel.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Delezoide AL, Lasselin-Benoist C, Legeai-Mallet L, Brice P, Senee V, Yayon A, Munnich A, Vekemans M, Bonaventure J. Abnormal FGFR 3 expression in cartilage of thanatophoric dysplasia fetuses. Hum Mol Genet. 1997;6:1899–906. doi: 10.1093/hmg/6.11.1899. [DOI] [PubMed] [Google Scholar]
- Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84:911–21. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- Durbin JE, Hackenmiller R, Simon MC, Levy DE. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–50. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- Erlebacher A, Filvaroff EH, Gitelman SE, Derynck R. Toward a molecular understanding of skeletal development. Cell. 1995;80:371–8. doi: 10.1016/0092-8674(95)90487-5. [DOI] [PubMed] [Google Scholar]
- Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–8. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- Garbers DL. Guanylate cyclase receptor family. Recent Prog Horm Res. 1990;46:85–96. doi: 10.1016/b978-0-12-571146-3.50008-0. discussion 96–7. [DOI] [PubMed] [Google Scholar]
- Goriely A, Hansen RM, Taylor IB, Olesen IA, Jacobsen GK, McGowan SJ, Pfeifer SP, McVean GA, Meyts ER, Wilkie AO. Activating mutations in FGFR3 and HRAS reveal a shared genetic origin for congenital disorders and testicular tumors. Nat Genet. 2009;41:1247–52. doi: 10.1038/ng.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Degnin CR, Laederich MB, Lunstrum GP, Holden P, Bihlmaier J, Krakow D, Cho YJ, Horton WA. Sprouty 2 disturbs FGFR3 degradation in thanatophoric dysplasia type II: a severe form of human achondroplasia. Cell Signal. 2008;20:1471–7. doi: 10.1016/j.cellsig.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadari YR, Kouhara H, Lax I, Schlessinger J. Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol Cell Biol. 1998;18:3966–73. doi: 10.1128/mcb.18.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner C, van Oers JM, Vogt T, Landthaler M, Stoehr R, Blaszyk H, Hofstaedter F, Zwarthoff EC, Hartmann A. Mosaicism of activating FGFR3 mutations in human skin causes epidermal nevi. J Clin Invest. 2006;116:2201–2207. doi: 10.1172/JCI28163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BD, Spranger J. Hypochondroplasia: clinical and radiological aspects in 39 cases. Radiology. 1979;133:95–100. doi: 10.1148/133.1.95. [DOI] [PubMed] [Google Scholar]
- Harada D, Yamanaka Y, Ueda K, Nishimura R, Morishima T, Seino Y, Tanaka H. Sustained phosphorylation of mutated FGFR3 is a crucial feature of genetic dwarfism and induces apoptosis in the ATDC5 chondrogenic cell line via PLC gamma-activated STAT1. Bone. 2007;41:273–81. doi: 10.1016/j.bone.2006.11.030. [DOI] [PubMed] [Google Scholar]
- Harada D, Yamanaka Y, Ueda K, Tanaka H, Seino Y. FGFR3-related dwarfism and cell signaling. J Bone Miner Metab. 2009;27:9–15. doi: 10.1007/s00774-008-0009-7. [DOI] [PubMed] [Google Scholar]
- Hart KC, Robertson SC, Donoghue DJ. Identification of tyrosine residues in constitutively activated fibroblast growth factor receptor 3 involved in mitogenesis, Stat activation, and phosphatidylinositol 3-kinase activation. Mol Biol Cell. 2001;12:931–42. doi: 10.1091/mbc.12.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart KC, Robertson SC, Kanemitsu MY, Meyer AN, Tynan JA, Donoghue DJ. Transformation and Stat activation by derivatives of FGFR1, FGFR3, and FGFR4. Oncogene. 2000;19:3309–20. doi: 10.1038/sj.onc.1203650. [DOI] [PubMed] [Google Scholar]
- Henderson JE, Naski MC, Aarts MM, Wang D, Cheng L, Goltzman D, Ornitz DM. Expression of FGFR3 with the G380R achondroplasia mutation inhibits proliferation and maturation of CFK2 chondrocytic cells. J Bone Miner Res. 2000;15:155–65. doi: 10.1359/jbmr.2000.15.1.155. [DOI] [PubMed] [Google Scholar]
- Horton WA, Hall JG, Hecht JT. Achondroplasia. Lancet. 2007;370:162–72. doi: 10.1016/S0140-6736(07)61090-3. [DOI] [PubMed] [Google Scholar]
