Abstract
Both high and low molecular weight hyaluronan (HMW-HA vs. LMW-HA) exist in various tissues and cells. In this study we investigated LMW-HA-mediated CD44 interaction with Toll-like receptors (TLRs), the actin filament-associated protein (AFAP-110) and a myeloid differentiation factor (MyD88) in breast tumor cells (MDA-MB-231 cells). Our data indicate that LMW-HA (but not HMW-HA) preferentially stimulates a physical association between CD44 and TLRs followed by a concomitant recruitment of AFAP-110 and MyD88 into receptor-containing complexes in breast tumor cells.
LMW-HA-activated AFAP-110 then binds to F-actin resulting in MyD88/NF-κB nuclear translocation, NF-κB-specific transcription and target gene (IL-1β and IL-8) expression. These signaling events lead to pro-inflammatory cytokine/chemokine production in the breast tumor cells. AFAP-110-F-actin (activated by LMW-HA) also promotes tumor cell invasion. Downregulation of AFAP-110 or MyD88 by transfecting breast tumor cells with AFAP-110 siRNA or MyD88 siRNA, respectively not only blocks the ability of LMW-HA to stimulate AFAP-110-actin function, but also impairs MyD88-NF-κB nuclear translocation and NF-κB transcriptional activation. Consequently, both IL-1β/IL-8 production and tumor cell invasion are impaired. Taken together, these findings suggest that LMW-HA plays an important role in CD44-TLR-associated AFAP-110-actin interaction and MyD88-NF-κB signaling required for tumor cell behaviors which may contribute to the progression of breast cancer.
INTRODUCTION
Hyaluronan (HA), an important structural component of the extracellular matrix (ECM) exists as high molecular weight HA polymers (HMW-HA) and low molecular weight HA fragments (LMW-HA) and is enriched in many types of tumors (Smith et al., 1991; Bourguignon, 2001; Toole et al., 2002; Haylock and Nilsson, 2006). In cancer patients, the level of HA is usually higher in malignant tumors than in corresponding benign or normal tissues, and in some tumor types the level of HA is predictive of malignancy (Delpech et al., 1990; Toole et al., 2002). In particular, HA levels have been shown to be elevated in the serum of breast cancer patients (Delpech et al., 1990; Toole et al., 2002). The aberrant HA production by HA synthases (Itano and Kimata, 1996; Weigel et al., 1997; Spicer and Nguyen, 1999) and HMW-HA degradation into LMW-HA (by hyaluronidases) (Stern and Jedrzejas, 2006) are thought to be closely associated with breast tumor cell progression (Toole et al., 2002).
HA interacts with CD44 which is a ubiquitous, abundant and functionally important receptor expressed on the surface of many different types of cells, including normal and transformed epithelial tumor cells (Lesley et al., 2000). The crystal structure of the HA-CD44 complex has been reported recently and a single HA binding site has been identified (Banerji et al., 2007). A class of low molecular weight HA (LMW-HA) polymers between 6 and 18 disaccharides serve as a monovalent ligand for CD44 binding, whereas another class of low molecular weight HA (LMW-HA) polymers between 22 and 38 disaccharides act as a multivalent ligand for CD44 binding (Lesley et al., 2000). Different sizes of HA polymers appear to contribute to the onset of distinct CD44 signaling pathways and biological activities. For example, high molecular weight HA (HMW-HA) polymers (larger than 1,000,000 dalton) antagonizes mitogen-dependent cyclin D1 expression in messenchymal cells (Kothapalli et al., 2007). However, HMW-HA with a molecular weight of ~500,000 dalton appears to promote CD44 interaction with a number of downstream effectors [e.g., the cytoskeletal protein, ankyrin (Bourguignon, 2001; Turley et al., 2002), ERM/merlin (Bretscher et al., 2002) and/or various GTPases (e.g., RhoA, Rac1 and Cdc42) (Bourguignon, 2008)] in coordinating intracellular signaling pathways [e.g., Ca2+ mobilization (Singleton and Bourguignon, 2002; Bourguignon et al., 2004a; Singleton and Bourguignon, 2004; Wang and Bourguignon, 2006a), Rho signaling (Bourguignon, 2008), PI3 kinase-AKT activation (Bourguignon et al., 2003), NHE1-mediated cellular acidification (Bourguignon et al., 2004b), transcriptional upregulation (Bourguignon et al., 2005; Bourguignon et al., 2007; Bourguignon et al., 2008; Bourguignon et al., 2009b) and cytoskeletal function (Bourguignon, 2001; Turley et al., 2002; Bourguignon, 2008)] and generating the concomitant onset of tumor cell activities (e.g., tumor cell adhesion, growth, survival, migration and invasion) and tumor progression (Bourguignon, 2001; Turley et al., 2002; Bourguignon, 2008). HMW-HA binding to CD44 is also involved in the stimulation of both receptor kinases [e.g., ErbB2 (Bourguignon et al., 1997; Bourguignon et al., 2001b), EGFR (Bourguignon et al., 2006; Wang and Bourguignon, 2006b) and TGFβ receptors (Bourguignon et al., 2002)] and non-receptor kinases [e.g., c-Src (Bourguignon et al., 2001a) and ROK (Bourguignon et al., 1999; Singleton and Bourguignon, 2002; Bourguignon et al., 2003; Bourguignon, 2009)] required for a variety of tumor cell-specific functions leading to chemoresistance and tumor progression.
Low molecular weight HA (LMW-HA) polymers containing 10–25 disaccharide units has been found to promote certain sets of gene expression, cell proliferation, migration, angiogenesis and chemosensitivity (Lokeshwar et al., 1996; Cuff et al., 2001; Bourguignon, 2001; Noble, 2002; Kothapalli et al., 2008; Toole and Slomiany, 2008; Slomiany et al., 2009a; Slomiany et al., 2009b). Our previous studies indicated a correlation between high affinity binding of LMW-HA (10–15 disaccharide units) to cells expressing CD44 and the occurrence of a mitogenic response and chemokine gene expression (Lokeshwar et al., 1996; Bourguignon, 2001). Since anti-CD44 antibody inhibits LMW-HA-mediated cell proliferation and chemokine production, it is generally accepted that CD44 serves as a HA receptor involved in LMW-HA-mediated functions (Lokeshwar et al., 1996; Bourguignon, 2001; Kothapalli et al., 2008).
Inflammation is one of the risk factors for tumor development and correlates with increased invasiveness and poor prognosis in breast cancer (Calogero et al., 2007; Radisky and Radisky, 2007; Woodward and Buchholz, 2008; Yamauchi et al., 2009). In particular, the inflammatory breast cancer is an aggressive form of breast cancer with long-term survival less than 50% (Woodward and Buchholz, 2008; Yamauchi et al., 2009). Pro-inflammatory cytokines [e.g., interleukin-1β (IL-1β)] and chemokines [interleukin 8, (IL-8)] detected in human breast cancer tissues have been linked to breast cancer formation and recurrence (Nicolini et al., 2006; Calogero et al., 2007; Premkumar et al., 2007). Therefore, understanding the mechanisms by which pro-inflammatory chemokines/cytokines might be regulated in the breast tumor cells will lead to new insights into anti-inflammatory strategies that may prevent the development and recurrence of breast cancer. Whether LMW-HA plays a role in regulating the production of pro-inflammatory cytokines and chemokines in breast tumor cells is the focus of this study.
The Toll gene was initially discovered during studies on the dorsoventricular polarization that occurs in Drosophila embryogenesis (Medzhitov et al., 1997; Takeda et al., 2003). Subsequently, mammalian members of the homologues, Toll-like receptors (TLRs) family have been identified, and are considered to be essential for the detection of pathogens for triggering immune responses against microbial infections (Medzhitov et al., 1997; Tsuji et al., 2000; Takeda et al., 2003; Uehori et al., 2003). TLRs expressed on antigen presenting cells such as macrophages and dendritic cells play an important in immune responses (Bauer et al., 2009). TLRs are transmembrane proteins which generally transmit signals via their cytosolic adaptor protein, myeloid differentiation factor (MyD)88, leading to NFκB activation and pro-inflammatory gene expression (Schmitz et al., 2004; Toubi and Shoenfeld, 2004; Bauer et al., 2009). Several studies showed that certain TLRs such as TLR2 and TLR4 are also expressed in a variety of human cancer cells (Kelly et al., 2006; Molteni et al., 2006; Xie et al., 2009). However, the involvement of TLRs in breast tumor cell signaling and activation has not been established. In immune cells, TLRs initiate MyD88-dependent NFκB signaling and pro-inflammatory cytokine/chemokine gene expression in response to LMW-HA following tissue injury (Jiang et al., 2005; Scheibner et al., 2006). Here, we investigated whether LMW-HA is capable of inducing TLR-related MyD88/NFκB signaling and pro-inflammatory cytokine/chemokine production in breast tumor cells.
The actin filament-associated protein, AFAP-110 (also called AFAP1) was first identified as an SH3-SH2 binding partner for the non-receptor tyrosine kinase, Src (Flynn et al., 1993). Two other AFAP-110 family members, called AFAP1L1 and AFAP1L2 have also been identified recently (Snyder et al., 2011). AFAP-110 contains a carboxy terminal actin-binding domain (Qian et al., 2000) with several amino terminal-binding motifs including an SH3 binding motif (Guappone and Flynn, 1997), two SH2 binding motifs (Guappone et al., 1998), two plesckstrin homology domains and a leucine zipper motif (Qian et al., 1998; Qian et al., 2004). These functional motifs are involved in AFAP-110 interaction with actin filaments and a variety of signaling molecules (Flynn et al., 1993; Guappone and Flynn, 1997; Guappone et al., 1998; Qian et al., 1998; Qian et al., 2000; Qian et al., 2004). For example, AFAP-110 directly binds to actin filaments via its C-terminal actin-binding domain. AFAP-110 also functions as an adaptor protein by recruiting Src family members and/or other signaling components to actin filaments (Flynn et al., 1993; Guappone and Flynn, 1997). Thus, AFAP-110 not only serves as a scaffolding protein (mediating multi-protein complex assembly), but also directly participates in actin-cytoskeleton activation and signaling coordination. The expression of AFAP-110 has been detected in breast tumor cells such as MDA-MB-231 cells (Dorfleutner et al., 2007). Our previous studies indicate that CD44 interaction with IQGAP1 and N-WASP serves as a signal integrator by modulating actin-cytoskeleton function in HA-activated tumor cells (Bourguignon et al., 2005; Bourguignon et al., 2007). Whether or not AFAP-110 participates in HA (LMW-HA vs. HMW-HA)-mediated CD44 regulation of the cytoskeleton function and oncogenic signaling in breast tumor cells is not known.
In this study we present novel findings to demonstrate that LMW-HA (molecular mass ~3,000–5,000 dalton) [but not HMW-HA (molecular mass 500,000~1,000,000 dalton)] mediates CD44 interaction with TLR2/TLR4, AFAP-110 and MyD88-dependent pathways. These signaling events lead to cytoskeleton activation, NFκB-specific transcriptional activation and IL-1β/IL-8 gene expression required for pro-inflammatory cytokine/chemokine secretion and cell invasion in breast tumor cells. This new information provides insights into the mechanism of CD44-TLR signaling-regulated malignancy of breast cancers induced by ECM components such as LMW-HA.
MATERIALS AND METHODS
Cell Culture
The breast tumor cell line (MDA-MB-231 cells) was obtained from the American Type Culture Collection (ATCC) and grown in Eagle's minimum essential medium (EMEM) supplemented with Earle's salt solution, essential and non-essential amino acids, vitamins and 10% fetal bovine serum.
Antibodies and Reagents
Monoclonal rat anti-human CD44 antibody (Clone: 020; Isotype: IgG2b) obtained from Millipore (Billerica, MA) recognizes a determinant of the HA-binding region common to CD44 and its principal variant isoforms. This rat anti-CD44 was routinely used for HA-related blocking experiments and immunoblotting/immunoprecipitation (Bourguignon et al., 1997; Bourguignon et al., 1999; Bourguignon et al., 2001a; Bourguignon et al., 2001b; Bourguignon et al., 2002; Singleton and Bourguignon, 2002; Bourguignon et al., 2003; Bourguignon et al., 2004a; Bourguignon et al., 2004b; Singleton and Bourguignon, 2004; Bourguignon et al., 2005; Bourguignon et al., 2006; Wang and Bourguignon, 2006a, b; Bourguignon et al., 2007; Bourguignon, 2008; Bourguignon et al., 2008; Bourguignon, 2009; Bourguignon et al., 2009b). Mouse anti-AFAP-110 antibody was obtained from BD Biosciences (San Jose, CA). Other immuno-reagents such as mouse anti-TLR2 antibody, rabbit anti-TLR4 antibody, rabbit anti-MyD88 antibody, mouse anti-NFκB-p65 antibody, goat anti-actin antibody and mouse anti-lamin A/C antibody were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). For CD44siRNA, a pool of 2-target-specific 20–25nt siRNAs designed to knock down CD44 gene expression as described previously (Bourguignon et al., 2004b) was used. TLR2 siRNA (a pool of 3-target-specific 20–25nt siRNAs designed to knock down TLR2 gene expression), TLR4 siRNA (a pool of 3-target-specific 20–25nt siRNAs designed to knock down TLR4 gene expression), MyD88 siRNA (a pool of 4-target-specific 20–25nt siRNAs designed to knock down MyD88 gene expression), AFAP-110 siRNA (a pool of 3-target-specific 20–25nt siRNAs designed to knock down AFAP-110 gene expression) and scrambled siRNAs were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
High molecular weight HA (HMW-HA) (molecular mass ~1,000,000–500,000 dalton) was prepared from Healon HA polymers (purchased from Pharmacia & Upjohn Company, Kalamazoo, MI) using gel filtration column chromatography-Sephacryl S1000 column. Both low molecular weight HA (LMW-HA) fragments (molecular mass ~3,000–5,000 dalton) and HA fragments (2–3 disaccharides) were obtained by digesting HMW-HA with bovine testicular hyaluronidase (PH20) according to the method described previously (Lokeshwar et al., 1996). Briefly, high molecular weight healon HA polymers (500mg) was dissolved in 50ml of 0.1M acetate buffer (pH 5.4) containing 0.15M NaCl and digested with 20,000 units of bovine testicular hyaluronidase (PH20) (Wyeth Laboratories Inc. Philadelphia, PA) at 37°C. Ten milliter aliquots were removed after 2, 4, 6, 8, and 24h intervals, and the reaction was terminated by adding trichloroacetic acid at 10% final concentration (v/v). After incubating at 4°C for at least 4h, any precipitate was removed by centrifugation at 2,500 × g for 30min. The supernatants were then pooled, dialyzed extensively against distilled water, recentrifuged, and freeze-dried. The HA fragment preparation was dissolved in 10ml of 0.1M acetic acid and applied to a column (2.0 × 150cm) of Sephadex G-50. The column was eluted in 0.1M acetic acid at the flow of 10ml/h, and 5-ml fractions were collected. Each fraction was assayed for hyaluronan content, and size ranges of the fragments were determined as described previously (Lokeshwar et al., 1996). The purity of both LMW-HA fragments and HMW-HA polymers used in our experiments was further verified by anion exchange high-performance liquid chromatography (HPLC) followed by protein and endotoxin analyses using BCA protein assay kit (Pierce Co., Rockford, IL) and an in vitro Limulus Amebocyte Lysate (LAL) assay (Cambrex Bio Science Walkersville Inc., Walkersville, MD), respectively. No protein or endotoxin contamination was detected in these LMW-HA and HMW-HA preparations. HMW-HA, LMW-HA and HA fragments were then analyzed by using 4–40% polyacrylamide gradient gel electrophoresis followed by Alcian blue 8GX and silver staining. Both Select-HA™ NanoLadder (in the range of molecular mass ~2,000 dalton to Mr ~5,000 dalton) and Select-HA™ LowLadder (in the range of Mr ~27,000 dalton to Mr 495,000 dalton) obtained from Hyalose (Oklahoma City, OK) were used as HA standards.
