Abstract
Gene delivery from hydrogels represents a versatile approach for localized expression of tissue inductive factors than can promote cellular processes that lead to regeneration. Lentiviral gene therapy vectors were entrapped within fibrin hydrogels, either alone or complexes with hydroxylapatite (HA) nanoparticles. The inclusion of HA into the hydrogel led to the formation of small aggregates distributed throughout the hydrogel, with no obvious alteration of the pore structure outside the aggregates. The presence of HA slowed hydrogel degradation by collagenase and plasmin relative to fibrin alone, and also decreased the rate of cell migration. Lentivirus had similar release from the fibrin hydrogels formed with or without HA. The altered hydrogel properties suggest an interaction between the nanoparticle and fibrin, which may displace the virus from the particle leading to similar release profiles. Transgene expression by cells migrating into the hydrogel in vitro was reduced in the presence of HA, consistent with the role of cell migration on transgene expression. In vivo, lentivirus loaded fibrin hydrogels promoted localized transgene expression that increased through day 9 and decreased through day 14. For the fibrin only hydrogels, expression continued to decline after day 14. However, hydrogels with HA maintained this transgene expression level for an additional two weeks before declining. Immunostaining identified transgene primarily outside the fibrin-HA gel at day 9; however, at day 21, transgene expression was observed primarily within the fibrin-HA gel. The localized delivery of lentivirus provides an opportunity to enhance the bioactivity of fibrin hydrogels for a wide range of applications in regenerative medicine.
Introduction
Fibrin hydrogels are employed for numerous applications in regenerative medicine, as the hydrogels support many cellular processes and are degradable by cell-secreted proteases thereby allowing them to be remodeled. Fibrin hydrogels have a high water content, support cell adhesion, can be used as a vehicle for cell transplantation, and possess mechanical properties similar to that of native soft tissues [1–3]. Fibrin is formed via thrombin mediated cleavage of the Aα and Bβ chains of fibrinogen followed by conformational changes and exposure of polymerization sites [1]. A 3D fibrin network, known as the fibrin clot, occurs when factor XIIIa introduces covalent bonds between lysines and glutamines between the γ-chains of the fibrin polymer [1]. This forms a stable network that can persist from days to weeks. Degradation of the fibrin network occurs through the action of plasmin in a process called fibrinolysis [4].
Strategies to enhance the functionality of fibrin, and other, hydrogels include the delivery of gene therapy vectors. Gene delivery is a versatile approach that can be employed to induce the expression of tissue inductive factors within the local microenvironment, or block expression of factors that inhibit regeneration [5]. Fibrin has been employed for gene delivery both in vitro [6–9] and in vivo [10, 11], as have a range of other hydrogels, such as gelatin [12, 13], collagen [14], PEG based hydrogels [15], and agarose [16]. For gene delivery to impact regenerative medicine, systems are needed that provide efficient gene delivery in vivo and that promote transgene expression by cells within the material. Transgene expression localized within the material can facilitate regeneration throughout the site in which the gel was delivered, yet is challenging as cells must infiltrate the matrix to access the vector. Additionally, cell infiltration typically occurs with hydrogel degradation, which can enhance vector release.
In this report, we investigate the delivery of lentiviral gene therapy vectors from fibrin hydrogels that contain hydroxyapatite (HA) nanoparticles. Fibrin hydrogels undergo mild gelation conditions that will not damage the incorporated vector, and supports rapid and robust cell ingrowth. HA nanoparticles were incorporated within the fibrin gel, as these particles can interact with fibrin and the lentivirus. The interaction of the HA with the fibrin may stabilize the hydrogels, which will influence the rate of cell infiltration and vector release. Additionally, HA can interact with lentiviral particles which can enhance and localize gene transfer within the hydrogel [17]. The activity of the lentiviral vector is determined as a function of encapsulation within the hydrogel and association with the nanoparticle association. The extent and localization of transgene expression is quantified both in vitro and in vivo. These studies demonstrate the potential of fibrin hydrogels to serve as a material support for regenerative medicine, and as a vehicle for the localized delivery of lentiviral vectors in vivo.