- Ibrahimi OA, Yeh BK, Eliseenkova AV, Zhang F, Olsen SK, Igarashi M, Aaronson SA, Linhardt RJ, Mohammadi M. Analysis of mutations in fibroblast growth factor (FGF) and a pathogenic mutation in FGF receptor (FGFR) provides direct evidence for the symmetric two-end model for FGFR dimerization. Mol Cell Biol. 2005;25:671–84. doi: 10.1128/MCB.25.2.671-684.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata T, Chen L, Li C, Ovchinnikov DA, Behringer RR, Francomano CA, Deng CX. A neonatal lethal mutation in FGFR3 uncouples proliferation and differentiation of growth plate chondrocytes in embryos. Hum Mol Genet. 2000;9:1603–13. doi: 10.1093/hmg/9.11.1603. [DOI] [PubMed] [Google Scholar]
- Iwata T, Li CL, Deng CX, Francomano CA. Highly activated Fgfr3 with the K644M mutation causes prolonged survival in severe dwarf mice. Hum Mol Genet. 2001;10:1255–64. doi: 10.1093/hmg/10.12.1255. [DOI] [PubMed] [Google Scholar]
- Johnson DE, Williams LT. Structural and functional diversity in the FGF receptor multi-gene family. Adv Cancer Res. 1993;60:1–41. doi: 10.1016/s0065-230x(08)60821-0. [DOI] [PubMed] [Google Scholar]
- Karaplis AC, Luz A, Glowacki J, Bronson RT, Tybulewicz VL, Kronenberg HM, Mulligan RC. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 1994;8:277–89. doi: 10.1101/gad.8.3.277. [DOI] [PubMed] [Google Scholar]
- Karsenty G, Ducy P, Starbuck M, Priemel M, Shen J, Geoffroy V, Amling M. Cbfa1 as a regulator of osteoblast differentiation and function. Bone. 1999;25:107–8. doi: 10.1016/s8756-3282(99)00111-8. [DOI] [PubMed] [Google Scholar]
- Kitoh H, Brodie SG, Kupke KG, Lachman RS, Wilcox WR. Lys650Met substitution in the tyrosine kinase domain of the fibroblast growth factor receptor gene causes thanatophoric dysplasia Type I. Mutations in brief no. 199. Online. Hum Mutat. 1998;12:362–3. [PubMed] [Google Scholar]
- Klint P, Claesson-Welsh L. Signal transduction by fibroblast growth factor receptors. Front Biosci. 1999;4:D165–77. doi: 10.2741/klint. [DOI] [PubMed] [Google Scholar]
- Koike M, Yamanaka Y, Inoue M, Tanaka H, Nishimura R, Seino Y. Insulin-like growth factor-1 rescues the mutated FGF receptor 3 (G380R) expressing ATDC5 cells from apoptosis through phosphatidylinositol 3-kinase and MAPK. J Bone Miner Res. 2003;18:2043–51. doi: 10.1359/jbmr.2003.18.11.2043. [DOI] [PubMed] [Google Scholar]
- Kolupaeva V, Laplantine E, Basilico C. PP2A-mediated dephosphorylation of p107 plays a critical role in chondrocyte cell cycle arrest by FGF. PLoS One. 2008;3:e3447. doi: 10.1371/journal.pone.0003447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosher RA, Kulyk WM, Gay SW. Collagen gene expression during limb cartilage differentiation. J Cell Biol. 1986;102:1151–6. doi: 10.1083/jcb.102.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouhara H, Hadari YR, Spivak-Kroizman T, Schilling J, Bar-Sagi D, Lax I, Schlessinger J. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell. 1997;89:693–702. doi: 10.1016/s0092-8674(00)80252-4. [DOI] [PubMed] [Google Scholar]
- Krejci P, Bryja V, Pachernik J, Hampl A, Pogue R, Mekikian P, Wilcox WR. FGF2 inhibits proliferation and alters the cartilage-like phenotype of RCS cells. Exp Cell Res. 2004;297:152–64. doi: 10.1016/j.yexcr.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Krejci P, Krakow D, Mekikian PB, Wilcox WR. Fibroblast growth factors 1, 2, 17, and 19 are the predominant FGF ligands expressed in human fetal growth plate cartilage. Pediatr Res. 2007a;61:267–72. doi: 10.1203/pdr.0b013e318030d157. [DOI] [PubMed] [Google Scholar]
- Krejci P, Masri B, Fontaine V, Mekikian PB, Weis M, Prats H, Wilcox WR. Interaction of fibroblast growth factor and C-natriuretic peptide signaling in regulation of chondrocyte proliferation and extracellular matrix homeostasis. J Cell Sci. 2005;118:5089–100. doi: 10.1242/jcs.02618. [DOI] [PubMed] [Google Scholar]
