Abstract
Ceruloplasmin (Cp) is a ferroxidase involved in iron metabolism by converting Fe2+ to Fe3+, and by regulating cellular iron efflux. In the ceruloplasmin knockout (CpKO) mouse, the deregulation of iron metabolism results in moderate liver and spleen hemosiderosis, but the impact of Cp deficiency on brain neurochemistry and behavior in this animal model is unknown. We found that in contrast to peripheral tissues, iron levels in the hippocampus are significantly reduced in CpKO mice. Although it does not cause any discernable deficits in motor function or learning and memory, Cp deficiency results in heightened anxiety-like behavior in the open field and elevated plus maze tests. This anxiety phenotype is associated with elevated levels of plasma corticosterone. Previous studies provided evidence that anxiety disorders and long-standing stress are associated with reductions in levels of serotonin and brain-derived neurotrophic factor (BDNF) in the hippocampus. We found that levels of serotonin and norepinephrine, and the expression of BDNF and its receptor trkB, are significantly reduced in the hippocampus of CpKO mice. Thus, Cp deficiency causes an anxiety phenotype by a mechanism that involves decreased levels of iron, serotonin, norepinephrine and BDNF in the hippocampus.
Introduction
The redox properties of iron and its incorporation into several proteins make it an essential nutrient required for many cellular functions (Valerio, 2007; Zhang and Enns, 2009). However, whereas Fe3+ is relatively innocuous, Fe2+ readily interacts with hydrogen peroxide to generate the highly destructive hydroxyl radical (Mattson, 2004). In order to promote the beneficial actions of iron while decreasing potential toxic effects, iron is tightly regulated by several proteins (Chua, et.al, 2007). Ceruloplasmin (Cp), an essential ferroxidase, converts ferrous iron (Fe2+) to ferric iron (Fe3+) and may also regulate cellular iron efflux (Hellman and Gitlin, 2002). Cp knock-out (KO) mice exhibit systemic iron overload in organs such as liver and spleen similar to that seen in the rare human disease, aceruloplasminemia in which iron also accumulates in the brain and neurodegeneration occurs in some brain regions in mid-life (Harris et.al, 1998). However, it is not known if iron dysregulation occurs in the brain of Cp-deficient mice and whether there are functional consequences of the perturbed iron metabolism.
Both iron deficiency (Parks and Wharton, 1989) and iron overload (Rao and Georgieff, 2007) can lead to altered behavior including deficits in learning and memory. The hippocampus plays a major role in mediating learning and memory, and affective behaviors such as anxiety and depression. (Neumeister et al., 2005; Engin and Treit, 2007). The hippocampus also has a high metabolic demand and a significant reliance on iron (Carlson et al., 2009). Serotonin and norepinephrine are two neurotransmitters that modulate affect; both are synthesized and metabolized by enzymes that require iron for their activity (Youdim and Green, 1978), suggesting that changes in brain iron levels might influence behaviors controlled by monoaminergic neurotransmitters. Aged CpKO mice have been a model for brain iron dsyregulation (Patel et al., 2002), but the possibility that perturbed brain iron metabolism may result in neurochemical and behavioral alterations in young mice has not been examined.
Brain-derived neurotrophic factor (BDNF) is produced by neurons and plays important roles in cognition and mood (for review see Mattson et al., 2004; Deltheil et al., 2008; Tapia-Arancibia et al., 2008). BDNF signaling enhances synaptic plasticity and stimulates neurogenesis (Pardon, 2010). Lower levels of BDNF are associated with depression and anxiety in humans, and in animal models of these psychiatric disorders (Hashimoto, 2010; Duman et al., 2008). Moreover, both serotonin (Martinowich and Lu, 2008) and norepinephrine (Chen, et. al, 2007) induce BDNF expression. On the other hand, elevated levels of glucocorticoids that occur during chronic stress and in anxiety disorders suppress the expression of BDNF and impair hippocampal synaptic plasticity and learning and memory (Schaaf et al., 2000; Murray et al., 2008; Stranahan et al., 2008; Sterner and Kalynchuk, 2010). In the present study we show that Cp deficiency in mice results in an anxiety phenotype in young adult mice without discernable effects on learning and memory or motor performance. The anxiety phenotype resulting from Cp deficiency involves reductions in levels of iron, serotonin and norepinephrine, and diminished BDNF expression, in the hippocampus.
Materials and Methods
Mice
Ceruloplasmin knock-out (CpKO) mice were generated as described previously (Harris, 1999); breeding pairs were a gift from Z.L. Harris. Wild type(WT) and CpKO, background C57BL/6 lines were maintained according to National Institutes of Health guidelines. All animal procedures were approved by the Animal Care and Use Committee of the National Institute on Aging Intramural Research Program. All mice were male and three months of age when behavioral testing was initiated.
Experimental Design
Twelve WT and 12 CpKO three month-old male mice were each evaluated in a series of behavioral tests performed in the following order: rotorod, open field, novel object recognition, water maze, elevated plus maze and fear conditioning. Approximately 24 hours of rest between behavioral tests was provided, except 48 hours elapsed between the end of water maze testing and beginning of elevated maze testing.
Separate groups of 12 WT and 12 CpKO mice were tested in the tail suspension test and 24 hours later in the forced swim test. On the day before behavioral testing, blood was taken from these animals for corticosterone measurements.
Hippocampi from a group of mice that had not been behaviorally tested (6 WT and 6 CpKO) were used for HPLC analysis and atomic absorption measures. Brains tissue from an additional 10 WT and 10 CpKO mice that had not been tested were analyzed by atomic absorption to gain statistical significance.
RNA transcripts from the hippocampi of a separate cohort of 5 mice for each group (WT and CpKO) were analyzed (24 hours prior to hippocampal harvest, blood was taken from these animals for corticosterone measurements and those data were combined with data from blood samples from 12 other mice per group. Thus, the final group numbers for corticosterone measurements were 17 WT mice and 17 CpKO mice.
Data Analysis
Graphs represent group means with error bars representing the standard error of the means. One-way repeated ANOVA was used for fear conditioning measures of the training period and contextual memory. All other statistical comparisons were made using student t-test assuming equal variance in which p-values and critical ratios were calculated from a two-tail analysis.
Open Field Test
Open field testing was performed using the MEDOFA-MS system (Med Associates). Mice (n= 12/group) were placed in the center of an open field, and exploration was assessed for 15 min. Cages were cleaned with ethanol immediately after testing each mouse. The dimensions of the arena were 40 cm × 40 cm, of which the outer 10 cm were considered the peripheral zone and the inner 30 cm were considered the central zone.