Materials and Methods
Virus Production
Cells (HEK 293T) were cultured at 37°C and 5% CO2 in cell growth medium (Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA)) unless otherwise indicated. Lentivirus was prepared for the studies using previously established techniques [18]. Briefly, lentiviral packaging vectors (pMDL-GagPol, pRSV-Rev, pIVS-VSV-G) were co-transfected along with plenti-CMV-luciferase or plenti-CMV-GFP into HEK-293T cells using Lipofectamine 2000 (Roche Biosciences, Palo Alto, CA, USA). The supernatant was collected and filtered (0.45 micron filter) after 48 hours of transfection. Viruses were then concentrated using PEG-it (System Biosciences, Mountain, CA, USA), with the precipitated lentiviruses resuspended with PBS. The virus titer (lentivirus particle, LP) was determined by HIV-1 p24 Antigen ELISA Kit (ZeptoMetrix Co., Buffalo, USA). Infectious titer (Infectious unit, IU) of lenti-GFP was determined by counting the number of cells expressing GFP at 2 days after incubation of serially diluted viruses with HEK-293T cells. Lentivirus suspended in PBS was used alone or complexed with hydoxylapatite nanoparticles (Sigma, Aldrich, Louis, MO). HA/virus complexes were formed by mixing lentivirus (1×106 LP) with HA in PBS (final concentration of 1 mg/mL) and allowed to incubate for 10 min on ice.
Fibrin Hydrogels
Hydrogels were formed from Tisseel™ (Baxter Healthcare, BioScience Division, Westlake Village, CA) fibrin sealant products that were kindly provided by Baxter International Inc. Fibrinogen was reconstituted in the provided aprotinin solution (100 mg/ml) and was diluted in Tris Buffered Saline (TBS) to the working concentration (25 mg/ml). Thrombin was reconstituted with 40 μM calcium chloride (500 IU/ml) and further diluted with 40 μM calcium chloride to working concentrations. Fibrin gels were formed either by mixing fibrinogen and thrombin solutions by pipette or using a 2 ml Duploject™ kit (Baxter International Inc.). Polymerization was allowed to proceed for 60 minutes at 37°C in a humid environment. Gels were formed at a final fibrinogen concentration of 25 mg/ml before polymerization. For encapsulation of virus within the gel, lentivirus or HA/virus complexes were mixed with the fibrinogen solution immediately prior to gelation, and virus was loaded at a dose of 107 particles.
Vector Release
Release studies were performed by incubation of fibrin gels containing lentivirus in serum containing media at 37°C in a humid environment, with samples (100 uL) collected with replacement at the indicated time points and stored at −80°C until sample concentration was determined. At the conclusion of the study, fibrin gels were degraded in collagenase solution (Sigma Aldrich, Louis, MO, 1 mg/ml) in order to isolate virus remaining within the gels. Viral particle concentrations were determined using a HIV-1 p24 Antigen ELISA (ZeptoMetrix, Franklin, MA).
Activity Studies
The activity of encapsulated virus as a function of time was investigated by encapsulating lentivirus within fibrin gels and incubating the gels in serum containing media at 37°C and 5% CO2. After 0, 24 and 48 hours, the gels were degraded by adding collagenase (Sigma Aldrich, Louis, MO) to the media for a final concentration of 1 mg/ml. Conditions that contained virus or complexes in media with collagenase added at 0, 24, and 48 hours served as control. To measure the relative activity of the virus, samples (10 μL) were added to HEK 293T cells growing in monolayer. After 48 hours, the cells were lysed with reporter lysis buffer (Promega, Madison, WI) and assayed for enzymatic activity using luciferase assay (Promega, Maison, WI). The activity of the virus was expressed as percentage of control conditions (virus in media).
Degradation
Hydrogel degradation was characterized as it may contribute both to cell migration and gene delivery. Fibrin gels (10 μL) containing 0, 1, and 5 mg/mL HA were allowed to polymerize for 1 hr at 37°C. Their initial swollen mass (in mg) was taken after allowing the gels to swell for one hour in PBS. The samples were placed in degradation solution that contained collagenase (0.4 mg/mL), plasmin (0.1 mg/mL, Sigma Aldrich, St. Louis, MO), or both. Gels were gently blotted to remove excess water before weighing. Gels were weighed every hour until dissolution of the gel. The mass was normalized to the initial swollen mass.