Quantitative PCR (Q-PCR)
Total RNA was isolated from MDA-MB-231 cells [untransfected or transfected with various siRNAs (e.g., MyD88 siRNA or AFAP-110 siRNA or CD44 siRNA or TLR2 siRNA or TLR4 siRNA or TLR2 siRNA plus TLR4 siRNA or scrambled siRNA) in the presence of LMW-HA (1µg/ml) or anti-CD44 antibody (1µg/ml) plus LMW-HA (1µg/ml) (or normal IgG alone or normal IgG plus LMW-HA) or HMW-HA (1µg/ml) or HA fragments (2–3 disaccharides) (1µg/ml) or no HA addition for 24h at 37°C] using Tripure Isolation Reagent kits (Roche Applied Science, Indianapolis, IN) as described above. First-stranded cDNAs were synthesized from RNA using Superscript First-Strand Synthesis system (Invitrogen, Carlsbad, CA). Gene expression was quantified using probe-based SYBR Green PCR Master Mix kits, ABI PRISM 7900HT sequence detection system, and SDS software (Applied Biosystems, Foster City, CA). A cycle threshold (minimal PCR cycles required for generating a fluorescent signal exceeding a preset threshold) was determined for each gene of interest and normalized to a cycle threshold for a housekeeping gene (36B4) determined in parallel. The 36B4 is a human acidic ribosomal phosphoprotein PO whose expression was not changed in MDA-MB-231 cells transfected with various siRNAs in the presence or absence of 24h 1µg/ml LMW-HA (or HMW-HA or no HA) treatment. The Q-PCR primers used for detecting gene expression of IL-1β and IL-8 were as follows: Specifically, two IL-1β-specific primers (the sense primer 5’-GCAACTGTTCCTGAACTCAAC-3’ and the antisense primer 5’-ATCTTTTGGGGTCCGTCAACT-3’); two IL-8-specific primers (the sense primer 5’-ATGACTTCCAAGCTGGCCGT-3’ and the antisense primer 5’-CCTCTTCAAAAACTTCTCCACACC-3’) were used. Finally, for detecting 36B4 gene expression, two 36B4-specific primers (the sense primer 5’-GCGACCTGGAAGTCCAACTAC-3’ and the antisense primer 5’-ATCTGCTGCATCTGCTTGG-3’) were used.
Measurement of Cytokine/Chemokine Production
MDA-MB-231 cells [untransfected or transfected with various siRNAs (e.g., MyD88 siRNA or AFAP-110 siRNA or CD44 siRNA or TLR2 siRNA or TLR4 siRNA or TLR2 siRNA plus TLR4 siRNA and scrambled siRNA)] were washed three times with serum-free DMEM and incubated in serum-free DMEM containing various reagents [e.g. LMW-HA (1µg/ml) or anti-CD44 antibody (1µg/ml) plus LMW-HA (1µg/ml) (or normal IgG alone or normal IgG plus LMW-HA) or HMW-HA (1µg/ml) or HA fragments (2–3 disaccharides) (1µg/ml) no HA treatment] for 24 hr at 37°C in a 5% CO2 humidified chamber. Subsequently, IL-1β and IL-8 concentrations secreted in the medium from MDA-MB-231 cells (treated as above) were determined using the Quantikine IL-1β and IL-8 immunoassay (R & D Systems). All data were expressed as the mean ± SEM.
Immunoprecipitation & Immunoblotting Techniques
MDA-MB-231 cells [untransfected or transfected with various siRNAs (e.g., MyD88 siRNA or AFAP-110 siRNA or CD44 siRNA or TLR2 siRNA or TLR4 siRNA or TLR2 siRNA plus TLR4 siRNA or scrambled siRNA) were treated with LMW-HA (1µg/ml) or anti-CD44 antibody (1µg/ml) plus LMW-HA (1µg/ml) (or normal IgG alone or normal IgG plus LMW-HA) or HMW-HA (1µg/ml) or HA fragments (2–3 disaccharides) (1µg/ml) or no HA addition at 37°C for various time intervals (e.g., 0, 10, 30 or 60min) followed by immunoprecipitation and/or immunoblotting analyses as described below.
Cell lysate was first prepared by solubilizing MDA-MB-231 cells (treated with various conditions as described above) with the lysis buffer [50mM HEPES (pH 7.5), 150mM NaCl, 20mM MgCl2, 0.5% Nonidet P-40 (NP-40), 0.2mM Na3VO4, 0.2mM phenylmethylsulfonyl fluoride, 10µg/ml leupeptin, and 5µg/ml aprotinin]. Immunoprecipitation was performed by incubating NP-40 solubilized cell lysate with rat anti-CD44 antibody (clone 020) (1µg/ml) [or mouse anti-AFAP-110 (2µg/ml) or mouse anti-TLR2 antibody (2µg/ml) or rabbit anti-TLR4 antibody (2µg/ml) or normal IgG], followed by adding specific immuno-agarose beads conjugated with goat anti-rat IgG or goat anti-mouse IgG or goat anti-rabbit IgG, respectively (Sigma-Aldrich, St. Louis, MO). Subsequently, these immuno-beads were extensively washed with the lysis buffer for at least three times (each time centrifuging at 4°C and removing the supernatant). The immunoprecipitated materials associated with various immune-beads were then solubilized in SDS sample buffer, electrophoresed (using a 7.5% SDS-PAGE) and blotted onto nitrocellulose. After blocking non-specific sites with 3% bovine serum albumin, the nitrocellulose filter was immunoblotted with different antibodies [e.g., mouse anti-AFAP-110 antibody (2µg/ml) or mouse anti-TLR2 antibody (2µg/ml) or rabbit anti-TLR-4 antibody (2µg/ml) or rabbit anti-MyD88 antibody (2µg/ml) or rat anti-CD44 antibody (1µg/ml)], respectively.
Preparations of Cytoplasmic and Nucleus Fractions
MDA-MB-231 cells [untransfected or transfected with various siRNAs (e.g., MyD88 siRNA or AFAP-110 siRNA or scrambled siRNA) in the presence of LMW-HA (1µg/ml) or anti-CD44 antibody (1µg/ml) plus LMW-HA (1µg/ml) or HMW-HA (1µg/ml) or no HA addition for various time intervals (e.g., 0, 5 or 30min) at 37°C] were first incubated with a lysis buffer [50mM HEPES (pH 7.5), 150mM NaCl, 20mM MgCl2, 0.5% Nonidet P-40 (NP-40), 0.2mM Na3VO4, 0.2mM phenylmethylsulfonyl fluoride, 10µg/ml leupeptin, and 5µg/ml aprotinin]. The lysate was then homogenized by 30 strokes in a tight fitting Dounce homogenizer. Both cytoplasmic and nuclear fractions were prepared using the extraction kit from Acitve Motif (Carsbad, CA) and immunoblotted with various immuno-reagents [e.g. anti-AFAP-110 antibody (2µg/ml) or anti-MyD88 antibody (2µg/ml) or anti-NFκB-p65 antibody (2µg/ml) or anti-Lamin A/C antibody (a nucleus-specific marker) (2µg/ml), respectively].
Luciferase Reporter Assays
Transactivation assays were conducted with MDA-MB-231 cells (untreated or pretreated with anti-CD44 antibody (1µg/ml) (or normal IgG alone or normal IgG plus LMW-HA) or transfected with various siRNAs (e.g., MyD88 siRNA or AFAP-110 siRNA or CD44 siRNA or TLR2 siRNA or TLR4 siRNA or TLR2 siRNA plus TLR4 siRNA and scrambled siRNA), as above. Following 24h LMW-HA (1µg/ml) treatment [or 1µg/ml HMW-HA or HA fragments (2–3 disaccharides) (1µg/ml) no HA treatment], these cells (or various siRNA-treated cells) maintained in 35 mm-diameter dishes were transfected with 1.0µg of a plasmid, pNFκB-Luc which contains five NFκB binding sites in front of a luciferase gene (obtained from Strategene, La Jolla, CA). A plasmid encoding β-galactosidase (1.0µg) was also co-transfected to enable normalization for transfection efficiency. After 24h, expression of the reporter (luciferase) and the control (β-galactosidase) genes were determined using enzyme assay systems from Promega as per the manufacturer’s instructions.
F-actin Binding Assays
To study the binding interaction between CD44-associated AFAP-110 and F-actin, CD44-associated AFAP-conjugated beads [isolated from MDA-MB-231 cells transfected with 50 pmole AFAP-110 siRNA or 50 pmole scrambled siRNA or no siRNA in the presence of 1µg/ml LMW-HA or HMW-HA or pretreated with anti-CD44 (1µg/ml) followed by LMW-HA addition (or normal IgG alone or normal IgG plus LMW-HA) or HA fragments (2–3 disaccharides) (1µg/ml) no HA as described above)] in 50µl of TKM buffer [50mM Tris-HCl (pH7.4), 134mM KCl and 1mM MgCl2] were mixed with an equal volume of 8µM of 125I-labeled F-actin [prepared by incubating 125I-labeled G-actin with an actin polymerization buffer containing 10mM HEPES (pH 7.9), 100mM KCl, 2mM MgCl2, 0.2mM CaCl2, 5mM EGTA and 60nM Arp2/3 and N-WASP complex as described previously (Bourguignon et al., 2007)] followed by a 30 min incubation at room temperature. Following binding, the CD44-associated AFAP-110-conjugated beads were washed extensively in the binding buffer and analyzed by liquid scintillation counting. The amount of F-actin binding to the CD44-associated AFAP-110-beads isolated from untreated MDA-MB-231 cells (control) or MDA-MB-231 cells treated with scrambled siRNA (with no HA treatment) is designated as 100%. Each assay was set up in triplicate and repeated at least 5 times.
In some cases, AFAP-associated with anti-CD44-conjugated beads (isolated from MDA-MB-231 cells transfected with 50 pmole AFAP-110 siRNA or 50 pmole scrambled siRNA in the presence or absence of 1µg/ml LMW-HA) was also incubated with the binding buffer containing F-actin. Following binding, the CD44-AFAP-110-F-actin complex was washed extensively in the binding buffer and analyzed by immunoblotting using anti-actin antibody or anti-AFAP antibody or anti-CD44 antibody, respectively.
Immunofluorescence Staining
MDA-MB-231 cells (untreated or treated with various drugs including 15µg/ml cytochalasin D or transfected with MyD88 siRNA or AFAP-110 siRNA or siRNAs with scrambled sequences) were incubated with LMW-HA (1µg/ml) or anti-CD44 antibody (1µg/ml) plus LMW-HA (1µg/ml) (or normal IgG alone or normal IgG plus LMW-HA) or HMW-HA (1µg/ml) HA fragments (2–3 disaccharides) (1µg/ml) or no HA at 37°C for various time intervals (e.g., 0, 10, 30 or 60min). These cells were then fixed with 2% formaldehyde. Subsequently, these cells were rendered permeable by ethanol treatment followed by incubation with fluorescein (FITC)-conjugated anti-MyD88 antibody or Texas Red-conjugated anti-NFκB-65 antibody followed by DAPI staining (a marker for nucleus). To detect non-specific antibody, DAPI-labeled cells were also incubated with FITC or Texas Red-conjugated normal IgG, respectively. No labeling was observed in control samples.
In some cases, MDA-MB-231 cells [treated with AFAP-110 siRNA or scrambled siRNA siRNAs or cytochalasin D (15µg/ml) or anti-CD44 antibody (1µg/ml) followed by LMW-HA (1µg/ml) or HMW-HA (1µg/ml) addition or HA fragments (2–3 disaccharides) (1µg/ml) or no HA at 37°C for various time intervals (e.g., 0, 10, 30 or 60min)] were fixed with 2% formaldehyde, permeabilized by permeable by ethanol treatment and stained with Texas Red-conjugated phalloidin alone (to locate F-actin) followed by DAPI staining (a marker for nucleus). These fluorescence-labeled samples were then examined with a confocal laser scanning microscope.
Tumor Cell Invasion Assays
Twenty-four transwell units were used for monitoring in vitro tumor cell invasion as described previously (20). Specifically, the 5µm porosity polycarbonate filters coated with the reconstituted basement membrane substance Matrigel (Collaborative Research, Lexington, MA) were used for the cell invasion assay (20). MDA-MB-231 cells [1 × 104 cells/well-treated with various drugs including 15µg/ml cytochalasin D or transfected with MyD88 siRNA or AFAP-110 siRNA or siRNAs with scrambled sequences] were placed in the upper chamber of the transwell unit containing LMW-HA (1µg/ml) or anti-CD44 antibody (1µg/ml) plus LMW-HA (1µg/ml) (or normal IgG alone or normal IgG plus LMW-HA) or HMW-HA (1µg/ml) or HA fragments (2–3 disaccharides) (1µg/ml) or no HA addition. The growth medium containing high glucose DMEM supplemented with 10% fetal bovine serum were placed in the lower chamber of the transwell unit. After 18h incubation at 37°C in a humidified 95% air/5% CO2 atmosphere, cells on the upper side of the filter were removed by wiping with a cotton swab. Cell invasion processes were determined by measuring the cells that migrated (invaded) to the lower side of the polycarbonate filters by standard cell number counting methods as described previously (Bourguignon et al., 2004b). The CD44-specific cell invasion was determined by subtracting non-specific cell invasion (i.e., cell that migrated (invaded) to the lower chamber in the presence of anti-CD44 antibody treatment). Each assay was performed in triplicate and repeated at least 5–6 times.
RESULTS
Induction of CD44-AFAP-110 Association and F-Actin Binding by LMW-HA in Breast Tumor Cells
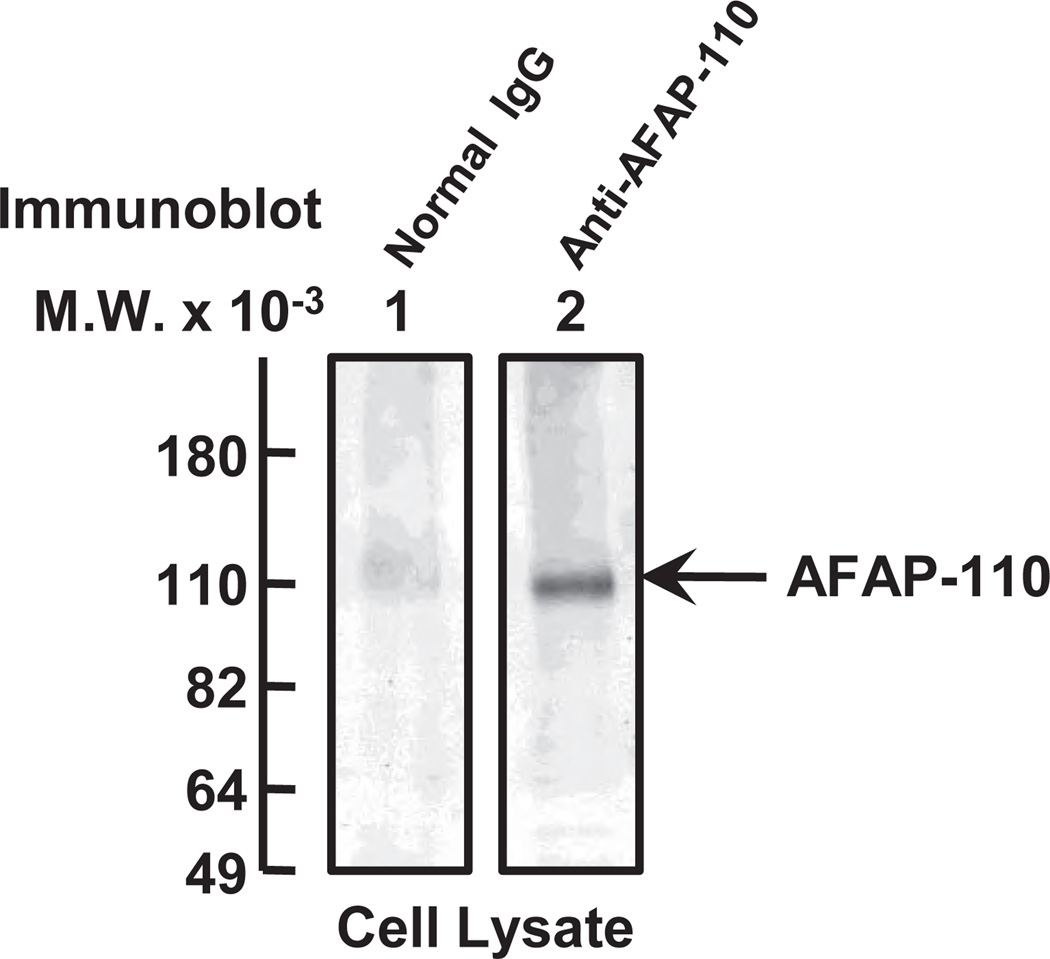
It has been shown that HA fragments (in particular, LMW-HA) promote a variety of biological activities including cell migration, invasion, proliferation and pro-inflammatory gene expression (Lokeshwar et al., 1996; Bourguignon, 2001; Noble, 2002). LMW-HA has also been shown to induce transmembrane interactions between CD44 and the cytoskeleton in vascular endothelial cells during tumor progression (Matou-Nasri et al., 2009). In the search for a new cytoskeleton regulator that correlates with breast tumor cell motility, an actin-binding protein, AFAP-110 [an 110kDa protein-known to interact with filamentous actin (F-actin)] (Flynn et al., 1993; Guappone and Flynn, 1997; Guappone et al., 1998; Qian et al., 1998; Qian et al., 2000; Qian et al., 2004) has been identified. Using a specific anti-AFAP-110-mediated immunoblot technique, we observed that significant amounts of AFAP-110 (molecular mass ~110 kDa) are expressed in MDA-MB-231 cells (Figure 1, lane 2). We believe that the AFAP-110 protein detected by anti-AFAP-110-mediated immunoblot is specific since no protein is detected in those cells incubated with normal mouse IgG (Figure 1, lane 1).
Figure 1.
Characterization of AFAP-110 in human breast tumor cells. MDA-MB-231 cells were solubilized and analyzed by a 7.5% SDS-PAGE followed by transferring to nitrocellulose sheets. Individual lane (from nitrocellulose sheets) was sliced separately and incubated with either control mouse IgG (lane 1); or with anti-AFAP-110 antibody (lane 2).