Rotarod Test
Rotarod testing was performed using the ENV-577M system (Med Associates). Rotarod acceleration was set to 2–20 rpm. Mice (n= 12/group) were placed on the Rotarod for three trials of 5 min each with a 15-min rest between trials. The apparatus was routinely cleaned with water and ethanol following each session. The number of falls (maximum of 10 falls) within a 5 min time period, time of the first fall, and the length of time spent on the rod were recorded. After 10 falls the test was terminated and the mouse was returned to its home cage.
Morris Water Maze
To test reference memory, mice (n= 12/group) were trained in the water maze as described previously (Stranahan et al., 2008) for 9 days, with four trials per day. The visual cues were black and white only to reduce the possible effects of color discrimination capabilities between strains. The platform was hidden 0.5 cm below the surface of the water, made opaque with white nontoxic paint, at a temperature of 27 °C ± 0.5 °C. The platform location was constant, and the starting points were changed every trial to avoid track memorizing. When trials ended, either when the mouse had found the platform or when 60 s had passed, mice were allowed to rest on the platform for 10 s. To test retention of the task, the platform was removed, and mice were given probe trials at 4 h after the last trial on the 9th day and 24 hours later on the 10th day.
Novel Object Recognition
Testing took place in 25-cm ×25-cm opaque-walled cages. For object familiarization, mice (n= 12/group) were allowed to explore their cage in the presence of two identical objects. Mice were then returned to their home cages for 2 h, followed by a 30-min exposure to one novel object and one familiar object. Mice had no observed baseline preference to the different objects. Object preferences were automatically analyzed using the ANYmaze video tracking software. Mice that spent less than 1 min total time exploring the objects were eliminated from the data analysis. An object preference index was determined by calculating the time spent near the novel object divided by the cumulative time spent with both familiar and novel objects. Cages were routinely cleaned with ethanol following testing/habituating of mice.
Fear Conditioning
In the training session, mice (n= 12/group) were placed in a contextual conditioning chamber (model MEDVFC-NIR-M; Med Associates) and allowed to explore the chamber for 2 min. At the end of 2 min, mice were subjected to three sessions of audio tone (CS, conditioned stimulus) and foot shock (US, unconditioned stimulus). Audio tone (5 kHz, 70 dB) was on for 30 s, followed immediately by a 0.5-mA, 2-s foot shock from the metal grid floor. Thirty seconds separated each session. Foot shock intensity was determined in a preliminary test on a separate cohort of animals for the minimal applicable intensity that elicited a response. On the following day, in the contextual fear session, mice were tested by being returned to the conditioning chamber for 5 min without any shock. The percentage of time freezing was recorded and used as an index of contextual memory. In the cued conditioning test (conducted 3 h following contextual conditioning) mice were tested by being returned to the chamber but in a different context. Mice were allowed to explore the chamber for 5 min without any audio tone. Following this, five audio tones were sounded for 30 s each. The percentage of time freezing until and after the audio tones was recorded and used as an index of cued memory. The test apparatus was cleaned with ethanol after testing each mouse.
Elevated Plus Maze
The apparatus consisted of a plus-shaped maze elevated 60 cm from the floor and comprising two opposite open arms, 25 cm × 5 cm each, crossed by two arms of the same dimensions enclosed by 30-cm-high walls with an open roof. In addition, a 1-cm-high edge made of clear Plexiglas surrounded the open arms to avoid falls. Each animal (n= 12/group) was placed in the middle of the maze facing the open arm. Following 5 min testing, animals were returned to their home cages. Arm preference was automatically analyzed using the ANYmaze video tracking software.
Forced Swim Test
Mice (n= 12/group) were tested for 5 min in a water-filled cylinder (50 cm depth × 20 cm diameter, with water level at a 40 cm height to prevent the mice from reaching the bottom with their tails). Water temperature was 27 °C ± 0.5 °C and was replaced between tests of different mice. Freezing periods of the mice were determined when mice were immobile with all four limbs.
Tail Suspension Test
Mice (n= 12/group) were tested for 6 min during which they were held by their tail. All test sessions were recorded in the dark using an infrared camera under infrared light and were subsequently analyzed. Freezing periods of the mice were defined as the time period during which the mouse hung without attempting to move.
Brain iron analysis
Brain regions (n= 16/group) were wet digested in 0.2% ultra-pure nitric acid using standard procedures and analyzed for iron concentration by atomic absorption spectrometry (Perkin Elmer AAnalyst 600, Perkin Elmer, Norwalk, CT) (Pinero et al., 2000). Standards were prepared by diluting a Perkin Elmer iron standard (PE#N9300126) in 0.2% ultra-pure nitric acid, and blanks were prepared with digesting and diluting reagents to control for possible contamination. All standard curves exceeded r > 0.99.
HPLC Analysis of Monoamines
Brain regions (n= 6/group) obtained from each animal were weighed, ultrasonicated in 0.1M perchloric acid, and stored at –70°C until extraction. The tissues obtained from each animal were homogenized in 0.1 M perchloric acid and centrifuged at 25,000 g for 12 min. Norepinephrine (NE), serotonin (5HT) and 5-hydroxyindol acetic acid (5HIAA) contents in supernatants of the brain structures were measured by HPLC with electrochemical detection. The analytical column was a SunFire C18 5 μm (4.6 × 150.0 mm) from Waters. The mobile phase was 0.01 M sodium dihydrogenphosphate, 0.01 M citric acid, 1.2 mM sodium EDTA, 1.2 mM sodium 1-heptane sulfonic acid, 10% methanol, pH 3.5; the flow rate was 1.0 ml/min and the column temperature was 34°C. The installation consisted of a Waters 717 Plus automated injection system, a Waters 1525 Binary pump, and a ESA Coulochem III detector. Waters Breeze system was used for analysis. Concentrations of NE, 5HT and 5HIAA were calculated as pg/mg of tissue weight
Corticosterone analysis
Thirty microliters of blood was taken via tail bleeds in the morning from naïve 3 month old mice (n= 17/group) that had not undergone behavior testing. The blood was spun at 2,500 g for 10 min and plasma was separated. Plasma was analyzed using a CORT RIA kit (MP Biomedicals, USA).