- Krejci P, Masri B, Salazar L, Farrington-Rock C, Prats H, Thompson LM, Wilcox WR. Bisindolylmaleimide I suppresses fibroblast growth factor-mediated activation of Erk MAP kinase in chondrocytes by preventing Shp2 association with the Frs2 and Gab1 adaptor proteins. J Biol Chem. 2007b;282:2929–36. doi: 10.1074/jbc.M606144200. [DOI] [PubMed] [Google Scholar]
- Krejci P, Murakami S, Prochazkova J, Trantirek L, Chlebova K, Ouyang Z, Aklian A, Smutny J, Bryja V, Kozubik A, et al. NF449 is a novel inhibitor of fibroblast growth factor receptor 3 (FGFR3) signaling active in chondrocytes and multiple myeloma cells. J Biol Chem. 2010a;285:20644–53. doi: 10.1074/jbc.M109.083626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejci P, Pejchalova K, Wilcox WR. Simple, mammalian cell-based assay for identification of inhibitors of the Erk MAP kinase pathway. Invest New Drugs. 2007c;25:391–5. doi: 10.1007/s10637-007-9054-7. [DOI] [PubMed] [Google Scholar]
- Krejci P, Prochazkova J, Bryja V, Jelinkova P, Pejchalova K, Kozubik A, Thompson LM, Wilcox WR. Fibroblast growth factor inhibits interferon gamma-STAT1 and inter-leukin 6-STAT3 signaling in chondrocytes. Cell Signal. 2009;21:151–60. doi: 10.1016/j.cellsig.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejci P, Prochazkova J, Smutny J, Chlebova K, Lin P, Aklian A, Bryja V, Kozubik A, Wilcox WR. FGFR3 signaling induces a reversible senescence phenotype in chondrocytes similar to oncogene-induced premature senescence. Bone. 2010b;47:102–10. doi: 10.1016/j.bone.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejci P, Salazar L, Goodridge HS, Kashiwada TA, Schibler MJ, Jelinkova P, Thompson LM, Wilcox WR. STAT1 and STAT3 do not participate in FGF-mediated growth arrest in chondrocytes. J Cell Sci. 2008a;121:272–81. doi: 10.1242/jcs.017160. [DOI] [PubMed] [Google Scholar]
- Krejci P, Salazar L, Kashiwada TA, Chlebova K, Salasova A, Thompson LM, Bryja V, Kozubik A, Wilcox WR. Analysis of STAT1 activation by six FGFR3 mutants associated with skeletal dysplasia undermines dominant role of STAT1 in FGFR3 signaling in cartilage. PLoS One. 2008b;3:e3961. doi: 10.1371/journal.pone.0003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg HM. PTHrP and skeletal development. Ann N Y Acad Sci. 2006;1068:1–13. doi: 10.1196/annals.1346.002. [DOI] [PubMed] [Google Scholar]
- L’Hote CG, Knowles MA. Cell responses to FGFR3 signalling: growth, differentiation and apoptosis. Exp Cell Res. 2005;304:417–31. doi: 10.1016/j.yexcr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Laederich MB, Degnin CR, Lunstrum GP, Holden P, Horton WA. Fibroblast growth factor receptor 3 (FGFR3) is a strong heat shock protein 90 (Hsp90) client: implications for therapeutic manipulation. J Biol Chem. 2011 doi: 10.1074/jbc.M110.206151. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, Karperien M, Defize LH, Ho C, Mulligan RC, et al. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273:663–6. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- Lax I, Wong A, Lamothe B, Lee A, Frost A, Hawes J, Schlessinger J. The docking protein FRS2alpha controls a MAP kinase-mediated negative feedback mechanism for signaling by FGF receptors. Mol Cell. 2002;10:709–19. doi: 10.1016/s1097-2765(02)00689-5. [DOI] [PubMed] [Google Scholar]
- Legeai-Mallet L, Benoist-Lasselin C, Delezoide AL, Munnich A, Bonaventure J. Fibroblast growth factor receptor 3 mutations promote apoptosis but do not alter chondrocyte proliferation in thanatophoric dysplasia. J Biol Chem. 1998;273:13007–14. doi: 10.1074/jbc.273.21.13007. [DOI] [PubMed] [Google Scholar]