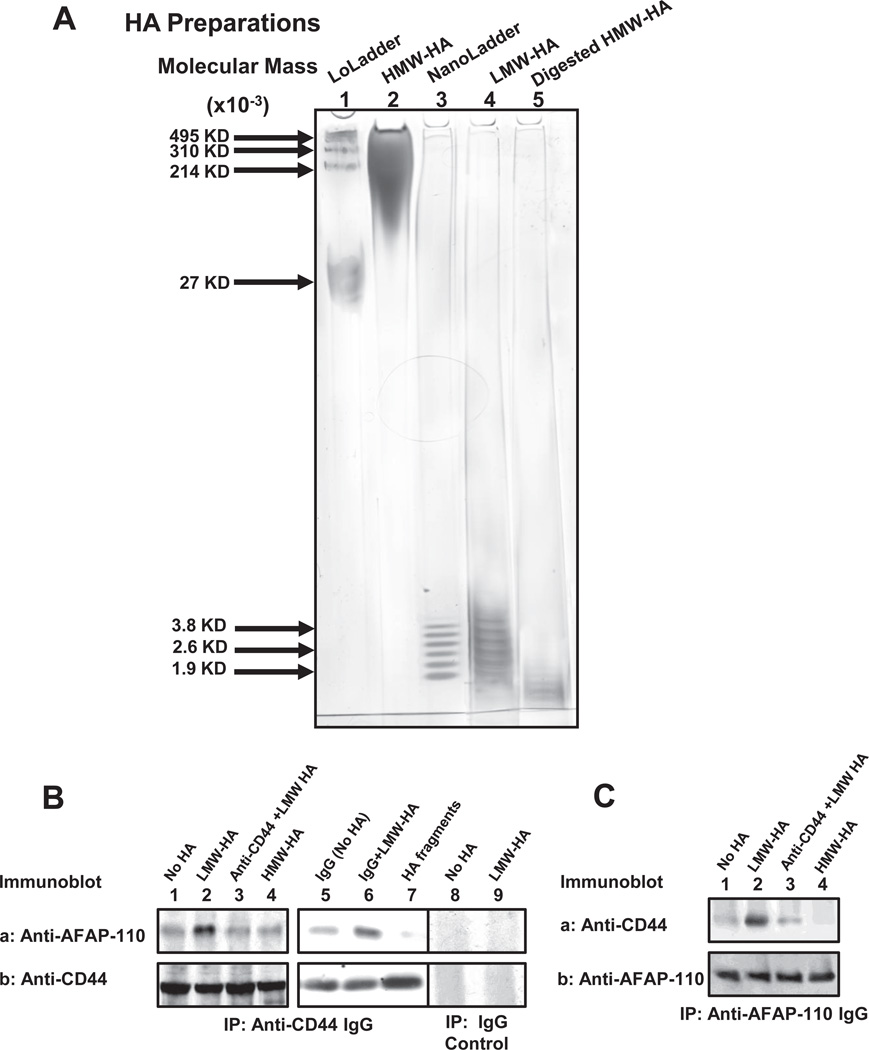
In order to further investigate the cellular and molecular processes underlying LMW-HA/CD44-induced cytoskeleton function in breast tumor cells, HMW-HA (Figure. 2A, lane 2), LMW-HA (Figure 2A, lane 4) and HA fragment with 2–3 disaccharides (Figure. 2A, lane 5) were prepared and analyzed by 4–40% polyacrylamide gradient gel electrophoresis. We then addressed the question of whether there is a physical linkage between CD44 and AFAP-110 in breast tumor cells (MDA-MB-231 cell line). To this end we first performed anti-CD44-mediated immunoprecipitation followed by anti-AFAP-110 immunoblot (Figure 2B-a) or anti-CD44 immunoblot (Figure 2B-b), respectively, using cells treated with either LMW-HA or HMW-HA or HA fragments (2–3 disaccharides) (or no HA). Our results indicate that LMW-HA treatment causes the recruitment of a significant amount of AFAP-110 into the CD44 complex (Figure 2B-a, b, lane 2). In contrast, a low level of AFAP-110-CD44 complex is detected in cells treated with no HA or HMW-HA or HA fragments (2–3 disaccharides) (Figure 2B-a,b, lane 1, lane 4 and lane 7). Pretreatment of cells with anti-CD44 antibody followed by LMW-HA treatment (Figure 2B-a,b, lane 3) significantly reduces AFAP-110 association with CD44 (Figure 2B-a,b, lane 3). However, normal IgG treatment does not appear to block LMW-HA-induced CD44-AFAP-110 association (Figure 2B-a,b, lane 6 vs. lane 5). Further analyses indicate that LMW-HA-induced AFAP-110 association with CD44 is specific since neither AFAP-110 nor CD44 is detected in IgG control-mediated precipitation experiments (Figure 2B, lane 8 and lane 9). These findings strongly suggest that the induction of CD44-AFAP-110 complex formation is CD44-specific and LMW-HA-dependent in breast tumor cells.
Figure 2.
Analyses of HA preparations and AFAP-110 interaction with CD44 in MDA-MB-231 cells.
A: HMW-HA (lane 2), LMW-HA (lane 4) and HA fragment with 2–3 disaccharides (lane 5) were analyzed by using 4–40% polyacrylamide gradient gel followed by Alcian blue 8GX and silver staining. Select-HA™ LowLadder (in the range of molecular mass ~495,000 dalton to 27,000 dalton) (Lane 1) and Select-HA™ NanoLadder (in the range of molecular mass ~4,000 dalton to Mr ~2,000 dalton) (lane 3) were used as HA-size marker standards.
B: MDA-MB-231 cells were solubilized by 1% Nonidet P-40 (NP-40) buffer and immunoprecipitated with anti-CD44 antibody followed by immunoblotting using anti-AFAP antibody (A-a) or reblotting with anti-CD44 antibody (A-b) as a loading control. [Cells treated with no HA (lane 1) or with LMW-HA (lane 2) or with anti-CD44 antibody plus LMW-HA (lane 3) or with HMW-HA (lane 4) or with normal IgG without HA (Lane 5) or with normal IgG plus LMW-HA (Lane 6) or with HA fragments (2–3 disaccharides) (lane 7)]. Cell lysate was also precipitated with normal IgG control followed by immunoblotting using anti-AFAP antibody (A-a) or reblotting with anti-CD44 antibody (A-b) as a loading control. [Cells treated with no HA (lane 8); LMW-HA (lane 9)].
C: Cell lysate was immunoprecipitated with anti-AFAP-110 antibody followed by immunoblotting using anti-CD44 antibody (B-a) or reblotting with anti-AFAP-110 antibody (B-b) as a loading control.
We also conducted the experiments involving anti-AFAP-110-mediated immunoprecipitation and anti-CD44-mediated immunoblotting (Figure 2C) using breast tumor cells (MDA-MB-231 cells). Our data clearly indicate that LMW-HA (Figure 2C-a,b, lane 2) promotes CD44 association with AFAP-110. In contrast, very little CD44-AFAP-110 complex is detected in cells treated with HMW-HA or anti-CD44 plus LMW-HA or without HA) (Figure 2C-a,b, lane 1, lane 3 and lane 4). These findings clearly establish that LMW-HA (to a lesser extent HMW-HA) promotes CD44-AFAP-110 complex formation in breast tumor cells
In response to external signals, AFAP-110 often directly interacts with actin filaments (Flynn et al., 1993; Guappone and Flynn, 1997; Guappone et al., 1998; Qian et al., 1998; Qian et al., 2000; Qian et al., 2004). The results of a filamentous actin (F-actin) binding assay indicate that the CD44-AFAP-110 complex induced by LMW-HA displays a high level of F-actin binding (Table 1A). In contrast, a lower level of F-actin binding to these CD44-AFAP-100 complexes is detected in those cells treated with no HA or HMW-HA or HA fragments (2–3 disaccharides) (Table 1A). Breast tumor cells pretreated with anti-CD44 antibody followed by LMW-HA addition also display a reduced amount of F-actin binding to AFAP-110 (Table 1A). However, normal IgG treatment does not appear to block LMW-HA-induced F-actin binding to AFAP-110 (Table 1A). The findings suggest that LMW-HA (but not HMW-HA) promotes AFAP-110 binding to F-actin in a CD44-dependent manner.
Table 1.
Measurement of F-actin binding to CD44-associated AFAP-110 complex isolated from MDA-MB-231 cells.
| (A) Effects of anti-CD44 antibody on HA-dependent 125I-F-actin binding to the CD44-AFAP-110 complex: | |
|---|---|
| Treatments |
125I-F-actin binding to the CD44-AFAP-110 complex (cpm) (% of control) |
| No treatment (control) | 12,742 ± 637 (100%) |
| LMW-HA treatment | 38,353 ± 3,068 (300%)a |
| Anti-CD44 IgG + LMW-HA treatment | 12,487 ± 250 (98%)a |
| HMW-HA treatment | 13,761 ± 550 (108%)a |
| HA fragment (2–3 disaccharide) treatment | 11,722 ± 423 (92%)a |
| Normal IgG + no HA treatment | 12,500 ± 573 (98%)a |
| Normal IgG + LMW-HA treatment | 37,981 ± 2,550 (298%)a |
| (B) Effect of AFAP-110 siRNA on HA-dependent 125I-F-actin binding to the CD44-AFAP-110 complex: | ||
|---|---|---|
| Treatments |
125I-F-actin binding to the CD44-AFAP-110 complex (cpm) (% of control) |
|
| Scrambled siRNA-treated cells | AFAP-110 siRNA treated cells | |
| No treatment (control) | 10,980 ± 439 (100%) | 9,309 ± 186 (85%)b |
| LMW-HA treatment | 31,842 ± 2,865 (290%)b | 9,529 ± 381 (87%)b |
| HMW-HA treatment | 11,529 ± 576 (105%)b | 9,200 ± 276 (84%)b |
| HA fragment (2–3 disaccharide) treatment | 10,138 ± 423 (92%)b | 9,187 ± 154 (84%)b |
The procedures for measuring 125I-F-actin binding to the CD44-AFAP-110 complex using MDA-MB-231 cells [treated with 1µg/ml LMW-HA or HMW-HA or HA fragment (2–3 disaccharide) (or pretreated with rat anti-CD44 IgG followed by adding 1µg/ml LMW-HA or no HA) (or normal IgG alone or normal IgG plus LMW-HA) or transfected with scrambled siRNA or AFAP-110 siRNA in the presence or absence of 1µg/ml LMW-HA or HMW-HA] are described in the Materials and Methods. The amount of F-actin binding to the CD44-associated AFAP-110 complex isolated from untreated cells (Table 1A-control) or MDA-MB-231 cells treated with scrambled siRNA without HA (Table 1B-control) is designated as 100%. All data represent mean ± SEM of the amount of 125I-F-actin binding to the CD44-AFAP-110 complex detected in each sample. The value represents an average of triplicate determinations of six experiments with an S.D. value less than ±5%.
Statistically significant (p<0.001; analysis of variance; n=6) as compared with control samples [e.g., untreated cells (control)].
Statistically significant (p<0.005; analysis of variance; n=6) as compared with control samples [e.g., scrambled siRNA-treated cells (control) without HA].
In addition, using cell invasion assays, we observed that MDA-MB-231 cells undergo active cell invasion following LMW-HA treatment (Table 2A). In contrast, treatment of MDA-MB-231 cells with HMW-HA or HA fragments (2–3 disaccharides) or no HA induces very little tumor cell invasion (Table 2A). Pretreatment of breast tumor cells with anti-CD44 antibody (Table 2A) followed by LMW-HA addition causes a significant inhibition of tumor cell invasion (Table 2A). However, normal IgG treatment fails to block LMW-HA-induced cell invasion (Table 2A). It is noted that LMW-HA-induced tumor cell invasion can also be effectively blocked by treating cells with cytochalasin D (an inhibitor known to impair F-actin polymerization). Together these findings indicate that LMW-HA induced breast tumor cell invasion is regulated by both CD44-specific and cytoskeleton-associated processes.
Table 2.
Measurement of HA-Mediated Breast Tumor Cell Invasion.
| (A) Effects of HA (LMW-HA vs. HMW-HA) on Breast Tumor Cell Invasion: | |
|---|---|
| Treatments | Tumor Cell Invasion (% of control) |
| No treatment (control) | 100 ± 5 |
| LMW-HA treatment | 289 ± 10a |
| LMW-HA + anti-CD44 antibody treatment | 102 ± 3a |
| LMW-HA + cytochalasin D treatment | 75 ± 2a |
| HMW-HA treatment | 108 ± 4a |
| HA fragment (2–3 disaccharide) treatment | 96 ± 2a |
| Normal IgG + no HA treatment | 102 ± 3a |
| Normal IgG + LMW-HA treatment | 279 ± 8a |
| (B) Effects of AFAP-110 siRNA on HA-mediated Breast Tumor Cell Invasion: | ||
|---|---|---|
| Treatments | Tumor Cell Invasion (% of control) |
|
| Scrambled siRNA-treated cells | AFAP-110 siRNA-treated cells | |
| No treatment (control) | 100 ± 4 | 88 ± 3b |
| LMW-HA treatment | 285 ± 8b | 82 ± 2b |
| HMW-HA treatment | 105 ± 5b | 84 ± 4b |
| HA fragment (2–3 disaccharide) treatment | 98 ± 3b | 86 ± 3b |
Twenty-four transwell units containing 5µm porosity polycarbonate filters coated with the reconstituted basement membrane substance Matrigel were used for monitoring in vitro tumor cell invasion. Cell invasion processes were determined by measuring the cells that migrated and invaded to the lower side of the polycarbonate filters by standard cell number counting methods. The CD44-specific cell invasion was determined by subtracting non-specific cell invasion [i.e., cell that migrated (invaded) to the lower chamber in the presence of anti-CD44 antibody treatment]. The CD44-specific cell invasion in untreated cells (Table 2-control) or scrambled siRNA-treated cells without HA (Table 2B-control) is designated as 100%. All data represent mean ±SEM of the number of cells undergoing invasion detected in each sample. The value represents an average of triplicate determinations of five experiments with an S.D. value less than ±5%.
Statistically significant (p<0.001; analysis of variance; n=5) as compared with control samples [e.g., untreated cells (control)].
Statistically significant (p<0.005; analysis of variance; n=5) as compared with control samples [e.g., scrambled siRNA-treated cells (control) without HA].