BDNF ELISA
Single hippocampi (n= 5/group) were homogenized in 300 μl of a modified Ripa buffer. Homogenates were spun at 20,000 g at 4°C. Supernatants were separated and protein concentrations were determined by Bradford assay. Samples were diluted to 2 mg/mL and 50 μl was loaded per well and analyzed using the quantikine BDNF ELISA kit (R&D Systems, USA)
RNA extraction and real-time PCR
RNA from the hippocampus (n= 5/group) was isolated using Trizol (Invitrogen) and purified with an RNA Micro Kit (Qiagen, Valencia, CA). Following treatment with DNase I, RNA was quantified and equal amounts were retro-transcribed using the SuperScript First Strand Synthesis System (Invitrogen Life Technologies). Real-time PCR analysis was performed with a PTC 200 Pelthier Thermo Cycler and Chromo 4 Fluorescent Detector (BioRad, Hercules,CA), and Sybr® Green PCR Master Mix according to the manufacturer's instructions (Applied Biosystems, Foster City, CA). Each reaction included 3 μl of diluted (1:4) cDNA and was performed in triplicate. PCR was performed under the following conditions: 10 min at 95 °C, followed by 40 cycles of 30 s at 95 °C, 30 s at 60 °C and 1 min at 72°C. The comparative Ct method was used to determine the normalized changes of the target gene relative to a calibrator reference. The primers used in this study were as follows: mBDNF1 (NM_007540), 5′- GCT TTG CGG ATA TTG CGA AGG GTT -3′ and 5′- ACC TGG TGG AAC ATT GTG GCT TTG -3′; mBDNF2 (NM_001048139), 5’- TGA AGT TGG CTT CCT AGC GGT GTA -3’ and 5’- TGG TGG AAC TTC TTT GCG GCT TAC -3’; mBDNF3 (NM_001048141) 5’- CCA GAG CAG CTG CCT TGA TGT TTA -3’ and 5’- CCG CCT TCA TGC AAC CGA AGT AT -3’; mBDNF4 (NM_001048142), 5’- TGA CAA CAA TGT GAC TCC ACT GCC -3’ and 5’- ATG GTC ATC ACT CTT CTC ACC TGG -3’; mTrkB long (NM_001025074), 5’- TTC AGC TGC TGT TGC TGC TTC T -3’ and 5’- AAC CGC TAA ACC GGC ACG AAT ATC -3’;mTrkB short (NM_008745), 5’- TCC TGC TCA AGT TGG CGA GAC ATT -3’ and 5’- ATA GGC AAC AGC AGT CCC AGA GTT -3’; mHPRT (NM_013556), 5’- CCT GCT GGA TTA CAT TAA AGC ACT G-3’ and 5’- CCT GAA GTA CTC ATT ATA GTC AAG G-3’.
Results
Ceruloplasmin-deficient mice exhibit an anxiety phenotype, without deficits in motor ability, memory retention or depression
Motor performance of WT and CpKO mice was tested using a Rotarod. No significant differences between the two groups were seen in the total number of falls (Fig. 1A), the time to the first fall, or total time spent on the rod (data not shown).
Figure 1.
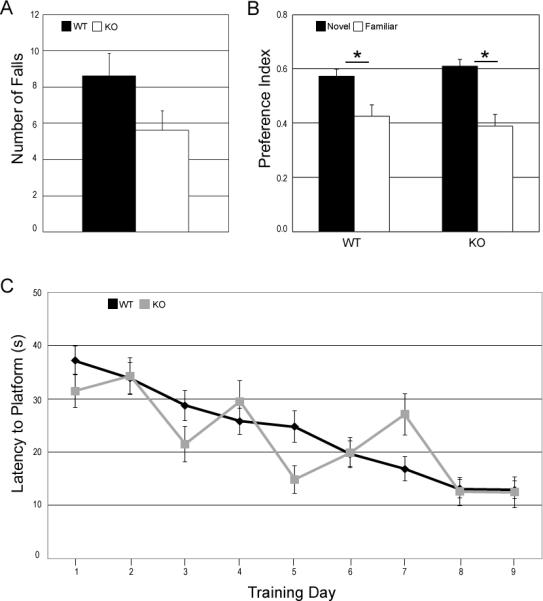
Motor ability and hippocampus-dependent memory are not impaired in ceruloplasmin-deficient mice. A. Results of rotarod testing showing total number of falls (maximum 10 falls) B. Novel object recognition preference index is calculated by dividing the time spent with the specific object (novel or familiar) by the total time spent investigating both objects. Both WT and CpKO mice showed preference for the novel object, *p<0.05 (n=12 mice). C. Morris Water Maze training over 9 days. Average of 4 trials a day. Mice that were passive and did not swim on all three trials were eliminated from final calculations. n's: WT, day1-9) = 11; KO, day1 = 10; KO day 2 = 8; KO, days 3 through 9 = 6.
Recognition memory, a hippocampus-dependent task, was tested in WT and CpKO mice using a novel object recognition test. Both groups of mice had a significant preference for the novel object over the familiar object with no significant differences between the groups (Fig. 1B). Another hippocampus-dependent task, spatial memory was tested in WT and CpKO mice in the Morris water maze. Over a period of nine training days both groups of mice decreased their latency to find the hidden platform. The CpKO performance was more variable from day to day and not significantly different from WT (Fig.1C). The variability seen in the CpKO group is possibly due to the increased passivity that occurred as the training progressed. Passivity was defined as a mouse that did not swim on all four trials in a given day. The number of passive animals was higher in the CpKO group on day one compared to the WT group and by training day three, 50% of the mice in the CpKO group (6 of 12 mice) were passive compared to less than 10% seen in the WT group (1 of 12 mice). The Morris water maze test is stressful to the mice and passivity is one response to elevated stress (Engelmann et al., 2006). Increased passivity in a certain group of mice can indicate a higher basal level of stress and anxiety (Francis et al., 1995).