- Legeai-Mallet L, Benoist-Lasselin C, Munnich A, Bonaventure J. Overexpression of FGFR3, Stat1, Stat5 and p21Cip1 correlates with phenotypic severity and defective chondrocyte differentiation in FGFR3-related chondrodysplasias. Bone. 2004;34:26–36. doi: 10.1016/j.bone.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Li C, Chen L, Iwata T, Kitagawa M, Fu XY, Deng CX. A Lys644Glu substitution in fibroblast growth factor receptor 3 (FGFR3) causes dwarfism in mice by activation of STATs and ink4 cell cycle inhibitors. Hum Mol Genet. 1999;8:35–44. doi: 10.1093/hmg/8.1.35. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhu YX, Plowright EE, Bergsagel PL, Chesi M, Patterson B, Hawley TS, Hawley RG, Stewart AK. The myeloma-associated oncogene fibroblast growth factor receptor 3 is transforming in hematopoietic cells. Blood. 2001;97:2413–9. doi: 10.1182/blood.v97.8.2413. [DOI] [PubMed] [Google Scholar]
- Lievens PM, Liboi E. The thanatophoric dysplasia type II mutation hampers complete maturation of fibroblast growth factor receptor 3 (FGFR3), which activates signal transducer and activator of transcription 1 (STAT1) from the endoplasmic reticulum. J Biol Chem. 2003;278:17344–9. doi: 10.1074/jbc.M212710200. [DOI] [PubMed] [Google Scholar]
- Lievens PM, Mutinelli C, Baynes D, Liboi E. The kinase activity of fibroblast growth factor receptor 3 with activation loop mutations affects receptor trafficking and signaling. J Biol Chem. 2004;279:43254–60. doi: 10.1074/jbc.M405247200. [DOI] [PubMed] [Google Scholar]
- Lievens PM, Roncador A, Liboi E. K644E/M FGFR3 mutants activate Erk1/2 from the endoplasmic reticulum through FRS2 alpha and PLC gamma-independent pathways. J Mol Biol. 2006;357:783–92. doi: 10.1016/j.jmb.2006.01.058. [DOI] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Liu Z, Xu J, Colvin JS, Ornitz DM. Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18. Genes Dev. 2002;16:859–69. doi: 10.1101/gad.965602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long F, Zhang XM, Karp S, Yang Y, McMahon AP. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 2001;128:5099–108. doi: 10.1242/dev.128.24.5099. [DOI] [PubMed] [Google Scholar]
- Logie A, Dunois-Larde C, Rosty C, Levrel O, Blanche M, Ribeiro A, Gasc JM, Jorcano J, Werner S, Sastre-Garau X, et al. Activating mutations of the tyrosine kinase receptor FGFR3 are associated with benign skin tumors in mice and humans. Hum Mol Genet. 2005;14:1153–60. doi: 10.1093/hmg/ddi127. [DOI] [PubMed] [Google Scholar]
- Macsai CE, Foster BK, Xian CJ. Roles of Wnt signalling in bone growth, remodelling, skeletal disorders and fracture repair. J Cell Physiol. 2008;215:578–87. doi: 10.1002/jcp.21342. [DOI] [PubMed] [Google Scholar]
- Maroteaux P, Lamy M, Robert JM. Thanatophoric dwarfism. Presse Med. 1967;75:2519–24. [PubMed] [Google Scholar]
- Martinez-Frias ML, de Frutos CA, Bermejo E, Nieto MA. Review of the recently defined molecular mechanisms underlying thanatophoric dysplasia and their potential therapeutic implications for achondroplasia. Am J Med Genet A. 2010;152A:245–55. doi: 10.1002/ajmg.a.33188. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Wilcox WR, Chan YY, Kawanami A, Bukulmez H, Balmes G, Krejci P, Mekikian PB, Otani K, Yamaura I, et al. FGFR3 promotes synchondrosis closure and fusion of ossification centers through the MAPK pathway. Hum Mol Genet. 2009;18:227–40. doi: 10.1093/hmg/ddn339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AN, McAndrew CW, Donoghue DJ. Nordihydroguaiaretic acid inhibits an activated fibroblast growth factor receptor 3 mutant and blocks downstream signaling in multiple myeloma cells. Cancer Res. 2008;68:7362–70. doi: 10.1158/0008-5472.CAN-08-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M, Froum S, Hamby JM, Schroeder MC, Panek RL, Lu GH, Eliseenkova AV, Green D, Schlessinger J, Hubbard SR. Crystal structure of an angiogenesis inhibitor bound to the FGF receptor tyrosine kinase domain. Embo J. 1998;17:5896–904. doi: 10.1093/emboj/17.20.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh BK, Hubbard SR, Schlessinger J. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276:955–60. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- Monsonego-Ornan E, Adar R, Feferman T, Segev O, Yayon A. The transmembrane mutation G380R in fibroblast growth factor receptor 3 uncouples ligand-mediated receptor activation from down-regulation. Mol Cell Biol. 2000;20:516–22. doi: 10.1128/mcb.20.2.516-522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsonego-Ornan E, Adar R, Rom E, Yayon A. FGF receptors ubiquitylation: dependence on tyrosine kinase activity and role in downregulation. FEBS Lett. 2002;528:83–9. doi: 10.1016/s0014-5793(02)03255-6. [DOI] [PubMed] [Google Scholar]