Cell Migration
A modified boyden chamber assay [19, 20] was used to evaluate cell infiltration into fibrin gels (50 μL) containing 0, 1, and 5 mg/mL HA. Hydrogels were polymerized in transwell culture inserts (24-well plate, 8.0 μm pore polyethylene terephthalate (PET) membrane, BD Biosciences, Bedford, MA), and placed in 24-well companion plates (BD Biosciences, Bedford, MA) and invasive HT1080 cells were seeded on the hydrogels (50,000 cells/gel). Inserts were filled with 500 μL starvation media (DMEM, 1% penicillin-streptomycin), and wells were filled with serum containing media (DMEM, 10% FBS, 1% penicillin-streptomycin). Media was changed every 24 hours. After 6 days in culture, cells were isolated from either side of the membrane using trypsin and counted with a hemocytometer. Cells isolated from the top of the membrane and inside the remaining hydrogel were counted as above the membrane. Cells isolated from the bottom of the membrane and on the bottom of the well were counted as below the membrane. The migration index was calculated as number of cells below the membrane divided by number of cells above the membrane.
Electron Microscopy
Fibrin hydrogels containing 0 or 5 mg/mL HA were formed and processed for visualization by scanning electron microscopy (SEM). Fibrin gels were fixed with 2% glutaraldehyde and 3% sucrose in PBS for 1 hour, rinsed twice with PBS at 4°C for 30 minutes, and washed with DI water for 5 minutes. The fixed gels were then dehydrated using a graded series of increasing ethanol concentrations, critical point dried, and sputter coated with Au/Pd. Samples were imaged using a Hitachi S-4500 with a cold field electron emission gun.
In Vitro Gene Delivery
The efficiency of gene delivery for the hydrogel was initially assessed by seeding of cells onto hydrogels. Fibrin hydrogels (200 μL) containing lentivirus or HA/virus complexes were deposited into 48 well plates using a 2 mL Duploject™ kit (Baxter International Inc.) and allowed to crosslink for 60 minutes at 37°C in a humid environment. HT 1080 cells were then seeded on the gels at 30,000 cells/gel. The gels seeded with cells were exposed to either serum containing media or to media supplemented with aprotinin (0.1 U/mL), which inhibits degradation of fibrin. Cells were cultured on the gels for 5 and 14 days with media changes (with and without aprotinin) every 2 days. After these time points, luciferase expression was measured as described above.
In Vivo Gene Delivery
In vivo expression was quantified by bioluminescence imaging of fibrin gels containing virus or HA/virus implanted subcutaneously into male CD-1 mice (20–22 g). Control studies consisted of lentivirus or the complexes injected into a subcutaneous pocket. Luciferase expression was quantified using an IVIS® imaging system (Caliper Corp., Alameda, CA) that contains a cooled CCD camera. Mice were injected i.p. with D-luciferin (Molecular Therapeutics, Inc., MI; 150 mg/kg body wt) using 28-gauge insulin syringes and placed in the chamber. Images were captured every 5 minutes for a total of 45 minutes until the peak intensity was confirmed. Gray-scale and bioluminescence images were superimposed using the Living Image software (Caliper Corp.). A constant size region of interest (ROI) was drawn, and the signal intensity was reported as an integrated light flux (photons/s) that was determined by IGOR software (WaveMetrics, OR). Background photon fluxes were determined by drawing a background ROI over regions removed from the implantation site. The background flux was subtracted from the integrated light flux obtained at the implantation sites. Care and use of the laboratory animals followed the guidelines established by the Northwestern University Institutional Animal Care and Use Committee (IACUC).
Histology
Histological analysis was performed on transplanted fibrin gels containing Lenti-GFP or complexes (Lenti-GFP and HA). On postoperative days 9 and 21, the fibrin gels and surrounding tissue were explanted from the subcutaneous pocket and frozen in isopentane (Fisher). Frozen specimens were embedded in Tissue-Tek O.C.T. compound (Miles Scientific, Elkhart, IN), and sectioned at 10 μm thickness. Infected cells were stained with polyclonal rabbit anti-EGFP (Invitrogen, Carlsbad, CA) as a primary antibody and Alexa Fluor 488 goat anti-rabbit (green) (Invitrogen, Carlsbad, CA) as a secondary antibody. A Hoechst stain was coincidently performed with the stain for EGFP to visualize cell nuclei.