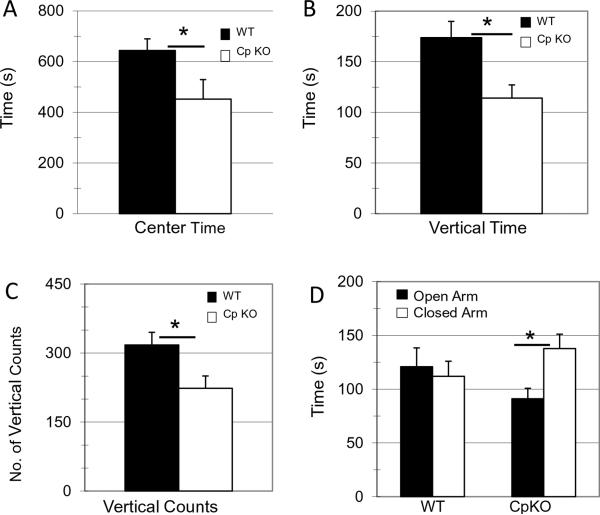
Mice exhibit a natural exploratory behavior, but are vulnerable in an open field and when vertically exploring their environment. Decreased vertical counts and center exploration is indicative of an increased basal anxiety level (Bailey and Crawley, 2009). Basal levels of anxiety were tested in the two mice groups using an open field test. The CpKO group spent significantly less time in the center of the field (t20=2.09, *p=0.04) (Fig. 2A), and had significantly less vertical time (t20=2.09,*p=0.009) (Fig. 2B) and counts (t20=2.09, *p=0.02) (Fig. 2C) compared to the WT group. Another test of anxiety performed on the WT and CpKO mice was the elevated plus maze. In this test the mice have the option to explore either two open arms of the maze or two closed arms of the maze. The WT mice spent equal amounts of time in both types of arms in the maze, whereas the CpKO mice spent significantly more time in the closed arms of the maze compared to the open arms (t22=2.07, *p=0.01) (Fig. 2D). Increased time spent in the closed arms of the elevated plus maze supports the increased anxiety phenotype demonstrated in previous behavior tests.
Figure 2.
CpKO mice exhibit an anxiety phenotype. A-C. Results of open field tests. Time spent in the center of the field (t22=2.09, df=20, *p<0.044) (A), time spent in vertical exploration (t22=2.09, df=20, *p<0.009) (B) and number of vertical exploration counts (t22=2.09, df=20, *p<0.02) (C) measured during a 30 minute exploration time. D. Elevated plus maze measures comparing time spent in open vs closed arm of the maze. CpKO mice spent significantly more time in the closed arm compared to the open arm (t22=2.07, df=22, *p<0.001).
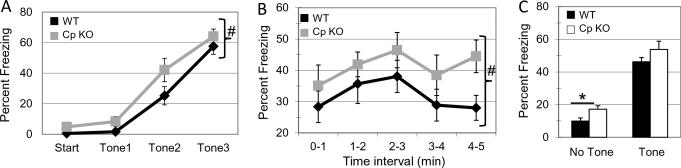
The anxiety phenotype seen in the CpKO group was further corroborated by the results of the fear conditioning tests. In both the training period (F(1,21)=5.2, p=0.033) (Fig. 3A) and contextual memory(F(1,20)=4.5, p=0.044) (Fig. 3B) testing period, the CpKO group showed significantly elevated percentages of time freezing. The hippocampus-dependent learning part of the task was performed equally well by WT and CpKO mice as demonstrated by the similar increased freezing due to the association made on the training day between the context and the foot shock. Cued fear, an amygdala- dependent memory task was also tested and was found to be unimpaired and similar in WT and CpKO mice. Under basal conditions in a novel environment for the cued fear test (t22=2.07, *p=0.02) (Fig. 3C), the CpKO mice exhibited significantly increased freezing time compared to the WT mice, consistent with an anxiety phenotype in the CpKO mice.
Figure 3.
Ceruloplasmin-deficient mice exhibit anxiety-like behavior in fear conditioning tests. A. Training day comparing % freezing at baseline (start) or during subsequent tones paired with a foot-shock. Over repeated measures CpKO mice show a significant increase in percentage of freezing (anova repeated measures #p<0.0001, n=12) (E). B. Contextual fear measures on day 2 comparing percent freezing over 5 min in intervals of 1 min. CpKO mice show a significant increase in percentage of freezing (ANOVA repeated measures; #p<0.007, n=12). C. Cued fear measures on day 2 comparing percent freezing at baseline or with a tone (cue). CpKO mice show a significant increase in percentage of freezing at baseline (t22=2.07, df=22, *p<0.02) but not in the cued portion of the test.
Many of the same molecular mechanisms that regulate anxiety behavior also play a role in depressive behavior. To test for depression we subjected both mouse groups to a tail suspension test and a forced swim test (data not shown). In these tests the percent of immobility seen during the test indicates the level of depression. There were no significant differences in percent immobility between the two groups of mice for either test. Thus, although the CpKO mice exhibit increased anxiety they do not show signs of depression.
Ceruloplasmin deficiency results in elevated plasma corticosterone levels
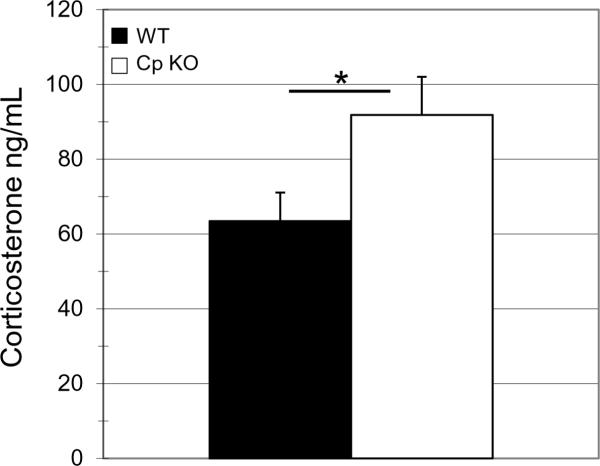
Elevated levels of anxiety are often associated with increased activation of the hypothalamic – pituitary – adrenal (HPA) stress system. One way to measure HPA axis activation state is by quantifying levels of circulating adrenal glucocorticoids. In rodents, the glucocorticoid corticosterone can be measured in plasma. The basal morning level of plasma corticosterone was significantly elevated in the CpKO mice compared to WT mice (t32=2.03*p=0.033) (Fig 4).
Figure 4.