LMW-HA-induced CD44 interaction with Toll-Like Receptor (TLR) and MyD88 in Breast Tumor Cells
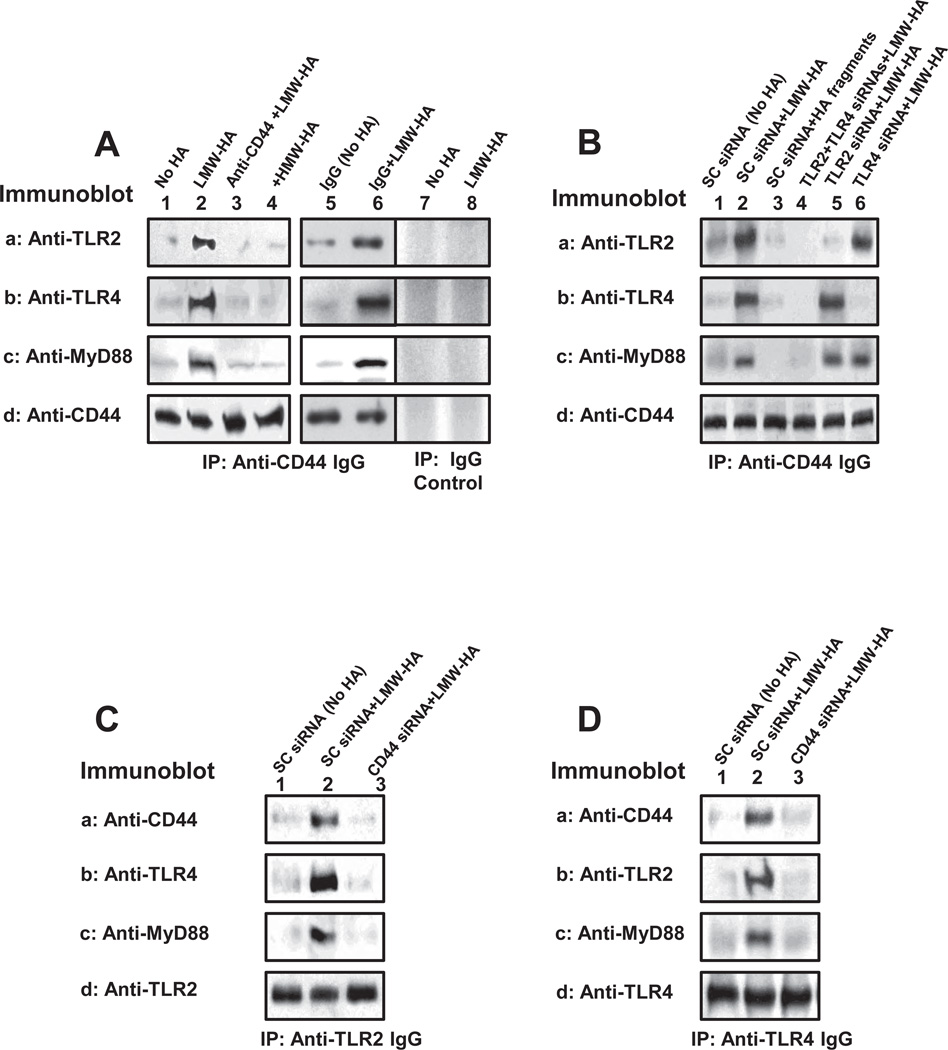
In immune cells, LMW-HA has been shown to interact with Toll-like receptors (TLRs) which in turn activate a MyD88-dependent pathway, resulting in nuclear factor-κB (NF-κB) signaling and cytokine/chemokine gene expression (Jiang et al., 2005; Scheibner et al., 2006). In order to investigate whether there is any interaction between CD44 and TLRs/MyD88 in MDA-MB-231 cells following LMW-HA treatment, we analyzed the anti-CD44-mediated immunoprecipitates from cell lysates by immunoblotting with anti-TLR-2 antibody (or anti-TLR-4 antibody), or anti-MyD88 antibody, respectively. Our results demonstrate that very little TLRs (e.g., TLR-2/TLR-4) (Figure 3A-a, b, lane 1) and MyD88 (Figure 3A-c, lane 1) are complexed with CD44 (Figure 3A-d, lane 1) isolated from MDA-MB-231 cells treated with HMW-HA or no HA (Figure 3A-a,b,c,d-lane 3 and lane 4). Furthermore, we observed that 10min LMW-HA treatment of MDA-MB-231 cells stimulates a significant increase in the amount of both TLRs [e.g., TLR-2 (Figure 3A-a, lane 2) and TLR-4 (Figure 3A-b, lane 2)] and MyD88 (Figure 3A-c, lane 2), recruited into the CD44-associated (Figure 3A-d, lane 2) signaling complex. Pretreatment of cells with anti-CD44 IgG followed by LMW-HA addition (Figure 3A-a,b,c,d, lane 3) display very little TLRs (e.g., TLR-2/TLR-4)/MyD88 complex formation with CD44. However, normal IgG treatment does not appear to block LMW-HA-induced TLRs/MyD88 association with CD44 (Figure 3A-a,b,c,d, lane 6 vs. lane 5). We also confirmed that LMW-HA-induced TLR2/TLR4 and MyD88 association with CD44 is specific since neither CD44 nor TLR2/TLR4/MyD88 is detected in IgG controlmediated precipitation experiments (Figure 3A, lane 7 and lane 8). These observations strongly support the notion that LMW-HA is involved in the recruitment of multi-molecular complex formation (containing TLRs and MyD88) in a CD44-specific fashion.
Figure 3.
Detection of CD44 interaction with TLR2, TLR4 and MyD88 in MDA-MB-231 cells.
A: Cell lysate was immunoprecipitated with anti-CD44 antibody followed by immunoblotting using anti-TLR2 antibody (a) or anti-TLR4 antibody (b) or anti-MyD88 antibody (c) or reblotting with anti-CD44 (d) as a loading control.
[Cells treated with no HA (lane 1) or with LMW-HA (lane 2) or with anti-CD44 antibody plus LMW-HA (lane 3) or with HMW-HA (lane 4) or with normal IgG without HA (Lane 5) or with normal IgG plus LMW-HA (Lane 6)]. Cell lysate was also precipitated with normal IgG control followed by immunoblotting using anti-AFAP antibody (A-a) or reblotting with anti-CD44 antibody (A-b) as a loading control. [Cells treated with no HA (lane 7); LMW-HA (lane 8)].
B: Cell lysate was immunoprecipitated with anti-CD44 antibody followed by immunoblotting using anti-TLR2 antibody (a) or anti-TLR4 antibody (b) or anti-MyD88 antibody (c) or reblotting with anti-CD44 (d) as a loading control.
[Cells treated with scrambled (SC) siRNA without HA (lane 1) or with LMW-HA (lane 2) or with HA fragments (2–3 disaccharides) (lane 3); or transfected with TLR2 siRNA and TLR4 siRNA (lane 4) plus LMW-HA or TLR2 siRNA plus LMW-HA (lane 5) or TLR4 siRNA plus LMW-HA (lane 6)].
C: Cell lysate was immunoprecipitated with anti-TLR2 antibody followed by immunoblotting using anti-CD44 antibody (a) or anti-TLR4 antibody (b) or anti-MyD88 antibody (c) or reblotting with anti-TLR2 (d) as a loading control.
[Cells treated with scrambled (SC) siRNA without HA (lane 1) or with LMW-HA (lane 2) or transfected with CD44 siRNA plus LMW-HA (lane 3)].
D: Cell lysate was immunoprecipitated with anti-TLR4 antibody followed by immunoblotting using anti-CD44 antibody (a) or anti-TLR2 antibody (b) or anti-MyD88 antibody (c) or reblotting with anti-TLR4 (d) as a loading control.
[Cells were treated with scrambled (SC) siRNA without HA (lane 1) or with LMW-HA (lane 2) or transfected with CD44 siRNA plus LMW-HA (lane 3)].
In addition, we conducted anti-CD44-mediated immunoprecipitation followed by anti-TLR2 (or anti-TLR4 or anti-MyD88)-mediated immunoblotting using cells treated with TLR2 siRNA (Figure 3B-a, b,c,d, lane 5) or TLR4 siRNA (Figure 3B-a,b,c,d, lane 6) or scrambled siRNA (Figure 3B-a,b,c,d, lane 1 and 2), respectively. Our results indicate that LMW-HA stimulates TLR2/TLR4/MyD88 complex formation with CD44 (Figure 3B-a,b,c,d, lane 1 and 2). In contrast, HA fragments (2–3 disaccharides) fail to induce TLRs/MyD88 association with CD44 (Figure 3B-a,b,c,d-lane 3). We also observed that (i) TLR2 downregulation (by treating cells with TLR2 siRNA, but not with scrambled siRNA) fails to block LMW-HA-mediated CD44 association with TLR4 and MyD88 (Figure 3B-a,b,c,d, lane 5 vs. lane 1 and 2); and (ii) TLR4 knock-down (by treating cells with TLR4 siRNA, but not with scrambled siRNA) also does not inhibit LMW-HA-mediated CD44 association with TLR2 and MyD88 (Figure 3B-a,b,c,d, lane 6 vs. lane 1 and 2). However, if both TLR2 and TLR4 are downregulated by treating cells with TLR2 siRNA and TLR4 siRNA, LMW-HA-induced TLR2/TLR4/MyD88 association with CD44 is greatly reduced (Figure 3B-a,b,c,d, lane 4 vs. lane 5 and lane 6). Thus, it is possible that both TLR2 and TLR4 (to lesser extent TLR2 alone or TLR4 alone) are required for LMW-HA signaling in breast tumor cells.
Moreover, we have treated MDA-MB-231 cells with CD44 siRNA (or scrambled siRNA) in the presence or absence of LMW-HA followed by anti-TLR2 or anti-TLR4-mediated immunoprecipitation and immunoblotting with CD44, TLR2/TLR4 or MyD88, respectively (Figure 3C-a,b,c,d, lane 3 vs. lane 1 and 2; and Figure 3D-a,b,c,d, lane 3 vs. lane 1 and 2). Our data indicate that downregulation of CD44 with CD44 siRNA (but not scrambled siRNA) significantly inhibits LMW-HA-induced complex formation containing TLR2, TLR4 and MyD88. These findings strongly suggest that CD44 (together with TLR2 and TLR4) plays an important role in recruiting multi-molecular complexes in breast tumor cells (MDA-MB-231 cells) following LMW-HA treatment.
NFκB Activation and Its Downstream Effector Functions by CD44/TRL-associated MyD88 Pathway in Breast Tumor Cells Treated with LMW-HA
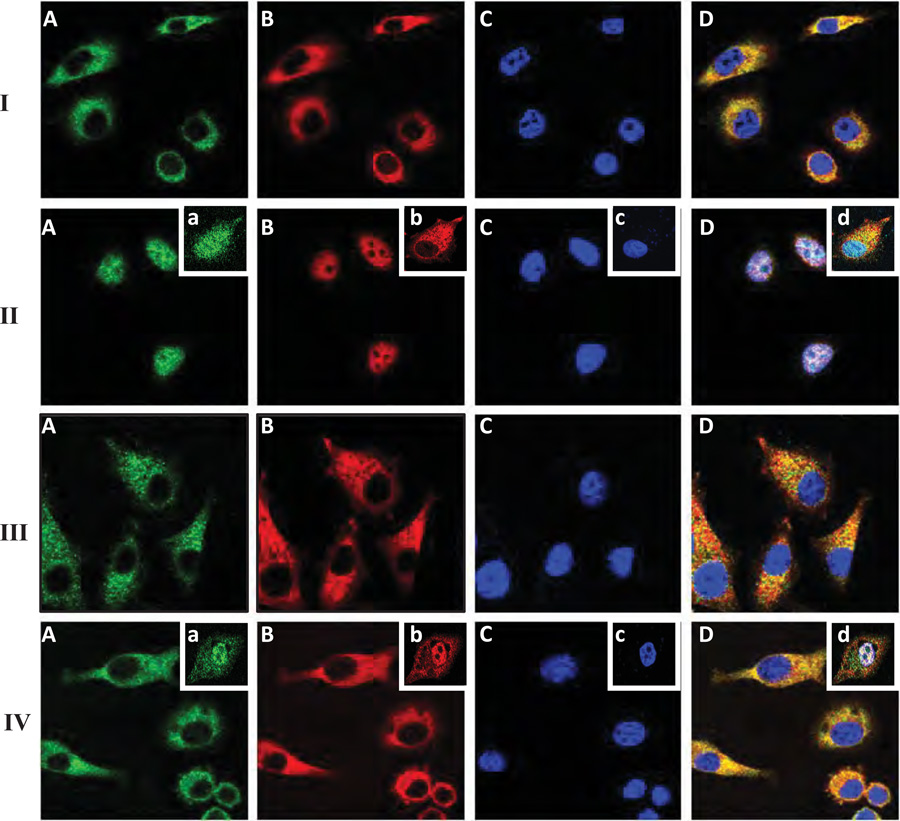
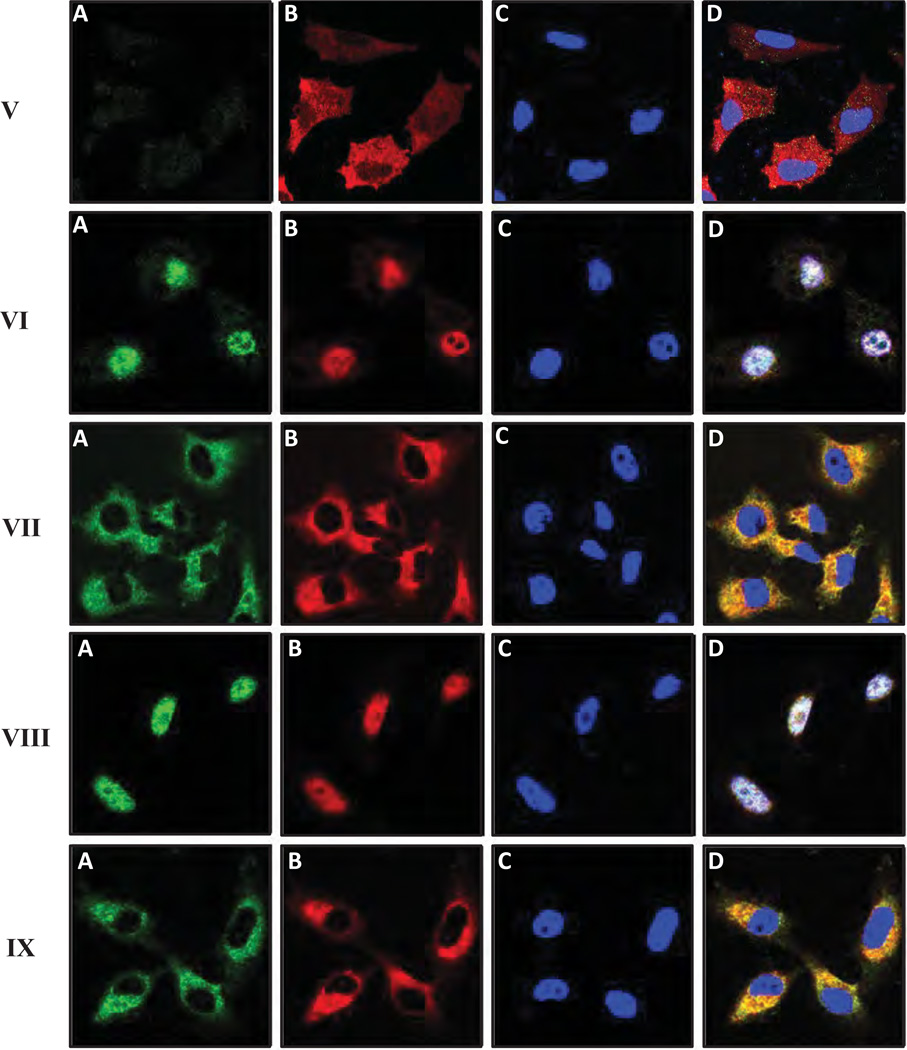
There is compelling evidence that MyD88 is linked to NF-κB-specific transcriptional upregulation in a number of cell types (Schmitz et al., 2004; Toubi and Shoenfeld, 2004). Consequently, we examined the potential impact of CD44-TLRs/MyD88 association on NF-κB signaling (e.g., NF-κB-p65 nuclear translocation and transcriptional activation) by LMW-HA in MDA-MB-231 cells. Using double immunofluorescence staining and confocal microscopic analyses, we confirmed that both MyD88 and NF-κB-p65 are primarily distributed in the cytosol of MDA-MB-231 in the absence of HA (Figure 4A-I, A, B & D). Very low levels of these molecules are detected in the nucleus (indicated by DAPI nuclear staining) in these cells without any HA treatment (Figure 4-I, C& D). However, thirty (30) min after LMW-HA (Figure 4-II, A–D), but not HMW-HA (Figure 4B-III, A–D) or HA fragment (2–3 disaccharide) treatment (Figure 4II inset, a–d), both MyD88 and NF-κB-p65 are co-translocated into the nucleus. Pretreatment of cells with anti-CD44 antibody followed by LWM-HA addition effectively blocks nuclear translocation of either MyD88 or NF-κB-p65 in these breast tumor cells (Figure 4-IV, A–D). However, normal IgG treatment does not appear to block LMW-HA-induced MyD88 or NF-κB-p65 (Figure 4-IV inset, a–d). These results suggest that LMW-HA-induced MyD88 and NF-κB-p65 nuclear accumulation is CD44-specific. We also noted that downregulation of MyD88 by transfecting MDA-MB-231 cells with MyD88 siRNA (Figure 4-V, A–D), but not scrambled sequence siRNA (Figure 4-VI, A–D) significantly inhibits LMW-HA-mediated NF-κB-p65 nuclear translocation in these cells. These observations suggest that MyD88 is closely linked to LMW-HA-induced NF-κB-p65 nuclear translocation events.
Figure 4.
Immunofluorescence staining of MyD88 and NFκB-p65 in MDA-MB-231 cells: MDA-MB-231 cells treated with various agents were fixed by 2% formaldehyde. Subsequently, cells were rendered permeable by ethanol treatment and stained with FITC-labeled anti-MyD88 antibody (green color) (A or a), Texas Red-labeled NFκB-p65 antibody (red color) (B or b) or DAPI (blue color) (C or c) and an overlay image (D or d) of (A, a) (B, b) and (C, c) as described in the Materials and Methods.
MDA-MB-231 cells were treated with no HA (I); or with LMW-HA (II) [or with HA fragments (2–3 disaccharides) (inset, a–d)]; or with HMW-HA (III); or with anti-CD44 antibody followed by LMW-HA (IV) [or with normal IgG plus LMW-HA, inset, a–d)]; or transfected with MyD88 siRNA plus LMW-HA (V); or with scrambled siRNA plus LMW-HA (VI); or with AFAP-110 siRNA plus LMW-HA (VII); or with scrambled siRNA plus LMW-HA (VIII); or with cytochalasin D plus LMW-HA (IX).