Statistics
Statistical significance between groups was analyzed by one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons. A value of P < 0.05 was accepted as significant.
Results
Hydrogel Properties
We initially characterized the impact of the hydrogel structure in the presence and absence of virus and HA-virus. SEM images of fibrin hydrogels loaded with virus displayed the characteristic fibrous structure (Figure 1A, C). However, with the incorporation of HA within the hydrogel, the fibrous structure was observed in portion of the hydrogel; however, other regions lacked the characteristic fibrous network and displayed an aggregation of particles (Figure 1B, D). The size of the particles is consistent with the size of the HA nanoparticles.
Figure 1. SEM of fibrin hydrogels.
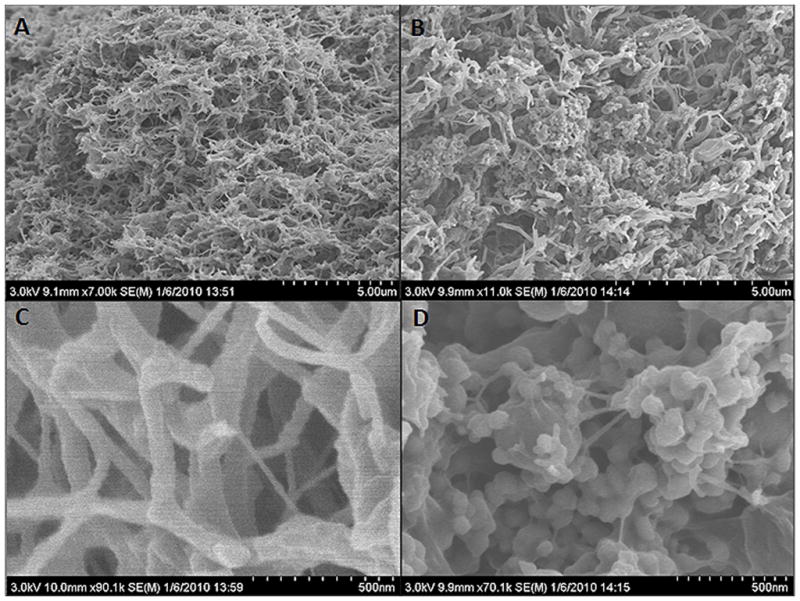
Images of fibrin hydrogels with lentivirus (A and C) and fibrin hydrogels with HA-lentivirus (B and D).
Matrix Degradation and Cell Migration
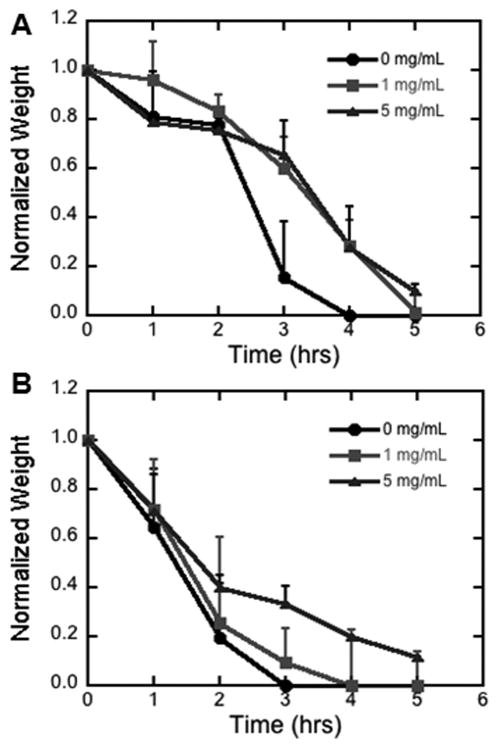
We subsequently investigated the impact of HA on the physical properties of the hydrogel, specifically the degradation rate of the matrix. Previous work suggested that HA can inhibit the activity of collagenase [21], which could reduce the degradation rate. Hydrogels loaded with two doses of HA and incubated with collagenase required approximately 25% more time for complete degradation relative to hydrogels lacking HA (Figure 2A). In the presence of plasmin, the greatest dose of HA slowed the degradation rate of the hydrogel relative to no HA or a small dose of HA (Figure 2B). Taken together, these results indicate that the presence of HA within fibrin hydrogels can slow degradation.