Serum corticosterone levels are elevated in ceruloplasmin-deficient mice. Serum taken from morning tail bleeds from 3 month old mice prior to behavior testing was assayed for corticosterone using a radioimmunoassay. Levels of corticosterone were significantly elevated in CpKO mice compared to WT animals (t22=2.03, df=32,*p<0.033)
Ceruloplasmin deficiency results in reduced levels of iron in the hippocampus
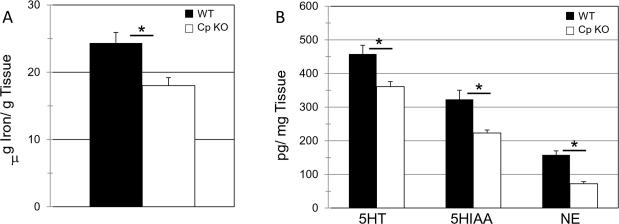
The main function of Cp is to regulate cellular iron metabolism. To determine if the absence of Cp altered brain iron levels, biopsy punches were taken from the hippocampus of both the WT and CpKO mice. These punches were analyzed for iron levels using atomic absorption spectroscopy. Levels of iron were normalized to the sample weight. The CpKO mice had a significantly lower concentration of iron in the hippocampus compared to WT mice (t30=2.04, *p=0.002) (Fig. 5A).
Figure 5.
CpKO mice have decreased concentrations of iron, serotonin (5HT), the serotonin metabolite (5HIAA) and norepinephrine (NE) in the hippocampus. A. Atomic absorption measures of hippocampal biopsy punches show significant decreases in iron concentrations in CpKO compared to wild type mice (t22=2.04, df=30, *p<0.0017). B. HPLC measures of hippocampal biopsy punches show significant reductions in levels of 5HT (t22=2.23, df=10, *p<0.009), 5HIAA (t22=2.23, df=10, *p<0.005). and NE (t22=2.23, df=10, *p<8.2x10-5). in CpKO compared to WT mice.
Ceruloplasmin deficiency results in reduced levels of serotonin and norepinephrine in the hippocampus
The elevation in anxiety levels and the decrease in hippocampal iron concentrations seen in the CpKO mouse prompted us to measure levels of serotonin (5HT) and norepinephrine (NE), two neurotransmitters for which iron plays important roles in their synthesis and metabolism. We found reduced levels of 5HT(t10=2.2, *p=0.009) and the 5HT metabolite 5HIAA(t10=2.2, *p=0.005) in the hippocampus of CpKO mice compared to WT mice (Fig. 5B). Levels of NE were also reduced significantly in the hippocampus of CpKO mice compared to WT mice (t10=2.2, *p=8.2×10-5) (Fig. 5B). Because deficiencies in 5HT and NE signaling are associated with anxiety (Dell'Osso et al., 2010), our findings suggest a role for reduced 5HT and NE levels in the anxiety phenotype of CpKO mice.
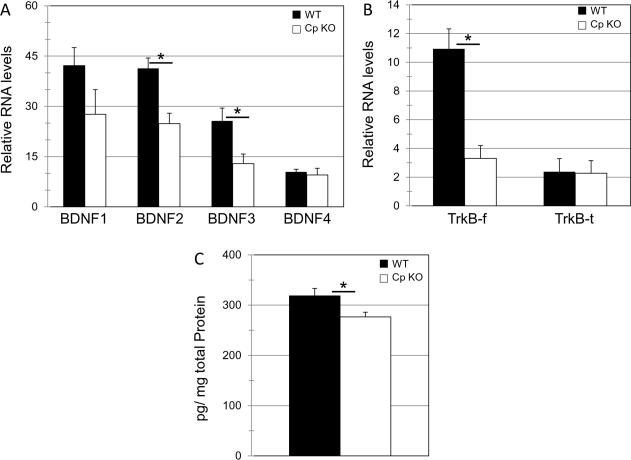
BDNF Signaling is impaired in ceruloplasmin-deficient mice
Changes in hippocampal BDNF signaling have been linked to altered 5HT (Martinowich and Lu, 2008) and NE levels (Chen, et.al, 2007). Moreover, reduced levels of hippocampal BDNF have been implicated in the manifestation of anxiety (Kikusui et al., 2009). We therefore analyzed the levels of the four BDNF mRNA transcripts by RT-PCR. We found that mRNA levels of transcripts 2(t8=2.3, *p=0.01) and 3 (t8=2.3, *p=0.03) were significantly reduced in the hippocampus of CpKO mice compared to WT mice (Fig, 6A). We also analyzed mRNA transcripts for the BDNF receptor TrkB. Two different splicing forms for TrkB exist, one for the full-length form (TrkB-f) which includes the tyrosine kinase intracellular domain and a truncated form (TrkB-t) that lacks the tyrosine kinase domain (Strohmaier, et al.,1996). The role of TrkB-t in BDNF signaling is not known but it may act in a dominant negative manner (Eide et al., 1996) or it may couple to a different intracellular signaling pathway (Cheng et al., 2007). We found that TrkB-f (t8=2.3, *p=0.003) was significantly reduced in the hippocampus of CpKO mice compared to WT mice, while TrkB-t was unchanged (Fig. 6B). Corroborating the RT-PCR data we found that protein levels of BDNF(t8=2.3, *p=0.03), measured by ELISA, were also significantly reduced in the hippocampus of the CpKO mouse (Fig. 6C). Together, these data provide evidence that ceruloplasmin deficiency results in impaired BDNF signaling in the hippocampus.
Figure 6.
The expression of BDNF and TrkB are reduced in the hippocampus of ceruloplasmin-deficient mice. A. RT-PCR measures of the 4 BDNF transcripts show a decrease in BDNF transcripts 2 (t22=2.31, df=8, *p<0.01) and 3 (t22=2.31, df=8, *p<0.03) in the hippocampus of the CpKO mouse. B. RT-PCR measures of 2 TrkB transcripts, a full-length form TrkBf and a short form TrkBt, show decreases in TrkBf, (t22=2.31, df=8, *p<0.003) but not TrkBt in the hippocampus of the CpKO mouse. C. Hippocampal BDNF protein levels were measured by ELISA. CpKO mice exhibit significantly decreased levels of BDNF protein in the hippocampus (t22=2.31, df=8, *p<0.03).
Discussion
The ferroxidase activity of CP facilitates its role in cellular iron efflux (Hellman and Gitlin, 2002). Some have speculated that in addition to efflux, Cp may be crucial in promoting cellular iron influx (Qian and Ke, 2001), and data suggest that Cp does play a role in iron influx in iron-deficient neurons (Ke et al., 2006). In the brain Cp is present in both a secreted form, and as a GPI-linked protein in astrocytes. Tight regulation of iron efflux and influx is essential to brain iron trafficking, and thus also to brain functions that rely heavily on optimal iron concentrations including energy metabolism, myelination and neurotransmitter production. In the present study we provide evidence that Cp contributes to cellular iron metabolism in the hippocampus, and that Cp deficiency has behavioral consequences. Cp-deficient mice exhibit reduced levels of hippocampal iron, 5HT, NE and BDNF compared to wild type control mice. The latter neurochemical alterations were associated with a heightened anxiety phenotype, without impairment of memory acquisition or retention. Our findings, discussed in detail below, suggest that perturbed cellular iron handling in Cp-deficient mice results in decreased levels of serotonin and norepinephrine and reduced BDNF expression, and heightened anxiety.