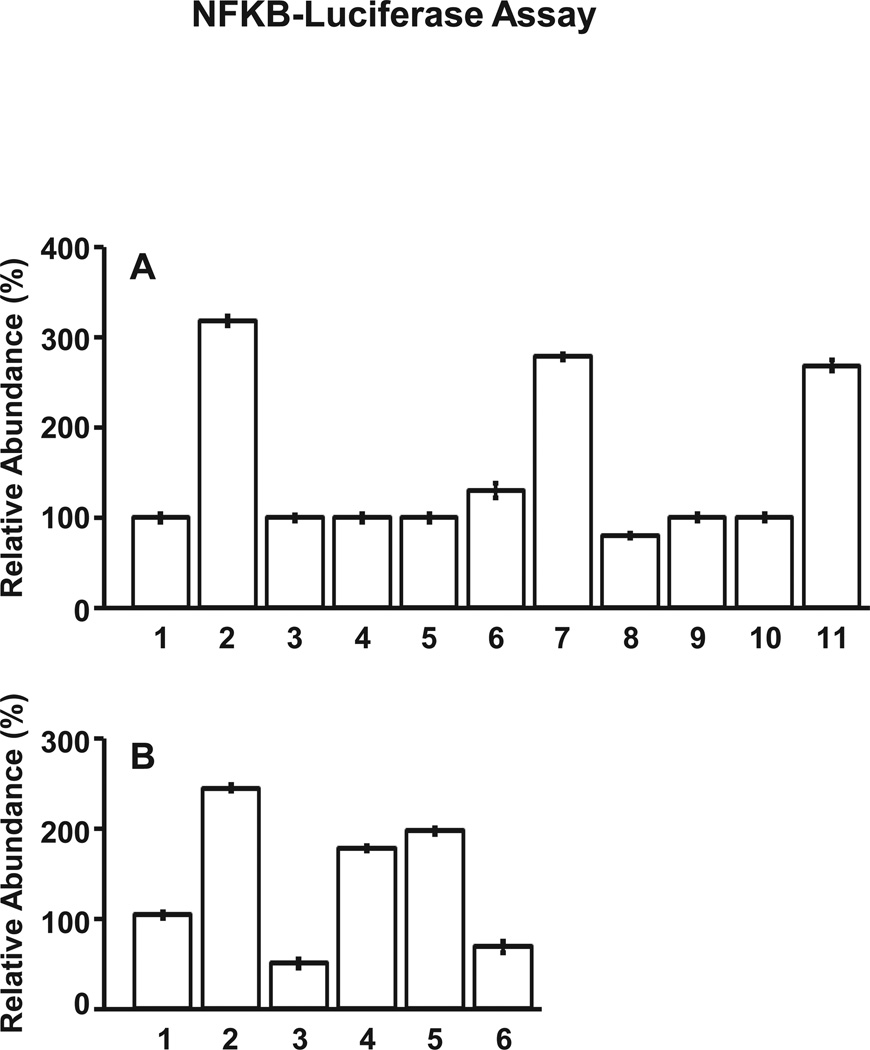
Many studies have shown that nuclear NF-κB-p65 functions as a transcription factor which binds to specific promoter elements of target genes and regulates transcriptional activities leading to gene expression encoding pro-inflammatory chemokine/cytokine (Jiang et al., 2005; Scheibner et al., 2006). In this study, we noted that NF-κB-associated transcriptional activation is greatly stimulated in MDA-MB-231 cells treated with LMW-HA (Figure 5A, bar 2) as compared to these cells treated with no HA (Figure 5A, bar 1) or HMW-HA (Figure 5A, bar 4) or HA fragments (2–3 disaccharides) (Figure 5A, bar 5). Pretreatment of cells with anti-CD44 antibody followed by LMW-HA significantly inhibits NF-κB-specific transcriptional activity (Figure 5A, bar 3). However, normal IgG treatment does not appear to reduce LMW-HA-induced NF-κB-specific transcriptional upregulation (Figure 5A, bar 10 and 11). Therefore, we believe that LMW-HA activation of NF-κB-mediated transcriptional regulation is CD44-specific. Moreover, we found that transfection of MDA-MB-231 cells with MyD88 siRNA (Figure 5A, bar 8), but not scrambled sequence siRNA (Figure 5A, bars 6 and 7) effectively impairs LMW-HA-activated NF-κB-transcriptional activity. These findings confirm the importance of MyD88 in regulating NF-κB-specific transcriptional activation in LMW-HA-treated breast tumor cells.
Figure 5.
Measurement of NFκB-specific transcriptional activation in MDA-MB-231 cells.
A: Cells were treated with no HA (bar 1) or with LMW-HA (bar 2) or with anti-CD44 plus LMW-HA (bar 3) or with HMW-HA (bar 4) or with HA fragments (2–3 disaccharides) (bar 5) or transfected with scrambled siRNA without HA (bar 6) or with scrambled siRNA plus LMW-HA (bar-7) or with MyD88 siRNA plus LMW-HA treatment (bar 8) or with AFAP-110 siRNA plus LMW-HA treatment (bar 9) or with normal IgG with no HA (bar 10) or with LMW-HA (bar 11)] were analyzed for NFκB-specific transcriptional activity.
B: Cells were treated with scrambled siRNA followed by no HA (bar 1) or with LMW-HA (bar 2) or transfected with CD44 siRNA plus LMW-HA (bar 3) or with TLR2 siRNA plus LMW-HA (bar 4) or with TLR4 siRNA plus LMW-HA (bar 5) or with TLR2 siRNA and TLR4 siRNA plus LMW-HA (bar 6)] were analyzed for NFκB-specific transcriptional activity.
The activity of NFκB-specific transcriptional activation in untreated cells (A) or scrambled siRNA-treated (without HA) (B) is designated as 100% (control). [All data represent mean ±SEM of the amount of NFκB-specific transcriptional activity detected in each sample. The value represents an average of triplicate determinations of five experiments with an S.D. value less than ±5%. The values expressed in bars 2–7 (A) or bars 2–5 (B) are statistically significant (p<0.001; analysis of variance; n=5) as compared with control samples [e.g., bar1-untreated cells (A-control) or bar1-scrambled siRNA cells without HA (B-control), respectively].
In addition, we found that LMW-HA promotes pro-inflammatory cytokine/chemokine (e.g., IL-1β and IL-8) gene expression and protein production (Table 3A). In contrast, very low level of IL-1β/IL-8 gene/protein expression is detected in breast tumor cells treated with HMW-HA, HA fragments (2–3 disaccharides) or no HA (Table 3A). We have also confirmed that treatment of breast tumor cells with anti-CD44 antibody significantly downregulates LMW-HA-activated pro-inflammatory cytokine and chemokine gene expression and protein secretion (Table 3A) in these tumor cells. However, normal IgG treatment does not appear to impair LMW-HA-induced IL-1β and IL-8 gene/protein expression (Table 3A). Thus, it is likely that LMW-HA-mediated NF-κB-p65 signaling and cytokine/chemokine production are functionally coupled in a CD44-dependent manner in breast tumor cells.
Table 3.
Detection of IL-1β and IL-8 gene expression and protein production in MDA-MB-231 cells.
| (A) Measurement of IL-1β and IL-8 gene expression and protein production in HA-treated MDA-MB-231 cells: | ||||
|---|---|---|---|---|
| Treatment | (a) Amount of Pro-inflammatory Cytokine & Chemokine Gene Expression (Relative Abundance)* |
(b) Amount of Pro-inflammatory Cytokine & Chemokine Protein Production** |
||
| IL-1β Gene Expression (% of control) |
IL-8 Gene Expression (% of control) |
IL-1β Protein Production (pg/ml) (% of control) |
IL-8 Protein Production (pg/ml) (% of control) |
|
| No HA-treated (Control) | 100 ± 3.0 | 100 ± 2.2 | 85 ± 4.1 (100%) | 88 ± 1.8 (100%) |
| LMW-HA-treated | 268 ± 15a | 295 ± 5.6b | 232 ± 10.8 (273%)c | 233 ± 8.4 (265%)d |
| LMW-HA +anti-CD44-reated | 98 ± 2.4a | 92 ± 1.2b | 70 ± 3.2 (82%)c | 73 ± 1.5 (83%)d |
| HMW-HA-treated | 102 ± 0.8a | 107 ± 1.6b | 84 ± 3.2 (99%)c | 91 ± 1.8 (102%)d |
| Scrambled siRNA-treated (No HA) | 105 ± 1.2a | 102 ± 1.0b | 86 ± 4.2 (101%)c | 90 ± 2.9 (102%)d |
| Scrambled siRNA-Treated (+LMW-HA) | 222 ± 9.0a | 238 ± 6.0b | 238 ± 12.2 (280%)c | 241 ± 14.2 (274%)d |
| CD44 siRNA-treated (+LMW-HA) | 44 ± 0.4a | 50 ± 0.3b | 44 ± 2.5 (52%)c | 42 ± 1.1 (47%)d |
| TLR2 siRNA-treated (+LMW-HA) | 210 ± 10.0a | 202 ± 16.0b | 207 ± 10.4 (243%)c | 199 ± 12 (226%)d |
| TLR4 siRNA-treated (+LMW-HA) | 217 ± 12.0a | 220 ± 18.0b | 221 ± 8.9 (261%)c | 200 ± 18 (228%)d |
| TLR2 siRNA+TLR siRNA-treated (+LMW-HA) | 62 ± 0.6a | 69 ± 0.7b | 47 ± 1.7 (55%)c | 56 ± 2.1 (63%)d |
| Normal IgG + no HA-treated | 102 ± 0.8a | 107 ± 1.6b | 84 ± 2.2 (99%)c | 91 ± 1.4 (103%)d |
| Normal IgG + LMW-HA-treated | 222 ± 0.9a | 238 ± 0.6b | 231 ± 3.2 (272%)c | 241 ± 2.8 (274%)d |
| HA fragment (2–3 disaccharide)-treated | 102 ± 0.8a | 107 ± 1.6b | 80 ± 2.4 (94%)c | 87 ± 1.5 (99%)d |
| (B) Effects of MyD88 siRNA treatment on IL-1β and IL-8 gene expression & protein production in HA-treated MDA-MB-231 cells: | ||||||||
|---|---|---|---|---|---|---|---|---|
| (a) Amount of Pro-inflammatory Cytokine & Chemokine Gene Expression (Relative Abundance)* |
(b) Amount of Pro-inflammatory Cytokine & Chemokine Protein Production** |
|||||||
| IL-1β Gene Expression (% of control) |
IL-8 Gene Expression (% of control) |
IL-1β Protein Production (pg/ml) (% of control) |
IL-8 Protein Production (pg/ml) (% of control) |
|||||
| Treatment | Scrambled siRNA-treated |
MyD88 siRNA-treated |
Scrambled siRNA-treated |
MyD88 siRNA-treated |
Scrambled siRNA- treated |
MyD88 siRNA-treated |
Scrambled siRNA-treated |
MyD88 siRNA-treated |
| No HA (control) | 100 ± 5 | 85± 2e | 100 ± 2 | 82 ± 2f | 84 ± 1.0 (100%) | 72 ± 0.2 (86%)g | 85 ± 1.1 (100%) | 70 ± 0.5 (82%)h |
| LMW-HA Treatment | 285 ± 10e | 80± 3e | 294 ± 12f | 84 ± 1f | 235 ± 4.8 (280%)g | 74 ± 0.3 (88%)g | 221 ± 2.2 (260%)h | 72 ± 0.6 (85%)h |
| HMW-HA Treatment | 105 ± 4e | 88± 2e | 104 ± 1f | 86 ± 2f | 88 ± 0.8 (105%)g | 71 ± 0.2 (84%)g | 92 ± 0.7 (108%)h | 75 ± 0.2 (88%)h |
| HA fragment (2–3 disaccharide) treatment | 98 ± 3e | 83± 3e | 96 ± 2f | 80 ± 3f | 85 ± 0.4 (101%)g | 68 ± 0.1 (81%)g | 82 ± 0.5 (97%)h | 77 ± 0.2 (91%)h |
| (C) Effects of AFAP-110 siRNA treatment on IL-1β and IL-8 gene expression & protein production in HA-treated MDA-MB-231 cells: | ||||||||
|---|---|---|---|---|---|---|---|---|
| (a) Amount of Pro-inflammatory Cytokine & Chemokine Gene Expression (Relative Abundance)* |
(b) Amount of Pro-inflammatory Cytokine & Chemokine Protein Production** |
|||||||
| IL-1β Gene Expression (% of control) |
IL-8 Gene Expression (% of control) |
IL-1β Protein Production (pg/ml) (% of control) |
IL-8 Protein Production (pg/ml) (% of control) |
|||||
| Treatment | Scrambled siRNA-treated |
AFAP-110 siRNA-treated |
Scrambled siRNA-treated |
AFAP-110 siRNA-treated |
Scrambled siRNA-treated |
AFAP-110 siRNA-treated |
Scrambled siRNA-treated |
AFAP-110 siRNA-treated |
| No Treatment (Control) | 100 ± 3 | 83 ± 3i | 100 ± 2 | 88 ± 2j | 84 ± 1.2 (100%) | 70 ± 0.3 (83%)k | 82 ± 1.2 (100%) | 72 ± 0.2 (86%)l |
| LMW-HA Treatment | 289 ± 13i | 89 ± 2i | 300 ± 16j | 85 ± 3j | 235 ± 1.8 (280%)k | 67 ± 0.2 (80%)k | 238 ± 1.6 (290%)l | 71 ± 0.1 (87%)l |
| HMW-HA Treatment | 104 ± 3i | 82 ± 2i | 106 ± 7j | 82 ± 2j | 90 ± 1.3 (107%)k | 69 ± 0.4 (82%)k | 96 ± 1.7 (117%)l | 68 ± 0.2 (83%)l |
| HA fragment (2–3 disaccharide) treatment | 98 ± 2i | 84 ± 1i | 96 ± 5j | 85 ± 3j | 86 ± 1.0 (102%)k | 71 ± 0.4 (85%)k | 86 ± 1.4 (105%)l | 65 ± 0.1 (79%)l |
The expression of NF-κB target genes (e.g., IL-1β and IL-8) was measured using specific primers and Q-PCR in MDA-MB-231 cells according to the procedures described in the Materials and Methods. Relative mRNA expression levels of IL-1β or IL-8 in various treatments were calculated after normalization with 36B4 mRNA levels as determined by Q-PCR. IL-1β or IL-8 gene expression from untreated cells (Table 3A-control) or scrambled siRNA-treated cells (Table 3B-control or Table 3C-control) is designated as 100%.
For the measurement of IL-1β and IL-8 protein production, MDA-MB-231 cells [untreated or pretreated with anti-CD44 or pretreated with various siRNAs (e.g., CD44siRNA or TLR2 siRNA or TLR4 siRNA or MyD88 siRNA or AFAP-110 siRNA or scrambled siRNA] were washed three times with serum free (SF)-DMEM and incubated in 3 ml of serum free-DMEM containing various reagents [e.g., LMW-HA or HMW-HA or HA fragment (2–3 disaccharide) no HA treatment] for 24 hr. Subsequently, concentrations of IL-1β or IL-8 secreted in the medium from these cells were determined using the Quantikine immunoassay for IL-1β or IL-8 (R & D Systems). IL-1β or IL-8 protein production in the medium from untreated cells (Table 3A-control) or scrambled siRNA-treated cells (Table 3B-control or Table 3C-control) is designated as 100%. All data represent mean ± SEM of the amount of IL-1β or IL-8 gene expression and protein production detected in each sample. The value represents an average of triplicate determinations of six experiments with an S.D. value less than ±5%.
Statistically significant (p<0.001; analysis of variance; n=6) as compared with control samples [e.g., untreated cells (control)].
Statistically significant (p<0.005; analysis of variance; n=6) as compared with control samples [e.g., scrambled siRNA-treated cells (control) with no HA].
Furthermore, we observed that LMW-HA promotes pro-inflammatory cytokine/chemokine (e.g., IL-1β and IL-8) gene expression and protein production (Table 3A) in MDA-231 cells transfected with scrambled siRNA. Downregulation of CD44 (by treating cells with CD44 siRNA) not only effectively blocks CD44 association with TLR2, TLR4 and MyD88 in MDA-231 cells treated with LMW-HA (Figure 3C-a,b,c,d, lane 3 vs. lane 1 & 2; and Figure 3D-a,b,c,d, lane 3 vs. lane 1 and 2), but also significantly inhibits LMW-HA-mediated signaling and functions [e.g., NF-κB -mediated transcriptional activation (Figure 5B-bar 3 vs. bar 1 and 2), IL-1β/IL-8 gene expression and protein production (Table 3A)]. In contrast, downregulation of either TLR2 alone (by treating cells with TLR2 siRNA alone) or TLR4 alone (by treating cells with TLR4 siRNA alone) fails to cause a significant inhibition of LMW-HA-induced CD44 recruitment with TLR4/MyD88 (Figure 3B-a,b,c,d, lane 5 vs. lane 1 and 2) or TLR2/MyD88 (Figure 3B-a,b,c,d, lane 6 vs. lane 1 and 2) as well as downstream signaling events (Figure 5B-bar 4, bar 5 vs. bar 1 and 2; and Table 3A). However, if the expression of both TLR2 and TLR4 is inhibited (by treating cells with TLR2 siRNA and TLR4 siRNA), LMW-HA-mediated signaling events [e.g., CD44 association with TLRs/MyD88 (Figure 3B-a,b,c,d, lane 5 vs. lane 1 and 2), NF-κB-mediated transcriptional activation (Figure 5B-bar 6 vs. bar 1 and 2), IL-1β/IL-8 gene expression and protein production (Table 3A)] are greatly impaired. These findings strongly suggest that CD44 and its associated TLR2/TLR4 are required for LMW-HA-mediated signaling and functions in breast tumor cells such as MDA-MB-231 cells.