- Murakami S, Balmes G, McKinney S, Zhang Z, Givol D, de Crombrugghe B. Constitutive activation of MEK1 in chondrocytes causes Stat1-independent achondroplasia-like dwarfism and rescues the Fgfr3-deficient mouse phenotype. Genes Dev. 2004;18:290–305. doi: 10.1101/gad.1179104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao K, Ogawa Y, Suga S, Imura H. Molecular biology and biochemistry of the natriuretic peptide system. I: Natriuretic peptides. J Hypertens. 1992;10:907–12. [PubMed] [Google Scholar]
- Nakashima T, Ishii T, Tagaya H, Seike T, Nakagawa H, Kanda Y, Akinaga S, Soga S, Shiotsu Y. New molecular and biological mechanism of antitumor activities of KW-2478, a novel nonansamycin heat shock protein 90 inhibitor, in multiple myeloma cells. Clin Cancer Res. 2010;16:2792–802. doi: 10.1158/1078-0432.CCR-09-3112. [DOI] [PubMed] [Google Scholar]
- Naski MC, Colvin JS, Coffin JD, Ornitz DM. Repression of hedgehog signaling and BMP4 expression in growth plate cartilage by fibroblast growth factor receptor 3. Development. 1998;125:4977–88. doi: 10.1242/dev.125.24.4977. [DOI] [PubMed] [Google Scholar]
- Naski MC, Wang Q, Xu J, Ornitz DM. Graded activation of fibroblast growth factor receptor 3 by mutations causing achondroplasia and thanatophoric dysplasia. Nat Genet. 1996;13:233–7. doi: 10.1038/ng0696-233. [DOI] [PubMed] [Google Scholar]
- Nowroozi N, Raffioni S, Wang T, Apostol BL, Bradshaw RA, Thompson LM. Sustained ERK1/2 but not STAT1 or 3 activation is required for thanatophoric dysplasia phenotypes in PC12 cells. Hum Mol Genet. 2005;14:1529–38. doi: 10.1093/hmg/ddi161. [DOI] [PubMed] [Google Scholar]
- Oberklaid F, Danks DM, Jensen F, Stace L, Rosshandler S. Achondroplasia and hypochondroplasia. Comments on frequency, mutation rate, and radiological features in skull and spine. J Med Genet. 1979;16:140–6. doi: 10.1136/jmg.16.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong SH, Hadari YR, Gotoh N, Guy GR, Schlessinger J, Lax I. Stimulation of phosphatidylinositol 3-kinase by fibroblast growth factor receptors is mediated by coordinated recruitment of multiple docking proteins. Proc Natl Acad Sci U S A. 2001;98:6074–9. doi: 10.1073/pnas.111114298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM. FGF signaling in the developing endochondral skeleton. Cytokine Growth Factor Rev. 2005;16:205–13. doi: 10.1016/j.cytogfr.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos-Bueno MR, Wilcox WR, Jabs EW, Sertie AL, Alonso LG, Kitoh H. Clinical spectrum of fibroblast growth factor receptor mutations. Hum Mutat. 1999;14:115–25. doi: 10.1002/(SICI)1098-1004(1999)14:2<115::AID-HUMU3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Pejchalova K, Krejci P, Wilcox WR. C-natriuretic peptide: an important regulator of cartilage. Mol Genet Metab. 2007;92:210–5. doi: 10.1016/j.ymgme.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman KG, et al. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17:1352–65. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters K, Ornitz D, Werner S, Williams L. Unique expression pattern of the FGF receptor 3 gene during mouse organogenesis. Dev Biol. 1993;155:423–30. doi: 10.1006/dbio.1993.1040. [DOI] [PubMed] [Google Scholar]
- Pfeifer A, Aszodi A, Seidler U, Ruth P, Hofmann F, Fassler R. Intestinal secretory defects and dwarfism in mice lacking cGMP-dependent protein kinase II. Science. 1996;274:2082–6. doi: 10.1126/science.274.5295.2082. [DOI] [PubMed] [Google Scholar]