While the hippocampus is best known for its role in learning and memory, it is also involved in affective behaviors including anxiety and depression (Engin and Treit, 2007; Schmidt and Duman, 2007). Reduced levels and altered signaling of the neurotransmitters serotonin (Wesolowska, 2010) and norepinephrine (Godard et al., 2010) are implicated in anxiety behavior. Selective serotonin reuptake inhibitors (SSRIs) have long been prescribed for treatment of anxiety disorders and, more recently, dual 5HT and NE reuptake inhibitors have been employed to treat anxiety disorders in humans (Ravindran, 2010). We found decreases in levels of both serotonin and norepinephrine in the hippocampus of CpKO mice, which is likely due to altered cellular iron metabolism as suggested by the decreased iron levels in the hippocampus of CpKO mice. The latter possibility is consistent with the fact that the rate-limiting enzymes for the synthesis of serotonin (tryptophan hydroxylase) and norepinephrine (tyrosine hydroxylase) require iron as a cofactor (Waldmeier et al., 1993; Ramsey et al., 1995). Studies in rats have shown that diet-induced iron deficiency can decrease brain iron stores and decrease serotonin levels (Beard et al., 2007). Other studies have shown that a reduction of cellular iron levels can reduce the activity of tryptophan hydroxylase (Hasegawa et al., 1996, 1999), which is consistent with our data showing that lower hippocampal iron levels in CpKO mice are associated with reduced serotonin levels. Norepinephrine levels are also influence by iron levels as demonstrated by the reduction in norepinephrine transporters in response to reduced iron levels in PC12 cells and rat brain (Beard et al., 2006). Iron-deficient rats also show decreases in norepinephrine level, together with reduced levels of norepinephrine receptor and transport proteins, indicating impaired intracellular trafficking of norepinephrine in addition to reduced norepinephrine synthesis (Anderson et al., 2009). Our data are therefore consistent with a scenario in which lack of Cp in the hippocampus leads to reduced cellular iron concentrations, resulting in reduced serotonin and norepinephrine signaling and increased anxiety.
Another factor that can influence emotional behaviors is the neurotrophic factor BDNF. There is considerable evidence to support a role for BDNF in the mechanism of action of antidepressant drugs, particularly serotonin-selective reuptake inhibitors such as paroxetine and fluoxetine, and dual serotonin and norepinephrine reuptake inhibitors such as duloxetine and venlafaxin (Martínez-Turrillas et al., 2005; Calabrese et al., 2007; Larsen et al., 2008; Castren and Rantamaki, 2010). BDNF has also been suggested to exert an anxiolytic activity (Martinowich et al., 2007). Mice heterozygous for BDNF (Koizumi et al., 2006), mice with a conditional BDNF knockout in the brain (Rios et al., 2001), and mice with a hippocampus-specific deletion of BDNF (Heldt et al., 2007) all exhibit increased anxiety phenotypes. Our data show elevated plasma corticosterone levels in the CpKO mice, indicative of increased stress, along with reduced levels of BNDF and TrkBf in the hippocampus. These findings are consistent with the anxiety phenotype in the Cp-deficient mice because elevated corticosterone levels can decrease BDNF expression in the hippocampus at both the mRNA and protein levels (Schaaf et al., 2000). Previous studies have shown decreases in BDNF mRNA and protein levels and TrkBf mRNA levels in iron-deficient animals (Tran et al., 2008, 2009). This implies that the reduced iron concentrations seen in the hippocampus of the CpKO mouse contributes to reduced BDNF signaling in their hippocampus. BDNF has been shown to have a positive influence on serotonin levels and signaling, and evidence also suggests that serotonin can, in turn, induce BDNF expression (Martinowich et al., 2008). Norepinephrine also stimulates BDNF production in hippocampal neurons (Chen et al., 2007). Thus, the reduced levels of serotonin and norepinephrine in the hippocampus, likely contribute to reduced BDNF production and the anxiety phenotype of CpKO mice.
It was previously reported that a different line of CpKO mice exhibits increased iron deposition in the brainstem and cerebellum, loss of brainstem dopaminergic neurons, and motor deficits which were evident only in old (16 month-old) mice (Patel et al., 2002). In contrast to the motor impairment in the old CpKO mice in the latter study, we did not detect a motor deficit in our young 3-4 month-old CpKO mice. Instead, we discovered an anxiety phenotype associated with deficits in hippocampal iron, monoamine and BDNF levels. We found no evidence for learning and memory impairment in young CpKO mice compared to wild type mice. Humans with aceruloplasminemia present with motor symptoms, cognitive impairment and psychiatric problems in mid-life (Xu et al., 2004). Brain iron trafficking is known to differ among brain regions, and in an age-dependent manner (Zecca et al., 2004) so it is likely that the effects of ceruloplasmin deficiency on iron metabolism are brain-region dependent and that functional deficits manifest at different ages depending upon the brain region affected. Our findings suggest a likely neurochemical basis for the anxiety phenotype of ceruloplasmin-deficient mice involving reductions in hippocampal serotonin, norepinephrine and BDNF production. It will be of considerable interest to determine whether similar neurochemical alterations occur in ceruloplasmin-deficient humans, and whether their neurological symptoms can be lessened by treatment with agents that increase serotoninergic and/or noradrenergic signaling.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute on Aging. We thank Henriette van Praag for valuable advice on behavioral testing procedures and Chris Earley for comments on the manuscript.