Finally, downregulation of MyD-88 (by treating cells with MyD88 siRNA) (but not scrambled sequenced siRNA) also effectively inhibits LMW-HA-stimulated IL-1β and IL-8 gene expression and protein secretion (Table 3B). Together, these findings strongly demonstrate that CD44 and TLR2/TLR4-associated MyD88 play an important role in regulating NF-κB-p65-specific transcriptional activation and cytokine/chemokine gene expression/protein production) in breast tumor cells (MDA-MB-231 cells) following LMW-HA treatment.
Effects of AFAP-110 Downregulation on LMW-HA mediated F-actin function and MyD88-NFκB Activation in Breast Tumor cells
(a) F-actin reorganization, binding and tumor cell invasion
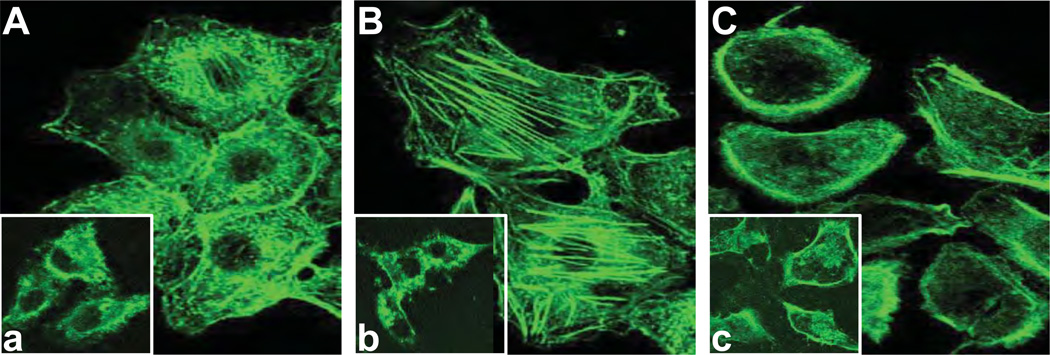
To further assess the importance of AFAP-110 in LMW-HA-mediated signaling events, we examined the effects of AFAP-110 on F-actin rearrangement in MDA-MB-231 cells induced by LMW-HA. Staining of MDA-MB-231 cells with fluorescent phalloidin reveals that the assembly of actin fibrils and stress fibers occurs in scrambled siRNA-treated cells following LMW-HA addition (Figure 6B). In the majority of those cells treated with LMW-HA for 15min, the actin filaments were present in numerous stress fibers, and/or in thick filaments associated with the plasma membrane and cellular projections as well as the perinuclear region (Figure 6B). In contrast, we have found that a small amount of F-actin fragments was randomly distributed in the cytosol in cells treated with scrambled siRNA without HA (Figure 6A) or with HMW-HA (Figure 6a) or with HA fragments (2–3 disaccharides) (Figure 6b). Furthermore, in cells treated with AFAP-110 siRNA followed by LMW-HA addition (Figure 6C), we determined that stress fibers or actin fibrils were no longer apparent, the total amount of actin was greatly reduced, and the small amount of remaining actin was primarily located at the cytoplasm (Figure 6C). The inability of LMW-HA to promote F-actin assembly and reorganization in AFAP-110 siRNA-treated cells is similar to those cells treated with cytochalasin D (a drug known to impair F-actin formation) (Figure 6c). These findings indicate that AFAP-110 is functionally linked to the rearrangement of actin filaments and fibers during LMW-HA-mediated CD44 signaling.
Figure 6.
Immunofluorescence staining of F-actin in MDA-MB-231 cells.
A: Localization of FITC-phalloidin labeled F-actin in scrambled siRNA-treated cells with no HA [a inset: localization of FITC-phalloidin labeled F-actin in scrambled siRNA-treated cells plus HMW-HA]. B: Localization of FITC-phalloidin labeled F-actin in scrambled siRNA-treated cells plus LMW-HA [b inset: localization of FITC-phalloidin labeled F-actin in scrambled siRNA-treated cells plus HA fragments (2–3 disaccharides)].
C: Localization of FITC-phalloidin labeled F-actin in AFAP siRNA-treated cells plus LMW-HA [c inset: localization of FITC-phalloidin labeled F-actin in scrambled siRNA-treated cells plus cytochalasin D and LMW-HA].
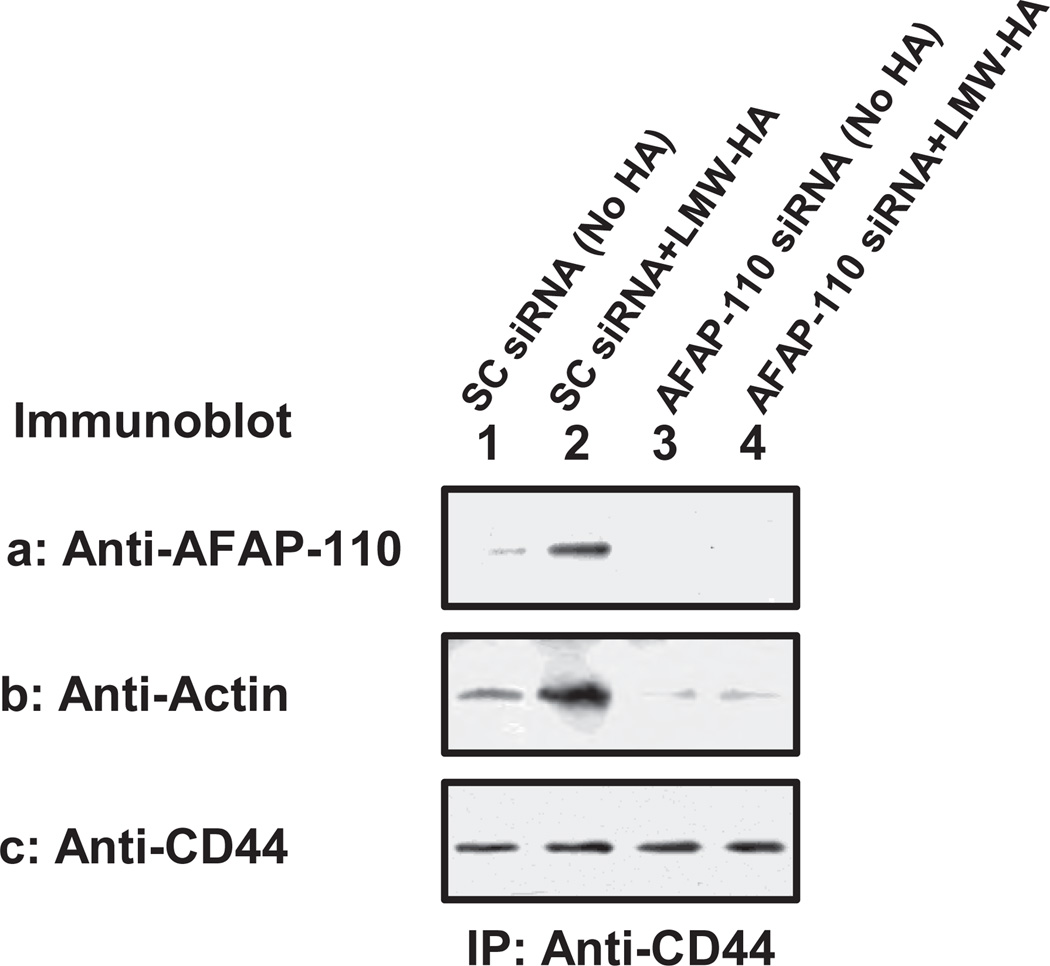
Our results also indicate that LMW-HA promotes CD44 association with AFAP-110 and F-actin binding (Figure 7a, b, c, lane 1 & 2 and Table 1B) in MDA-MB-231 cells treated with scrambled siRNA. We also observed that LMW-HA [but not HMW-HA or HA fragments (2–3 disaccharides)] stimulates breast tumor cells invasion using scrambled siRNA-treated cells (Table 2B). In contrast, neither CD44-AFAP-110 association nor F-actin binding (Figure 7a, b, c, lane 3 & 4 and Table 1B) is detected in MDA-MB-231 cells treated with AFAP-110 siRNA in the presence and absence of LMW-HA. Breast tumor cell invasion is also significantly reduced in cells treated with AFAP siRNA followed by LMW-HA (Table 2B). All of these results support the contention that AFAP-110 serves as an essential F-actin binding protein required for LMW-HA/CD44-mediated breast tumor cell invasion.
Figure 7.
Analyses of LMW-HA-induced AFAP-110 interaction with CD44 and F-actin in MDA-MB-231 cells treated with AFAP siRNA or scrambled siRNA: MDA-MB-231 cells (treated with AFAP-110 siRNA or scrambled siRNA) were solubilized by 1% Nonidet P-40 (NP-40) buffer and immunoprecipitated with anti-CD44 antibody followed by immunoblotting using anti-AFAP antibody (a) or incubated with F-actin followed by anti-actin-mediated immunoblot (b) or reblotting with anti-CD44 (c) as a loading control.
[Lane 1: Scrambled siRNA-treated cells with no HA; Lane 2: Scrambled siRNA-treated cells incubated with LMW-HA (1µg/ml) for 5min; Lane 3: AFAP-110 siRNA-treated cells with no HA; Lane 4: AFAP-110 siRNA-treated cells incubated with LMW-HA (1µg/ml) for 5min].
(b) MyD88-linked NF-κB activation
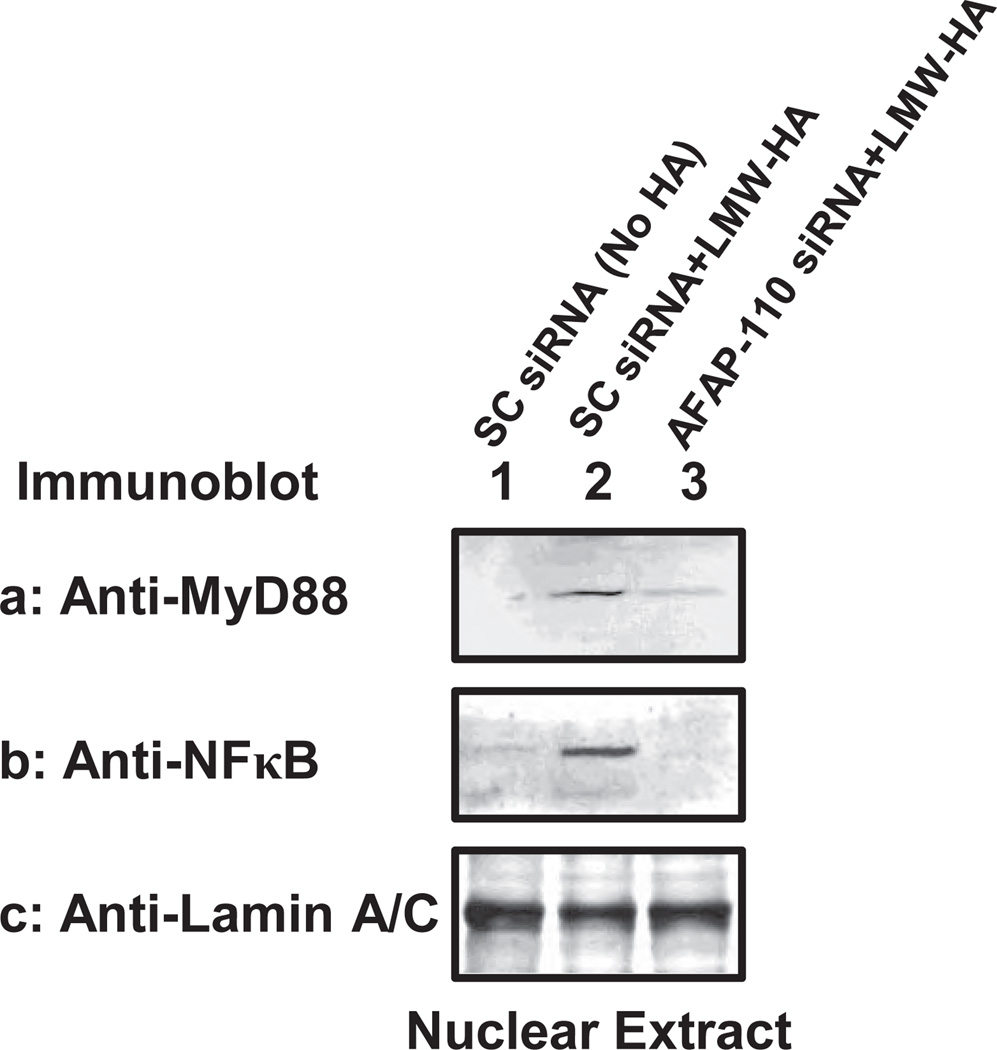
To assess the effects of AFAP-110 downregulation on MyD88-mediated NF-κB signaling in breast tumor cells, we also transfected MDA-MB-231 cells with AFAP-110 siRNA (or scrambled sequenced siRNA). Our immunofluorescence staining data indicate that LMW-HA is capable of promoting MyD88-NF-κB nuclear translocation (Figure 4-VIII, A–D) in scrambled sequence siRNA-treated cells. In contrast, LMW-HA fails to induce MyD88 and NF-κB nuclear translocation (Figure 4-VII, A–D) in AFAP-110 siRNA-treated cells. Pretreatment of breast tumor cells with cytochalasin D (an inhibitor known to impair F-actin polymerization) also causes a significant inhibition of LMW-HA/CD44-mediated MyD88/NF-κB nuclear translocation (Figure 4-IX, A–D). Furthermore, we have observed that a 30 min LMW-HA treatment of scrambled siRNA-treated cells stimulates a significant increase in the amount of MyD88 (Figure 8a, lane 2) and NF-κB (Figure 8b, lane 2) in the nuclear fractions of these cells as compared with those cells without HA treatment (Figure 8a,b, lane 1). When these tumor cells transfected with AFAP-110 siRNA followed by a 30min LMW-HA treatment, both MyD88 (Figure 8a, lane 3) and NF-κB (Figure 8b, lane 3) in the nuclear fraction are greatly reduced in these cells. These findings suggest that AFAP-110 is capable of regulating MyD88 and NF-κB nuclear translocation. The question of whether additional signaling pathway(s) may be involved in MyD88/NFκB nuclear translocation remains to be investigated.
Figure 8.
Detection of LMW-HA-mediated MyD88 and NF-κB-p65 nuclear localization in MDA-MB-231 cells: The nuclear fraction isolated from MDA-MB-231 cells [treated with scrambled sequence siRNA and incubated with no HA (1µg/ml) (lane 1) or LMW-HA (1µg/ml) for 30min (lane 2); or treated with AFAP-110 siRNA and incubated with LMW-HA (1µg/ml) (lane 3)] were immunoblotted with anti-MyD88 antibody (a) or anti-NFκB-p65 antibody (b) or anti-lamin A/C antibody (c) (as a loading control).
Subsequently, we found that NF-κB-specific transcriptional activation (Figure 5A, bar 8) and cytokine/chemokine gene/protein expression (Table 3C) in AFAP-110 siRNA-treated cells followed by LMW-HA treatment are also greatly inhibited as compared to scrambled siRNA-treated cells incubated with LMW-HA (Figure 5A, bars 5 and 6; Table 3C). Together, these findings strongly suggest that AFAP-110 plays an important role not only in linking F-actin for cytoskeleton-regulated tumor cell invasion (Table 1B), but also in regulating MyD88/NF-κB nuclear translocation required for MyD88-mediated NF-κB transcriptional activation, pro-inflammatory cytokine and chemokine gene expression/protein production in breast tumor cells.
DISCUSSION
HA is thought to provide stem cell niches that facilitate the invasion of tumor cells into the extracellular matrix (ECM) materials. HA interacts with a specific cell surface receptor, CD44, which belongs to a family of multifunctional transmembrane glycoproteins expressed in numerous cells and tissues including breast cancer stem cells, and breast carcinoma cells/tissues (Iida and Bourguignon, 1995; Iida and Bourguignon, 1997; Al-Hajj et al., 2003; Auvinen et al., 2005). One recent concept which has emerged from cancer biological studies is that low molecular weight-HA (LMW-HA) and high molecular weight (HMW-HA) may display distinct chemotherapy responses (chemosensitivity vs. chemoresistance; (Bourguignon et al., 2008; Toole and Slomiany, 2008; Bourguignon et al., 2009a; Bourguignon et al., 2009b; Slomiany et al., 2009a; Slomiany et al., 2009b). In this study we investigated which oncogenic pathways are directly involved in regulating LMW-HA-mediated CD44 signaling and breast tumor cell behaviors.