- Plowright EE, Li Z, Bergsagel PL, Chesi M, Barber DL, Branch DR, Hawley RG, Stewart AK. Ectopic expression of fibroblast growth factor receptor 3 promotes myeloma cell proliferation and prevents apoptosis. Blood. 2000;95:992–8. [PubMed] [Google Scholar]
- Pogue R, Lyons K. BMP signaling in the cartilage growth plate. Curr Top Dev Biol. 2006;76:1–48. doi: 10.1016/S0070-2153(06)76001-X. [DOI] [PubMed] [Google Scholar]
- Prinos P, Costa T, Sommer A, Kilpatrick MW, Tsipouras P. A common FGFR3 gene mutation in hypochondroplasia. Hum Mol Genet. 1995;4:2097–101. doi: 10.1093/hmg/4.11.2097. [DOI] [PubMed] [Google Scholar]
- Priore R, Dailey L, Basilico C. Downregulation of Akt activity contributes to the growth arrest induced by FGF in chondrocytes. J Cell Physiol. 2006;207:800–8. doi: 10.1002/jcp.20620. [DOI] [PubMed] [Google Scholar]
- Qing J, Du X, Chen Y, Chan P, Li H, Wu P, Marsters S, Stawicki S, Tien J, Totpal K, et al. Antibody-based targeting of FGFR3 in bladder carcinoma and t(4;14)-positive multiple myeloma in mice. J Clin Invest. 2009;119:1216–29. doi: 10.1172/JCI38017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffioni S, Zhu YZ, Bradshaw RA, Thompson LM. Effect of transmembrane and kinase domain mutations on fibroblast growth factor receptor 3 chimera signaling in PC12 cells. A model for the control of receptor tyrosine kinase activation. J Biol Chem. 1998;273:35250–9. doi: 10.1074/jbc.273.52.35250. [DOI] [PubMed] [Google Scholar]
- Raucci A, Laplantine E, Mansukhani A, Basilico C. Activation of the ERK1/2 and p38 mitogen-activated protein kinase pathways mediates fibroblast growth factor-induced growth arrest of chondrocytes. J Biol Chem. 2004;279:1747–56. doi: 10.1074/jbc.M310384200. [DOI] [PubMed] [Google Scholar]
- Ronchetti D, Greco A, Compasso S, Colombo G, Dell’Era P, Otsuki T, Lombardi L, Neri A. Deregulated FGFR3 mutants in multiple myeloma cell lines with t(4;14): comparative analysis of Y373C, K650E and the novel G384D mutations. Oncogene. 2001;20:3553–62. doi: 10.1038/sj.onc.1204465. [DOI] [PubMed] [Google Scholar]
- Roovers K, Assoian RK. Integrating the MAP kinase signal into the G1 phase cell cycle machinery. Bioessays. 2000;22:818–26. doi: 10.1002/1521-1878(200009)22:9<818::AID-BIES7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Rousseau F, Bonaventure J, Legeai-Mallet L, Pelet A, Rozet JM, Maroteaux P, Le Merrer M, Munnich A. Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature. 1994;371:252–4. doi: 10.1038/371252a0. [DOI] [PubMed] [Google Scholar]
- Rousseau F, Bonaventure J, Legeai-Mallet L, Schmidt H, Weissenbach J, Maroteaux P, Munnich A, Le Merrer M. Clinical and genetic heterogeneity of hypochondroplasia. J Med Genet. 1996;33:749–52. doi: 10.1136/jmg.33.9.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau F, Saugier P, Le Merrer M, Munnich A, Delezoide AL, Maroteaux P, Bonaventure J, Narcy F, Sanak M. Stop codon FGFR3 mutations in thanatophoric dwarfism type 1. Nat Genet. 1995;10:11–2. doi: 10.1038/ng0595-11. [DOI] [PubMed] [Google Scholar]
- Sahni M, Ambrosetti DC, Mansukhani A, Gertner R, Levy D, Basilico C. FGF signaling inhibits chondrocyte proliferation and regulates bone development through the STAT-1 pathway. Genes Dev. 1999;13:1361–6. doi: 10.1101/gad.13.11.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni M, Raz R, Coffin JD, Levy D, Basilico C. STAT1 mediates the increased apoptosis and reduced chondrocyte proliferation in mice overexpressing FGF2. Development. 2001;128:2119–29. doi: 10.1242/dev.128.11.2119. [DOI] [PubMed] [Google Scholar]
- Sanjay A, Horne WC, Baron R. The Cbl family: ubiquitin ligases regulating signaling by tyrosine kinases. Sci STKE. 2001;2001:pe40. doi: 10.1126/stke.2001.110.pe40. [DOI] [PubMed] [Google Scholar]