References
- Anderson JG, Fordahl SC, Cooney PT, Weaver TL, Colyer CL, Erikson KM. Extracellular norepinephrine, norepinephrine receptor and transporter protein and mRNA levels are differentially altered in the developing rat brain due to dietary iron deficiency and manganese exposure. Brain Res. 2009;1281:1–14. doi: 10.1016/j.brainres.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KR, Crawley JN. Anxiety-Related Behaviors in Mice. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. CRC Press; Boca Raton (FL): 2009. [PubMed] [Google Scholar]
- Beard JL, Wiesinger JA, Jones BC. Cellular iron concentrations directly affect the expression levels of norepinephrine transporter in PC12 cells and rat brain tissue. Brain Res. 2006;1092:47–58. doi: 10.1016/j.brainres.2006.03.071. [DOI] [PubMed] [Google Scholar]
- Beard JL, Unger EL, Bianco LE, Paul T, Rundle SE, Jones BC. Early postnatal iron repletion overcomes lasting effects of gestational iron deficiency in rats. J Nutr. 2007;137:1176–1182. doi: 10.1093/jn/137.5.1176. [DOI] [PubMed] [Google Scholar]
- Calabrese F, Molteni R, Maj PF, Cattaneo A, Gennarelli M, Racagni G, Riva MA. Chronic duloxetine treatment induces specific changes in the expression of BDNF transcripts and in the subcellular localization of the neurotrophin protein. Neuropsychopharmacology. 2007;32:2351–2359. doi: 10.1038/sj.npp.1301360. [DOI] [PubMed] [Google Scholar]
- Carlson ES, Tkac I, Magid R, O'Connor MB, Andrews NC, Schallert T, Gunshin H, Georgieff MK, Petryk A. Iron is essential for neuron development and memory function in mouse hippocampus. J Nutr. 2009;139:672–679. doi: 10.3945/jn.108.096354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Developmental Neurobiology. 2010;70:289–297. doi: 10.1002/dneu.20758. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Nguyen TV, Pike CJ, Russo-Neustadt AA. Norepinephrine induces BDNF and activates the PI-3K and MAPK cascades in embryonic hippocampal neurons. Cell Signal. 2007;19:114–128. doi: 10.1016/j.cellsig.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Cheng A, Coksaygan T, Tang H, Khatri R, Balice-Gordon RJ, Rao MS, Mattson MP. Truncated tyrosine kinase B brain-derived neurotrophic factor receptor directs cortical neural stem cells to a glial cell fate by a novel signaling mechanism. J Neurochem. 2007;100:1515–1530. doi: 10.1111/j.1471-4159.2006.04337.x. [DOI] [PubMed] [Google Scholar]
- Chua AC, Graham RM, Trinder D, Olynyk JK. The regulation of cellular iron metabolism. Crit Rev Clin Lab Sci. 2007;44:413–459. doi: 10.1080/10408360701428257. [DOI] [PubMed] [Google Scholar]
- Dell'Osso B, Buoli M, Baldwin DS, Altamura AC. Serotonin norepinephrine reuptake inhibitors (SNRIs) in anxiety disorders: a comprehensive review of their clinical efficacy. Hum Psychopharmacol. 2010;25:17–29. doi: 10.1002/hup.1074. [DOI] [PubMed] [Google Scholar]
- Deltheil T, Guiard BP, Cerdan J, David DJ, Tanaka KF, Repérant C, Guilloux JP, Coudoré F, Hen R, Gardier AM. Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice. Neuropharmacology. 2008;55:1006–1014. doi: 10.1016/j.neuropharm.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide FF, Vining ER, Eide BL, Zang K, Wang XY, Reichardt LF. Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J Neurosci. 1996;16:3123–3129. doi: 10.1523/JNEUROSCI.16-10-03123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Ebner K, Landgraf R, Wotjak CT. Effects of morris water maze testing on the neuroendocrine stress response and intrahypothalamic release of vasopressin and oxytocin in the rat. Horm Behav. 2006;50:496–501. doi: 10.1016/j.yhbeh.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D. The role of hippocampus in anxiety: intracerebral infusion studies. Behav Pharmacol. 2007;18:365–374. doi: 10.1097/FBP.0b013e3282de7929. [DOI] [PubMed] [Google Scholar]
- Francis DD, Zaharia MD, Shanks N, Anisman H. Stress-induced disturbances in morris water-maze performance: Interstrain variability. Physiol Behav. 1995;58:57–65. doi: 10.1016/0031-9384(95)00009-8. [DOI] [PubMed] [Google Scholar]
- Goddard AW, Ball SG, Martinez J, Robinson MJ, Yang CR, Russell JM, Shekhar A. Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress Anxiety. 2010;27:339–350. doi: 10.1002/da.20642. [DOI] [PubMed] [Google Scholar]
- Harris ZL, Klomp LW, Gitlin JD. Aceruloplasminemia: an inherited neurodegenerative disease with impairment of iron homeostasis. Am J Clin Nutr. 1998;67:972S–977S. doi: 10.1093/ajcn/67.5.972S. [DOI] [PubMed] [Google Scholar]
- Harris ZL, Durley AP, Man TK, Gitlin JD. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci U S A. 1999;96:10812–10817. doi: 10.1073/pnas.96.19.10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H, Iida Y, Oguro K, Kojima M, Ichiyama A. Tryptophan hydroxylase activity in serotonin producing mast cells. dependence on intracellular iron concentration manipulated by permeable chelators. Adv Exp Med Biol. 1996;398:513–517. [PubMed] [Google Scholar]
- Hasegawa H, Oguro K, Naito Y, Ichiyama A. Iron dependence of tryptophan hydroxylase activity in RBL2H3 cells and its manipulation by chelators. Eur J Biochem. 1999;261:734–739. doi: 10.1046/j.1432-1327.1999.00316.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. Brain-derived neurotrophic factor as a biomarker for mood disorders: an historical overview and future directions. Psychiatry Clin Neurosci. 2010;64:341–357. doi: 10.1111/j.1440-1819.2010.02113.x. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman NE, Gitlin JD. Ceruloplasmin metabolism and function. Annu Rev Nutr. 2002;22:439–458. doi: 10.1146/annurev.nutr.22.012502.114457. [DOI] [PubMed] [Google Scholar]
- Ke Y, Ho K, Du J, Zhu L, Xu Y, Wang Q, Wang CY, Li L, Ge X, Chang YZ, Qian ZM. Role of soluble ceruloplasmin in iron uptake by midbrain and hippocampus neurons. J Cell Biochem. 2006;98:912–919. doi: 10.1002/jcb.20740. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Ichikawa S, Mori Y. Maternal deprivation by early weaning increases corticosterone and decreases hippocampal BDNF and neurogenesis in mice. Psychoneuroendocrinology. 2009;34:762–772. doi: 10.1016/j.psyneuen.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Koizumi H, Hashimoto K, Iyo M. Dietary restriction changes behaviours in brain-derived neurotrophic factor heterozygous mice: Role of serotonergic system. Eur J Neurosci. 2006;24:2335–2344. doi: 10.1111/j.1460-9568.2006.05094.x. [DOI] [PubMed] [Google Scholar]