Activation of TLRs also appears to be closely associated with tumor progression. Here, we found that both TLR2 and TLR4 are expressed in MDA-MB-231 cells. These results are consistent with a previous finding showing TLR2 is greatly upregulated in MDA-MB-231 cells (displaying a high invasive potential) as compared to MCF-7 cells (displaying poor invasive phenotype) (Xie et al., 2009). Moreover, we observed that LMW-HA induces CD44 interaction with Toll-like receptors (TLR2/4) (Figure 3), which are known to initiate pro-inflammatory signal transduction-related pathways in breast tumor cells, similar to immune cells. Although CD44 activates cytoskeleton pathways (Bourguignon, 2001; Turley et al., 2002; Bretscher, et al., 2002; Bourguignon, 2008), TLRs generally signal through MyD88 (a scaffold protein)-dependent pathway, resulting in early activation of nuclear factor-κB (NF-κB) and pro-inflammatory gene expression and cytokine/chemokine production.
Inflammation has been thought to contribute to the development of cancer. Cancer-associated inflammation is thought to be linked to immune suppression that allows tumor cells to escape immune surveillance. Cancer-associated inflammatory mediators are also involved in promoting the malignant cancer cell activity by acting as growth factors (Ben-Baruch, 2003). Within the tumor microenvironment, certain inflammatory mediators, such as cytokines (IL-1β) and chemokines (IL-8) often play a fundamental role in regulating tumor subpopulation expression, and breast cancer invasion. For examples, IL-1β has been shown to up-regulate aromatase expression and subsequent local production of estrogen in the breast tissues leading to breast cancer progression. Overexpression of IL-1β is also associated with 90% of estrogen receptor-negative invasive breast carcinomas, and that it is localized to both tumor cells and stromal cells (Pantschenko et al., 2003; Snoussi et al., 2005; Nicolini et al., 2006; Frasor et al., 2008). Expression of chemokines such as IL-8 has been detected in human breast cancer tissues. The increased expression of IL-8 may be involved in the development and progression of breast cancer (Green et al., 1997; Miller et al., 1998; De Larco et al., 2001). Our new data indicate that LMW-HA (but not HMW-HA) preferentially stimulates CD44 association with TLR2/TLR4 and MyD88 (Figure 3) leading to nuclear translocation of both MyD88 and NF-κB-p65 (Figs. 4 and 7) in MDA-MB-231 cells. These events result in an activation of NF-κB-specific transcriptional activation (Figure 5) and the expression of both IL-1β (pro-inflammatory cytokine) and IL-8 (pro-inflammatory chemokine) genes and proteins in breast tumor cells (Table 3). The fact that treatment of MDA-MB -231 cells with anti-CD44 antibody and MyD88siRNA (but not scrambled sequence siRNA) largely inhibits LMW-HA-mediated NF-κB transcriptional activity (Figure 5) and the production of both IL-1β and IL-8 genes/proteins (Table 3) suggests that LMW-HA stimulates CD44-TLRs signaling “cross-talk” and cytokine/chemokine production in a CD44-specific and MyD88-dependent manner.
Both oncogenic signaling and cytoskeleton functions are directly involved in breast tumor cell-specific behaviors (Bourguignon, 2001, 2008). As part of our continuing effort to identify CD44-linked cytoskeleton components that correlate with certain metastatic behaviors, a new candidate molecule, named AFAP-110 has been identified. The AFAP-110 has been shown to be involved in stress fiber formation and cell adhesion in breast tumor cells (Dorfleutner et al., 2007). Using an AFAP-110-specific antibody, we have confirmed the presence of AFAP-110 in human breast cells (MDA-MB-231 cells) (Figure 1). In addition, we have observed that AFAP-110 and the cell surface molecule, CD44 are closely associated as a complex in human breast tumor cells following HA stimulation (Figure 2). We have also demonstrated that the LMW-HA-induced CD44-AFAP-110 complex binds to F-actin (Table 1). These findings are consistent with previous reports showing that AFAP-110 plays an important role in controlling the dynamics of actin assembly. The physiological significance of the F-actin binding properties of purified CD44-AFAP-110 complex is further suggested by the observation that the downregulation of AFAP-110 by treating cells with AFAP-110 siRNA results in the disappearance of the CD44-AFAP-110 complex (Figure 7), the reduction of F-actin binding to the CD44-AFAP-110 signaling complex (Table 1B), and the inhibition of HA/CD44-mediated breast tumor cell invasion (Table 2B).
Our data also show that CD44-AFAP pathway influences actin-cytoskeleton organization (Figure 6B). The fact that disruption of actin polymerization through cytoskeleton D treatment (Figure 6c) or through AFAP knockdown (Fig. 6C) greatly reduces the ability of CD44-AFAP-induced MyD88/NFκB signaling events [e.g., MyD88/NFκB nuclear translocation (Figure 4, IX) and invasion (Table 2)] strongly suggests the importance of the CD44-AFAP pathway in breast tumor cell activation. These observations further support the likelihood that CD44-AFAP-110 complex formation is one of the critical steps in regulating F-actin binding required for LMW-HA/CD44-mediated cytoskeleton function and breast tumor cell invasion.
Emerging evidence regarding the close connection between cytoskeleton activation and oncogenic signaling in multiple cell systems lead us to also investigate the possible role of AFAP-110-cytoskeleton in regulating MyD88-NF-κB signaling pathways during LMW-HA-mediated CD44-TLR activation. Our results indicate that downregulation of AFAP-110 (by treating breast tumor cells with AFAP-110 siRNA) inhibits LMW-HA stimulation of MyD88 and NF-κB nuclear translocation, transcriptional activation and cytokine/chemokine production (Figs. 4, 5 & 8; Table 3C). Thus, these results provide strong evidence that LMW-HA-induced AFAP-110-F-actin binding not only regulates tumor cell invasion, but also plays an important role in regulating nuclear translocation of both MyD88 and NF-κB leading to transcriptional upregulation and target gene (IL-1β and IL-8) expression as well as cytokine/chemokine production. These observations strongly suggest that AFAP-110 participates in both cytoskeleton activation and CD44-TLR signaling interaction processes. This LMW-HA/CD44-TLR-mediated interplay between breast tumor cell invasion and inflammatory cytokine/chemokine production may be a crucial step leading to the malignant and metastasis (also possible recurrence processes) in breast cancer.
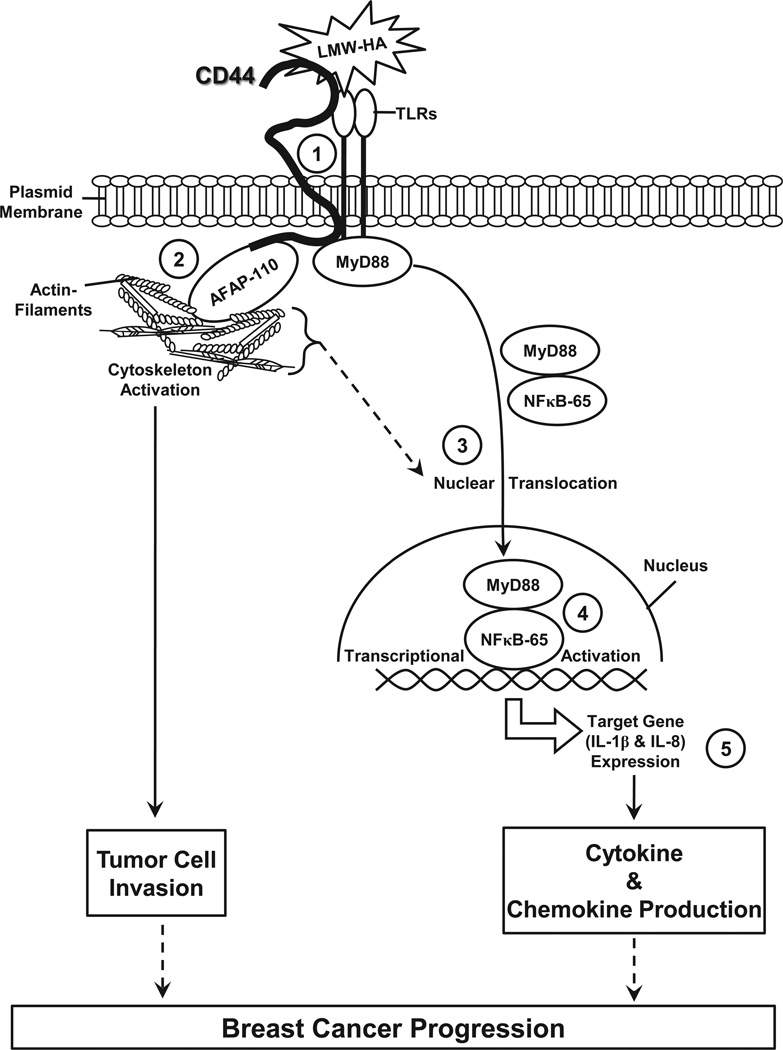
Presently, the binding interactions between CD44 and LMW-HA (or HMW-HA) are not fully understood. It has been suggested that the LMW-HA serves as a monovalent ligand with a low affinity binding to CD44, whereas the HMW-HA acts as a multivalent ligand exhibiting a high affinity binding and the ability to cross-link several CD44 molecules (Lesley et al., 2000). If a large amount of LMW-HA is accumulated in the matrix environment, LMW-HA may successfully compete for HMW-HA binding to CD44 leading to the onset of a unique CD44 signaling pathway and subsequent biological activities. As summarized in Figure 9, we propose that binding of LMW-HA to MDA-MB-231 cells induces CD44 association with AFAP-110, TLR2/TLR4 and MyD88 in a multi-molecular complex (step 1), which then promotes AFAP-110-F-actin binding and cytoskeleton activation (step 2), resulting in tumor cell invasion. At the same time, MyD88 and NF-κB, due to LMW-HA/CD44-TLR-mediated activation (together with cytoskeleton function), promotes both MyD-88 and NF-κB nuclear translocation (step 3) and NF-κB-specific transcriptional activation (step 4). NF-κB is known to be involved in transcription of specific target genes such as pro-inflammatory cytokines (IL-1β) chemokines (IL-8) (step 5). Consequently, the production of these pro-inflammatory cytokines and chemokines cause inflammatory responses, critical components in the tumorigenesis pathway. Taken together, the results presented here clearly indicate that LMW-HA-CD44 interaction with AFAP-110 and TLRs plays a pivotal role in stimulating F-actin-mediated cytoskeleton function and MyD88-NF-κB signaling leading to the concomitant stimulation of cell invasion and pro-inflammatory cytokine/chemokine production in breast tumor cells. These unique LMW-HA/CD44 signaling events may be instrumental in fostering an environment supportive of tumor invasion and progression.
Figure 9.
A proposed model for the LMW-HA-induced CD44 interaction with TLRs and MyD88/NF-κB signaling in breast tumor cells: The interaction between LMW-HA and CD44 induces a multi-molecular complex containing CD44, AFAP-110, TLR2/TLR4 and MyD88 (step 1) which then promotes AFAP-110-F-actin binding and cytoskeleton activation (step 2) resulting in tumor cell invasion. At the same time, MyD88 and NF-κB, due to LMW-HA/CD44-TLR-mediated activation (together with cytoskeleton function), promotes both MyD-88 and NF-κB nuclear translocation (step 3) and NF-κB-specific transcriptional activation (step 4). NF-κB is known to be involved in transcription of specific target genes such as IL-1β (cytokine) and IL-8 (chemokine) (step 5). Consequently, the production of pro-inflammatory cytokines (IL-1β) and chemokines (IL-8) causes inflammatory responses, critical components in the tumorigenesis pathway. Taken together, these results clearly indicate that LMW-HA-CD44 interaction with AFAP-110 and TLRs plays a pivotal role in stimulating F-actin-mediated cytoskeleton function and MyD88-NF-κB signaling leading to the concomitant stimulation of cell invasion and pro-inflammatory cytokine/chemokine production in breast tumor cells.
ACKNOWLEDGEMENTS
We gratefully acknowledge the assistance of Drs. Gerard J. Bourguignon and Walter M. Holleran in the preparation and review of this manuscript. We are grateful for Ms. Christina Camacho for her assistance in preparing graphs and illustrations. We would also like to thank Dr. Eli Gilad for his help in preparing LMW-HA and HMW-HA reagents. This work was supported by Veterans Affairs (VA) Merit Review grant and United States Public Health grants (R01 CA66163, R01 CA78633 and P01 AR39448). L.Y.W.B is a VA Senior Research Career Scientist.