- Scuto A, Krejci P, Popplewell L, Wu J, Wang Y, Kujawski M, Kowolik C, Xin H, Chen L, Wang Y, et al. The novel JAK inhibitor AZD1480 blocks STAT3 and FGFR3 signaling, resulting in suppression of human myeloma cell growth and survival. Leukemia. 2011;25:538–50. doi: 10.1038/leu.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian A, Matsushita T, Kawanami A, Mackem S, Landreth GE, Murakami S. Genetic inactivation of ERK1 and ERK2 in chondrocytes promotes bone growth and enlarges the spinal canal. J Orthop Res. 2011;29:375–9. doi: 10.1002/jor.21262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev O, Chumakov I, Nevo Z, Givol D, Madar-Shapiro L, Sheinin Y, Weinreb M, Yayon A. Restrained chondrocyte proliferation and maturation with abnormal growth plate vascularization and ossification in human FGFR-3(G380R) transgenic mice. Hum Mol Genet. 2000;9:249–58. doi: 10.1093/hmg/9.2.249. [DOI] [PubMed] [Google Scholar]
- Shiang R, Thompson LM, Zhu YZ, Church DM, Fielder TJ, Bocian M, Winokur ST, Wasmuth JJ. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994;78:335–42. doi: 10.1016/0092-8674(94)90302-6. [DOI] [PubMed] [Google Scholar]
- Sims NA, Jenkins BJ, Quinn JM, Nakamura A, Glatt M, Gillespie MT, Ernst M, Martin TJ. Glycoprotein 130 regulates bone turnover and bone size by distinct downstream signaling pathways. J Clin Invest. 2004;113:379–89. doi: 10.1172/JCI19872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su WC, Kitagawa M, Xue N, Xie B, Garofalo S, Cho J, Deng C, Horton WA, Fu XY. Activation of Stat1 by mutant fibroblast growth-factor receptor in thanatophoric dysplasia type II dwarfism. Nature. 1997;386:288–92. doi: 10.1038/386288a0. [DOI] [PubMed] [Google Scholar]
- Suhasini M, Li H, Lohmann SM, Boss GR, Pilz RB. Cyclic-GMP-dependent protein kinase inhibits the Ras/Mitogen-activated protein kinase pathway. Mol Cell Biol. 1998;18:6983–94. doi: 10.1128/mcb.18.12.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavormina PL, Bellus GA, Webster MK, Bamshad MJ, Fraley AE, McIntosh I, Szabo J, Jiang W, Jabs EW, Wilcox WR, et al. A novel skeletal dysplasia with developmental delay and acanthosis nigricans is caused by a Lys650Met mutation in the fibroblast growth factor receptor 3 gene. Am J Hum Genet. 1999;64:722–31. doi: 10.1086/302275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavormina PL, Shiang R, Thompson LM, Zhu YZ, Wilkin DJ, Lachman RS, Wilcox WR, Rimoin DL, Cohn DH, Wasmuth JJ. Thanatophoric dysplasia (types I and II) caused by distinct mutations in fibroblast growth factor receptor 3. Nat Genet. 1995;9:321–8. doi: 10.1038/ng0395-321. [DOI] [PubMed] [Google Scholar]
- Toydemir RM, Brassington AE, Bayrak-Toydemir P, Krakowiak PA, Jorde LB, Whitby FG, Longo N, Viskochil DH, Carey JC, Bamshad MJ. A novel mutation in FGFR3 causes camptodactyly, tall stature, and hearing loss (CATSHL) syndrome. Am J Hum Genet. 2006;79:935–41. doi: 10.1086/508433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel S, Li ZH, Wei E, Wiesmann M, Chang H, Chen C, Reece D, Heise C, Stewart AK. CHIR-258, a novel, multitargeted tyrosine kinase inhibitor for the potential treatment of t(4;14) multiple myeloma. Blood. 2005;105:2941–8. doi: 10.1182/blood-2004-10-3913. [DOI] [PubMed] [Google Scholar]
- Trudel S, Stewart AK, Rom E, Wei E, Li ZH, Kotzer S, Chumakov I, Singer Y, Chang H, Liang SB, et al. The inhibitory anti-FGFR3 antibody, PRO-001, is cytotoxic to t(4;14) multiple myeloma cells. Blood. 2006;107:4039–46. doi: 10.1182/blood-2005-10-4179. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Kunieda T. A loss-of-function mutation in natriuretic peptide receptor 2 (Npr2) gene is responsible for disproportionate dwarfism in cn/cn mouse. J Biol Chem. 2005;280:14288–92. doi: 10.1074/jbc.C500024200. [DOI] [PubMed] [Google Scholar]