- Larsen MH, Hay-Schmidt A, Rønn LC, Mikkelsen JD. Temporal expression of brain-derived neurotrophic factor (BDNF) mRNA in the rat hippocampus after treatment with selective and mixed monoaminergic antidepressants. Eur J Pharmacol. 2008;578:114–122. doi: 10.1016/j.ejphar.2007.08.050. [DOI] [PubMed] [Google Scholar]
- Martínez-Turrillas R, Del Río J, Frechilla D. Sequential changes in BDNF mRNA expression and synaptic levels of AMPA receptor subunits in rat hippocampus after chronic antidepressant treatment. Neuropharmacology. 2005;49:1178–1188. doi: 10.1016/j.neuropharm.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Lu B. Interaction between BDNF and serotonin: Role in mood disorders. Neuropsychopharmacology. 2008;33:73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Metal-catalyzed disruption of membrane protein and lipid signaling in the pathogenesis of neurodegenerative disorders. Ann N Y Acad Sci. 2004;1012:37–50. doi: 10.1196/annals.1306.004. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Murray F, Smith DW, Hutson PH. Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. Eur J Pharmacol. 2008;583:115–127. doi: 10.1016/j.ejphar.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Daher RJ, Charney DS. Anxiety disorders: noradrenergic neurotransmission. Handb Exp Pharmacol. 2005;169:205–223. doi: 10.1007/3-540-28082-0_8. [DOI] [PubMed] [Google Scholar]
- Pardon MC. Role of neurotrophic factors in behavioral processes: implications for the treatment of psychiatric and neurodegenerative disorders. Vitam Horm. 2010;82:185–200. doi: 10.1016/S0083-6729(10)82010-2. [DOI] [PubMed] [Google Scholar]
- Parks YA, Wharton BA. Iron deficiency and the brain. Acta Paediatr Scand Suppl. 1989;361:71–77. doi: 10.1111/apa.1989.78.s361.71. [DOI] [PubMed] [Google Scholar]
- Patel BN, Dunn RJ, Jeong SY, Zhu Q, Julien JP, David S. Ceruloplasmin regulates iron levels in the CNS and prevents free radical injury. J Neurosci. 2002;22:6578–6586. doi: 10.1523/JNEUROSCI.22-15-06578.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian ZM, Ke Y. Rethinking the role of ceruloplasmin in brain iron metabolism. Brain Res Rev. 2001;35:287–294. doi: 10.1016/s0165-0173(01)00056-x. [DOI] [PubMed] [Google Scholar]
- Ramsey AJ, Daubner SC, Ehrlich JI, Fitzpatrick PF. Identification of iron ligands in tyrosine hydroxylase by mutagenesis of conserved histidinyl residues. Protein Sci. 1995;4:2082–2086. doi: 10.1002/pro.5560041013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R, Georgieff MK. Iron in fetal and neonatal nutrition. Semin Fetal Neonatal Med. 2007;12:54–63. doi: 10.1016/j.siny.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran LN, Stein MB. The pharmacologic treatment of anxiety disorders: A review of progress. J Clin Psychiatry. 2010;71:839–854. doi: 10.4088/JCP.10r06218blu. [DOI] [PubMed] [Google Scholar]
- Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan RM, Jaenisch R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, De Kloet ER, Vreugdenhil E. Corticosterone effects on BDNF expression in the hippocampus. implications for memory formation. Stress. 2000;3:201–208. doi: 10.3109/10253890009001124. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- Sterner EY, Kalynchuk LE. Behavioral and neurobiological consequences of prolonged glucocorticoid exposure in rats: relevance to depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:777–790. doi: 10.1016/j.pnpbp.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohmaier C, Carter BD, Urfer R, Barde YA, Dechant G. A splice variant of the neurotrophin receptor trkB with increased specificity for brain-derived neurotrophic factor. EMBO J. 1996;15:3332–3337. [PMC free article] [PubMed] [Google Scholar]
- Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev. 2008;59:201–220. doi: 10.1016/j.brainresrev.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Tran PV, Carlson ES, Fretham SJ, Georgieff MK. Early-life iron deficiency anemia alters neurotrophic factor expression and hippocampal neuron differentiation in male rats. J Nutr. 2008;138:2495–2501. doi: 10.3945/jn.108.091553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PV, Fretham SJ, Carlson ES, Georgieff MK. Long-term reduction of hippocampal brain-derived neurotrophic factor activity after fetal-neonatal iron deficiency in adult rats. Pediatr Res. 2009;65:493–498. doi: 10.1203/PDR.0b013e31819d90a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio LG. Mammalian iron metabolism. Toxicol Mech Methods. 2007;17:497–517. doi: 10.1080/15376510701556690. [DOI] [PubMed] [Google Scholar]
- Waldmeier PC, Buchle AM, Steulet AF. Inhibition of catechol-O-methyltransferase (COMT) as well as tyrosine and tryptophan hydroxylase by the orally active iron chelator, 1,2-dimethyl-3-hydroxypyridin-4-one (L1, CP20), in rat brain in vivo. Biochem Pharmacol. 1993;45:2417–2424. doi: 10.1016/0006-2952(93)90222-i. [DOI] [PubMed] [Google Scholar]
- Wesolowska A. Potential role of the 5-HT6 receptor in depression and anxiety: An overview of preclinical data. Pharmacol Rep. 2010;62:564–577. doi: 10.1016/s1734-1140(10)70315-7. [DOI] [PubMed] [Google Scholar]
- Xu X, Pin S, Gathinji M, Fuchs R, Harris ZL. Aceruloplasminemia: An inherited neurodegenerative disease with impairment of iron homeostasis. Ann N Y Acad Sci. 2004;1012:299–305. doi: 10.1196/annals.1306.024. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Green AR. Iron deficiency and neurotransmitter synthesis and function. Proc Nutr Soc. 1978;37:173–179. doi: 10.1079/pns19780022. [DOI] [PubMed] [Google Scholar]
- Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- Zhang AS, Enns CA. Iron homeostasis: recently identified proteins provide insight into novel control mechanisms. J Biol Chem. 2009;284:711–715. doi: 10.1074/jbc.R800017200. [DOI] [PMC free article] [PubMed] [Google Scholar]