Abbreviations Used
- HA
Hyaluronan
- LMW-HA
Low Molecular Weight Hyaluronan
- HMW-HA
High Molecular Weight Hyaluronan
- TLRs
Toll-Like Receptors
- AFAP-110
Actin Filament-Associated Protein 110)
- MyD88
Myeloid Differentiation Factor 88
- NF-κB
Nuclear Factor-κB
- IL-1β
Interleukine-1β
- IL-8
Interleukine-8
- F-actin
Filamentous actin
REFERENCES
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auvinen P, Tammi R, Tammi M, Johansson R, Kosma VM. Expression of CD44s, CD44v3 and CD 44v6 in benign and malignant breast lesions: correlation and colocalization with hyaluronan. Histopathology. 2005;47:420–428. doi: 10.1111/j.1365-2559.2005.02220.x. [DOI] [PubMed] [Google Scholar]
- Banerji S, Wright AJ, Noble M, Mahoney DJ, Campbell ID, Day AJ, Jackson DG. Structures of the Cd44-hyaluronan complex provide insight into a fundamental carbohydrate-protein interaction. Nat Struct Mol Biol. 2007;14:234–239. doi: 10.1038/nsmb1201. [DOI] [PubMed] [Google Scholar]
- Bauer S, Muller T, Hamm S. Pattern recognition by Toll-like receptors. Adv Exp Med Biol. 2009;653:15–34. doi: 10.1007/978-1-4419-0901-5_2. [DOI] [PubMed] [Google Scholar]
- Ben-Baruch A. Host microenvironment in breast cancer-development: inflammatory cells, cytokines and chemokines in breast cancer progression: reciprocal tumor-microenvironment interactions. Breast Cancer Res. 2003;5:31–36. doi: 10.1186/bcr554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon LY. CD44-mediated oncogenic signaling and cytoskeleton activation during mammary tumor progression. J Mammary Gland Biol Neoplasia. 2001;6:287–297. doi: 10.1023/a:1011371523994. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY. Hyaluronan-mediated CD44 activation of RhoGTPase signaling and cytoskeleton function promotes tumor progression. Semin Cancer Biol. 2008;18:251–259. doi: 10.1016/j.semcancer.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon LY. Hyaluronan-Mediated CD44 Interaction with Receptor and Non-Receptor Kinases Promotes Oncogenic Signaling, Cytoskeleton Activation and Tumor Progression. In: Stern R, editor. Hyaluronan in Cancer Biology. Elsevier Publication Co.; 2009. pp. 89–107. [Google Scholar]
- Bourguignon LY, Gilad E, Brightman A, Diedrich F, Singleton P. Hyaluronan-CD44 interaction with leukemia-associated RhoGEF and epidermal growth factor receptor promotes Rho/Ras co-activation, phospholipase C epsilon-Ca2+ signaling, and cytoskeleton modification in head and neck squamous cell carcinoma cells. J Biol Chem. 2006;281:14026–14040. doi: 10.1074/jbc.M507734200. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Gilad E, Rothman K, Peyrollier K. Hyaluronan-CD44 interaction with IQGAP1 promotes Cdc42 and ERK signaling, leading to actin binding, Elk-1/estrogen receptor transcriptional activation, and ovarian cancer progression. J Biol Chem. 2005;280:11961–11972. doi: 10.1074/jbc.M411985200. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Peyrollier K, Gilad E, Brightman A. Hyaluronan-CD44 interaction with neural Wiskott-Aldrich syndrome protein (N-WASP) promotes actin polymerization and ErbB2 activation leading to beta-catenin nuclear translocation, transcriptional up-regulation, and cell migration in ovarian tumor cells. J Biol Chem. 2007;282:1265–1280. doi: 10.1074/jbc.M604672200. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Peyrollier K, Xia W, Gilad E. Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J Biol Chem. 2008;283:17635–17651. doi: 10.1074/jbc.M800109200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon LY, Singleton PA, Diedrich F. Hyaluronan-CD44 interaction with Rac1-dependent protein kinase N-gamma promotes phospholipase Cgamma1 activation, Ca(2+) signaling, and cortactin-cytoskeleton function leading to keratinocyte adhesion and differentiation. J Biol Chem. 2004a;279:29654–29669. doi: 10.1074/jbc.M403608200. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Singleton PA, Diedrich F, Stern R, Gilad E. CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J Biol Chem. 2004b;279:26991–27007. doi: 10.1074/jbc.M311838200. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Singleton PA, Zhu H, Diedrich F. Hyaluronan-mediated CD44 interaction with RhoGEF and Rho kinase promotes Grb2-associated binder-1 phosphorylation and phosphatidylinositol 3-kinase signaling leading to cytokine (macrophage-colony stimulating factor) production and breast tumor progression. J Biol Chem. 2003;278:29420–29434. doi: 10.1074/jbc.M301885200. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Singleton PA, Zhu H, Zhou B. Hyaluronan promotes signaling interaction between CD44 and the transforming growth factor beta receptor I in metastatic breast tumor cells. J Biol Chem. 2002;277:39703–39712. doi: 10.1074/jbc.M204320200. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Spevak CC, Wong G, Xia W, Gilad E. Hyaluronan-CD44 interaction with protein kinase C(epsilon) promotes oncogenic signaling by the stem cell marker Nanog and the Production of microRNA-21, leading to down-regulation of the tumor suppressor protein PDCD4, anti-apoptosis, and chemotherapy resistance in breast tumor cells. J Biol Chem. 2009a;284:26533–26546. doi: 10.1074/jbc.M109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon LY, Xia W, Wong G. Hyaluronan-mediated CD44 interaction with p300 and SIRT1 regulates beta-catenin signaling and NFkappaB-specific transcription activity leading to MDR1 and Bcl-xL gene expression and chemoresistance in breast tumor cells. J Biol Chem. 2009b;284:2657–2671. doi: 10.1074/jbc.M806708200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon LY, Zhu H, Chu A, Iida N, Zhang L, Hung MC. Interaction between the adhesion receptor, CD44, and the oncogene product, p185HER2, promotes human ovarian tumor cell activation. J Biol Chem. 1997;272:27913–27918. doi: 10.1074/jbc.272.44.27913. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Zhu H, Shao L, Chen YW. CD44 interaction with c-Src kinase promotes cortactin-mediated cytoskeleton function and hyaluronic acid-dependent ovarian tumor cell migration. J Biol Chem. 2001a;276:7327–7336. doi: 10.1074/jbc.M006498200. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Zhu H, Zhou B, Diedrich F, Singleton PA, Hung MC. Hyaluronan promotes CD44v3-Vav2 interaction with Grb2-p185(HER2) and induces Rac1 and Ras signaling during ovarian tumor cell migration and growth. J Biol Chem. 2001b;276:48679–48692. doi: 10.1074/jbc.M106759200. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Zhu H, Shao L, Zhu D, Chen YW. Rho-kinase (ROK) promotes CD44v(3,8–10)-ankyrin interaction and tumor cell migration in metastatic breast cancer cells. Cell Motil Cytoskeleton. 1999;43:269–287. doi: 10.1002/(SICI)1097-0169(1999)43:4<269::AID-CM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- Calogero RA, Cordero F, Forni G, Cavallo F. Inflammation and breast cancer. Inflammatory component of mammary carcinogenesis in ErbB2 transgenic mice. Breast Cancer Res. 2007;9:211. doi: 10.1186/bcr1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuff CA, Kothapalli D, Azonobi I, Chun S, Zhang Y, Belkin R, Yeh C, Secreto A, Assoian RK, Rader DJ. The adhesion receptor CD44 promotes atherosclosis by mediating inflammatory cell recruitment and vascular cell activation. J. Clin Invest. 2001;108:1031–1040. doi: 10.1172/JCI12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Larco JE, Wuertz BR, Rosner KA, E GD, Manivel JC, Furcht LT. A potential role for interleukin-8 in the metastatic phenotype of breast carcinoma cells. Am J of Pathol. 2001;158:639–646. doi: 10.1016/S0002-9440(10)64005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpech B, Chevallier B, Reinhardt N, Julien JP, Duval C, Maingonnat C, Bastit P, Asselain B. Serum hyaluronan (hyaluronic acid) in breast cancer patients. Int J Cancer. 1990;46:388–390. doi: 10.1002/ijc.2910460309. [DOI] [PubMed] [Google Scholar]
- Dorfleutner A, Stehlik C, Zhang J, Gallick GE, Flynn DC. AFAP-110 is required for actin stress fiber formation and cell adhesion in MDA-MB-231 breast cancer cells. J Cell Physiol. 2007;213:740–749. doi: 10.1002/jcp.21143. [DOI] [PubMed] [Google Scholar]
- Flynn DC, Leu TH, Reynolds AB, Parsons JT. Identification and sequence analysis of cDNAs encoding a 110-kilodalton actin filament-associated pp60src substrate. Mol Cell Biol. 1993;13:7892–7900. doi: 10.1128/mcb.13.12.7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasor J, Weaver AE, Pradhan M, Mehta K. Synergistic up-regulation of prostaglandin E synthase expression in breast cancer cells by 17beta-estradiol and proinflammatory cytokines. Endocrinology. 2008;149:6272–6279. doi: 10.1210/en.2008-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR, Green VL, White MC, Speirs V. Expression of cytokine messenger RNA in normal and neoplastic human breast tissue: identification of interleukin-8 as a potential regulatory factor in breast tumours. Int J Cancer. 1997;72:937–941. doi: 10.1002/(sici)1097-0215(19970917)72:6<937::aid-ijc3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Guappone AC, Flynn DC. The integrity of the SH3 binding motif of AFAP-110 is required to facilitate tyrosine phosphorylation by, and stable complex formation with, Src. Mol Cell Biochem. 1997;175:243–252. doi: 10.1023/a:1006840104666. [DOI] [PubMed] [Google Scholar]
- Guappone AC, Weimer T, Flynn DC. Formation of a stable src-AFAP-110 complex through eithee an amino-terminal or a carboxy-terminal SH2-binding motif. Mol Carcinog. 1998;22:110–119. doi: 10.1002/(sici)1098-2744(199806)22:2<110::aid-mc6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Haylock DN, Nilsson SK. The role of hyaluronic acid in hemopoietic stem cell biology. Regen Med. 2006;1:437–445. doi: 10.2217/17460751.1.4.437. [DOI] [PubMed] [Google Scholar]
- Iida N, Bourguignon LY. New CD44 splice variants associated with human breast cancers. J Cell Physiol. 1995;162:127–133. doi: 10.1002/jcp.1041620115. [DOI] [PubMed] [Google Scholar]
- Iida N, Bourguignon LY. Coexpression of CD44 variant (v10/ex14) and CD44S in human mammary epithelial cells promotes tumorigenesis. J Cell Physiol. 1997;171:152–160. doi: 10.1002/(SICI)1097-4652(199705)171:2<152::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Itano N, Kimata K. Expression cloning and molecular characterization of HAS protein, a eukaryotic hyaluronan synthase. J Biol Chem. 1996;271:9875–9878. doi: 10.1074/jbc.271.17.9875. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, Visintin I, Rutherford T, Mor G. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006;66:3859–3868. doi: 10.1158/0008-5472.CAN-05-3948. [DOI] [PubMed] [Google Scholar]
- Kothapalli D, Zhao L, Hawthorne EA, Cheng Y, Lee E, Pure E, Assoian RK. Hyaluronan and CD44 antagonize mitogen-dependent cyclin D1 expression in mesenchymal cells. J. Cell Biol. 2007;176:535–544. doi: 10.1083/jcb.200611058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothapalli D, Floers J, Xu T, Pure E, Assoian RK. Differential activation of ERK and Rac mediates the proliferative and anti-proliferative effects of hyaluronan and Cd44. J. Biol Chem. 2008;283:31823–31829. doi: 10.1074/jbc.M802934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J, Hascall VC, Tammi M, Hyman R. Hyaluronan binding by cell surface CD44. J Biol Chem. 2000;275:26967–26975. doi: 10.1074/jbc.M002527200. [DOI] [PubMed] [Google Scholar]
- Lokeshwar VB, Iida N, Bourguignon LY. The cell adhesion molecule, GP116, is a new CD44 variant (ex14/v10) involved in hyaluronic acid binding and endothelial cell proliferation. J Biol Chem. 1996;271:23853–23864. doi: 10.1074/jbc.271.39.23853. [DOI] [PubMed] [Google Scholar]
- Matou-Nasri S, Gaffney J, Kumar S, Slevin M. Oligosaccharides of hyaluronan induce angiogenesis through distinct CD44 and RHAMM-mediated signalling pathways involving Cdc2 and gamma-adducin. Int J Oncol. 2009;35:761–773. doi: 10.3892/ijo_00000389. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Miller LJ, Kurtzman SH, Wang Y, Anderson KH, Lindquist RR, Kreutzer DL. Expression of interleukin-8 receptors on tumor cells and vascular endothelial cells in human breast cancer tissue. Anticancer Res. 1998;18:77–81. [PubMed] [Google Scholar]
- Molteni M, Marabella D, Orlandi C, Rossetti C. Melanoma cell lines are responsive in vitro to lipopolysaccharide and express TLR-4. Cancer Lett. 2006;235:75–83. doi: 10.1016/j.canlet.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Nicolini A, Carpi A, Rossi G. Cytokines in breast cancer. Cytokine Growth Factor Rev. 2006;17:325–337. doi: 10.1016/j.cytogfr.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Noble PW. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002;21:25–29. doi: 10.1016/s0945-053x(01)00184-6. [DOI] [PubMed] [Google Scholar]
- Pantschenko AG, Pushkar I, Anderson KH, Wang Y, Miller LJ, Kurtzman SH, Barrows G, Kreutzer DL. The interleukin-1 family of cytokines and receptors in human breast cancer: implications for tumor progression. Int J Oncol. 2003;23:269–284. [PubMed] [Google Scholar]
- Premkumar VG, Yuvaraj S, Vijayasarathy K, Gangadaran SG, Sachdanandam P. Serum cytokine levels of interleukin-1beta,-6,-8, tumour necrosis factor-alpha and vascular endothelial growth factor in breast cancer patients treated with tamoxifen and supplemented with co-enzyme Q(10), riboflavin and niacin. Basic Clin Pharmacol Toxicol. 2007;100:387–391. doi: 10.1111/j.1742-7843.2007.00065.x. [DOI] [PubMed] [Google Scholar]
- Qian Y, Baisden JM, Westin EH, Guappone AC, Koay TC, Flynn DC. Src can regulate carboxy terminal interactions with AFAP-110, which influence self-association, cell localization and actin filament integrity. Oncogene. 1998;16:2185–2195. doi: 10.1038/sj.onc.1201753. [DOI] [PubMed] [Google Scholar]
- Qian Y, Baisden JM, Zot HG, Van Winkle WB, Flynn DC. The carboxy terminus of AFAP-110 modulates direct interactions with actin filaments and regulates its ability to alter actin filament integrity and induce lamellipodia formation. Exp Cell Res. 2000;255:102–113. doi: 10.1006/excr.1999.4795. [DOI] [PubMed] [Google Scholar]
- Qian Y, Gatesman AS, Baisden JM, Zot HG, Cherezova L, Qazi I, Mazloum N, Lee MY, Guappone-Koay A, Flynn DC. Analysis of the role of the leucine zipper motif in regulating the ability of AFAP-110 to alter actin filament integrity. J Cell Biochem. 2004;91:602–620. doi: 10.1002/jcb.10725. [DOI] [PubMed] [Google Scholar]
- Radisky ES, Radisky DC. Stromal induction of breast cancer: inflammation and invasion. Rev Endocr Metab Disord. 2007;8:279–287. doi: 10.1007/s11154-007-9037-1. [DOI] [PubMed] [Google Scholar]
- Sales KM, Winslet MC, Seifalian AM. Stem cells and cancer: an overview. Stem Cell Rev. 2007;3:249–255. doi: 10.1007/s12015-007-9002-0. [DOI] [PubMed] [Google Scholar]
- Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177:1272–1281. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- Schmitz F, Mages J, Heit A, Lang R, Wagner H. Transcriptional activation induced in macrophages by Toll-like receptor (TLR) ligands: from expression profiling to a model of TLR signaling. EuR J Immunol. 2004;34:2863–2873. doi: 10.1002/eji.200425228. [DOI] [PubMed] [Google Scholar]
- Singleton PA, Bourguignon LY. CD44v10 interaction with Rho-kinase (ROK) activates inositol 1,4,5-triphosphate (IP3) receptor-mediated Ca2+ signaling during hyaluronan (HA)-induced endothelial cell migration. Cell Motil Cytoskeleton. 2002;53:293–316. doi: 10.1002/cm.10078. [DOI] [PubMed] [Google Scholar]
- Singleton PA, Bourguignon LY. CD44 interaction with ankyrin and IP3 receptor in lipid rafts promotes hyaluronan-mediated Ca2+ signaling leading to nitric oxide production and endothelial cell adhesion and proliferation. Exp Cell Res. 2004;295:102–118. doi: 10.1016/j.yexcr.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Slomiany MG, Dai L, Bomar PA, Knackstedt TJ, Kranc DA, Tolliver L, Maria BL, Toole BP. Abrogating drug resistance in malignant peripheral nerve sheath tumors by disrupting hyaluronan-CD44 interactions with small hyaluronan oligosaccharides. Cancer Res. 2009a;69:4992–4998. doi: 10.1158/0008-5472.CAN-09-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomiany MG, Dai L, Tolliver LB, Grass GD, Zeng Y, Toole BP. Inhibition of Functional Hyaluronan-CD44 Interactions in CD133-positive Primary Human Ovarian Carcinoma Cells by Small Hyaluronan Oligosaccharides. Clin Cancer Res. 2009b;15:7593–7601. doi: 10.1158/1078-0432.CCR-09-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HS, Stern R, Liu E, Benz C. Early and late events in the development of human breast cancer. Basic Life Sci. 1991;57:329–337. doi: 10.1007/978-1-4684-5994-4_27. [DOI] [PubMed] [Google Scholar]
- Snoussi K, Strosberg AD, Bouaouina N, Ben Ahmed S, Chouchane L. Genetic variation in pro-inflammatory cytokines (interleukin-1beta, interleukin-1alpha and interleukin-6) associated with the aggressive forms, survival, and relapse prediction of breast carcinoma. Eur Cytokine Netw. 2005;16:253–260. [PubMed] [Google Scholar]
- Snyder BN, Cho Y, Qian Y, Coad JE, Flynn DC, Cunnick JM. FAP1L1 is a novel adaptor protein of the AFAP family that interacts with cortactin and localizes to invadosomes. EuRJ Cell Biol. 2011;90:376–389. doi: 10.1016/j.ejcb.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer AP, Nguyen TK. Mammalian hyaluronan synthases: investigation of functional relationships in vivo. Biochem Soc Trans. 1999;27:109–115. doi: 10.1042/bst0270109. [DOI] [PubMed] [Google Scholar]
- Stern R, Jedrzejas MJ. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem Rev. 2006;106:818–839. doi: 10.1021/cr050247k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Toole BP, Slomiany MG. Hyaluronan: a constitutive regulator of chemoresistance and malignancy in cancer cells. Semin Cancer Biol. 2008;18:244–250. doi: 10.1016/j.semcancer.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole BP, Wight TN, Tammi MI. Hyaluronan-cell interactions in cancer and vascular disease. J Biol Chem. 2002;277:4593–4596. doi: 10.1074/jbc.R100039200. [DOI] [PubMed] [Google Scholar]
- Toubi E, Shoenfeld Y. Toll-like receptors and their role in the development of autoimmune diseases. Autoimmunity. 2004;37:183–188. doi: 10.1080/08916930410001704944. [DOI] [PubMed] [Google Scholar]
- Tsuji S, Matsumoto M, Takeuchi O, Akira S, Azuma I, Hayashi A, Toyoshima K, Seya T. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guerin: involvement of toll-like receptors. Infect Immun. 2000;68:6883–6890. doi: 10.1128/iai.68.12.6883-6890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277:4589–4592. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- Uehori J, Matsumoto M, Tsuji S, Akazawa T, Takeuchi O, Akira S, Kawata T, Azuma I, Toyoshima K, Seya T. Simultaneous blocking of human Toll-like receptors 2 and 4 suppresses myeloid dendritic cell activation induced by Mycobacterium bovis bacillus Calmette-Guerin peptidoglycan. Infect Immun. 2003;71:4238–4249. doi: 10.1128/IAI.71.8.4238-4249.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SJ, Bourguignon LY. Hyaluronan-CD44 promotes phospholipase C–mediated Ca2 signaling and cisplatin resistance in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2006a;132:19–24. doi: 10.1001/archotol.132.1.19. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Bourguignon LY. Hyaluronan and the interaction between CD44 and epidermal growth factor receptor in oncogenic signaling and chemotherapy resistance in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2006b;132:771–778. doi: 10.1001/archotol.132.7.771. [DOI] [PubMed] [Google Scholar]
- Weigel PH, Hascall VC, Tammi M. Hyaluronan synthases. J Biol Chem. 1997;272:13997–14000. doi: 10.1074/jbc.272.22.13997. [DOI] [PubMed] [Google Scholar]
- Woodward WA, Buchholz TA. The role of locoregional therapy in inflammatory breast cancer. Semin Oncol. 2008;35:78–86. doi: 10.1053/j.seminoncol.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Xie W, Wang Y, Huang Y, Yang H, Wang J, Hu Z. Toll-like receptor 2 mediates invasion via activating NF-kappaB in MDA-MB-231 breast cancer cells. Biochem Biophys Res Commun. 2009;379:1027–1032. doi: 10.1016/j.bbrc.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Cristofanilli M, Nakamura S, Hortobagyi GN, Ueno NT. Molecular targets for treatment of inflammatory breast cancer. Nat Rev Clin Oncol. 2009;6:387–394. doi: 10.1038/nrclinonc.2009.73. [DOI] [PubMed] [Google Scholar]