- Ueda K, Yamanaka Y, Harada D, Yamagami E, Tanaka H, Seino Y. PTH has the potential to rescue disturbed bone growth in achondroplasia. Bone. 2007;41:13–8. doi: 10.1016/j.bone.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613–22. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- Waller DK, Correa A, Vo TM, Wang Y, Hobbs C, Langlois PH, Pearson K, Romitti PA, Shaw GM, Hecht JT. The population-based prevalence of achondroplasia and thanatophoric dysplasia in selected regions of the US. Am J Med Genet A. 2008;146A:2385–9. doi: 10.1002/ajmg.a.32485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MK, D’Avis PY, Robertson SC, Donoghue DJ. Profound ligand-independent kinase activation of fibroblast growth factor receptor 3 by the activation loop mutation responsible for a lethal skeletal dysplasia, thanatophoric dysplasia type II. Mol Cell Biol. 1996;16:4081–7. doi: 10.1128/mcb.16.8.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MK, Donoghue DJ. Constitutive activation of fibroblast growth factor receptor 3 by the transmembrane domain point mutation found in achondroplasia. Embo J. 1996;15:520–7. [PMC free article] [PubMed] [Google Scholar]
- Weizmann S, Tong A, Reich A, Genina O, Yayon A, Monsonego-Ornan E. FGF upregulates osteopontin in epiphyseal growth plate chondrocytes: implications for endochondral ossification. Matrix Biol. 2005;24:520–9. doi: 10.1016/j.matbio.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Wilcox WR, Tavormina PL, Krakow D, Kitoh H, Lachman RS, Wasmuth JJ, Thompson LM, Rimoin DL. Molecular, radiologic, and histopathologic correlations in thanatophoric dysplasia. Am J Med Genet. 1998;78:274–81. doi: 10.1002/(sici)1096-8628(19980707)78:3<274::aid-ajmg14>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Wong A, Lamothe B, Lee A, Schlessinger J, Lax I. FRS2 alpha attenuates FGF receptor signaling by Grb2-mediated recruitment of the ubiquitin ligase Cbl. Proc Natl Acad Sci U S A. 2002;99:6684–9. doi: 10.1073/pnas.052138899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin X, Abrams TJ, Hollenbach PW, Rendahl KG, Tang Y, Oei YA, Embry MG, Swinarski DE, Garrett EN, Pryer NK, et al. CHIR-258 is efficacious in a newly developed fibroblast growth factor receptor 3-expressing orthotopic multiple myeloma model in mice. Clin Cancer Res. 2006;12:4908–15. doi: 10.1158/1078-0432.CCR-06-0957. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Nagano T, Takehara S, Hibi M, Aizawa S. Shisa promotes head formation through the inhibition of receptor protein maturation for the caudalizing factors, Wnt and FGF. Cell. 2005;120:223–35. doi: 10.1016/j.cell.2004.11.051. [DOI] [PubMed] [Google Scholar]
- Yamanaka Y, Tanaka H, Koike M, Nishimura R, Seino Y. PTHrP rescues ATDC5 cells from apoptosis induced by FGF receptor 3 mutation. J Bone Miner Res. 2003;18:1395–403. doi: 10.1359/jbmr.2003.18.8.1395. [DOI] [PubMed] [Google Scholar]
- Yasoda A, Komatsu Y, Chusho H, Miyazawa T, Ozasa A, Miura M, Kurihara T, Rogi T, Tanaka S, Suda M, et al. Overexpression of CNP in chondrocytes rescues achondroplasia through a MAPK-dependent pathway. Nat Med. 2004;10:80–6. doi: 10.1038/nm971. [DOI] [PubMed] [Google Scholar]
- Yu CF, Liu ZX, Cantley LG. ERK negatively regulates the epidermal growth factor-mediated interaction of Gab1 and the phosphatidylinositol 3-kinase. J Biol Chem. 2002;277:19382–8. doi: 10.1074/jbc.M200732200. [DOI] [PubMed] [Google Scholar]
- Zelzer E, Mamluk R, Ferrara N, Johnson RS, Schipani E, Olsen BR. VEGFA is necessary for chondrocyte survival during bone development. Development. 2004;131:2161–71. doi: 10.1242/dev.01053. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Qu Z, Kim S, Shi V, Liao B, Kraft P, Bandaru R, Wu Y, Greenberger LM, Horak ID. Down-modulation of cancer targets using locked nucleic acid (LNA)-based antisense oligonucleotides without transfection. Gene Ther. 2011;18:326–33. doi: 10.1038/gt.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Somlo G, Zhou B, Shao J, Bedell V, Slovak ML, Liu X, Luo J, Yen Y. Fibroblast growth factor receptor 3 inhibition by short hairpin RNAs leads to apoptosis in multiple myeloma. Mol Cancer Ther. 2005;4:787–98. doi: 10.1158/1535-7163.MCT-04-0330. [DOI] [PubMed] [Google Scholar]