Figure 2. Degradation of fibrin hydrogels.
Fibrin gels were loaded with multiple dosages of HA (0, 1, 5 mg/mL). Degradation was characterized in the presence of A) collagenase (0.4 mg/mL), B) plasmin (0.1 mg/mL).
This impact of HA on matrix degradation was subsequently investigated for an influence on cell migration, which has a dependence on matrix degradation [21]. Cell infiltration into fibrin gels containing 0, 1, and 5 mg/mL HA was investigated using a modified Boyden chamber assay (Figure 3). The presence of HA in the hydrogels reduced the migration index approximately 3-fold relative to hydrogels formed with fibrin alone.
Figure 3. Cell migration through fibrin hydrogels.
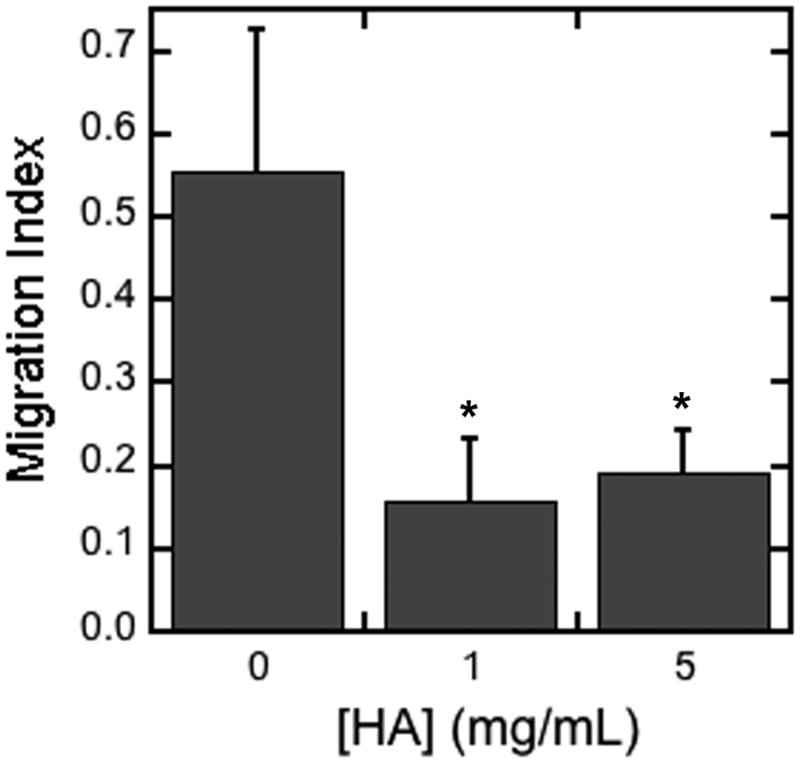
Cell migration was determined for hydrogels loaded with multiple dosages of HA (0, 1, 5 mg/mL). The migration index was calculated as number of cells that migrated through the gel and membrane divided by the number of cells that remained on top or in the gel. The symbol * indicates statistical significance relative to control group (without HA) at p < 0.05.
Vector Release and Stability
We subsequently investigated the release rate of vector from the hydrogel, and the impact of encapsulation on the virus activity. The impact of HA on release and the contribution of matrix degradation to release was initially investigated (Figure 4). Matrix degradation was inhibited through the addition of aprotinin to the media. Release was not significantly impacted by the incorporation of HA or the addition of aprotinin. During the initial 4 hours, approximately 39% of the entrapped vector was released, which gradually increased to 61% by 1 day, 70% by day 3 and 75% by day 6. Release profiles were also determined for multiple concentrations of thrombin (1, 25, and 100 U/mL), which can impact the mesh size and fibril thickness [22]. However, release was similar for the multiple thrombin concentrations (data not shown).
Figure 4. Cumulative vector release.
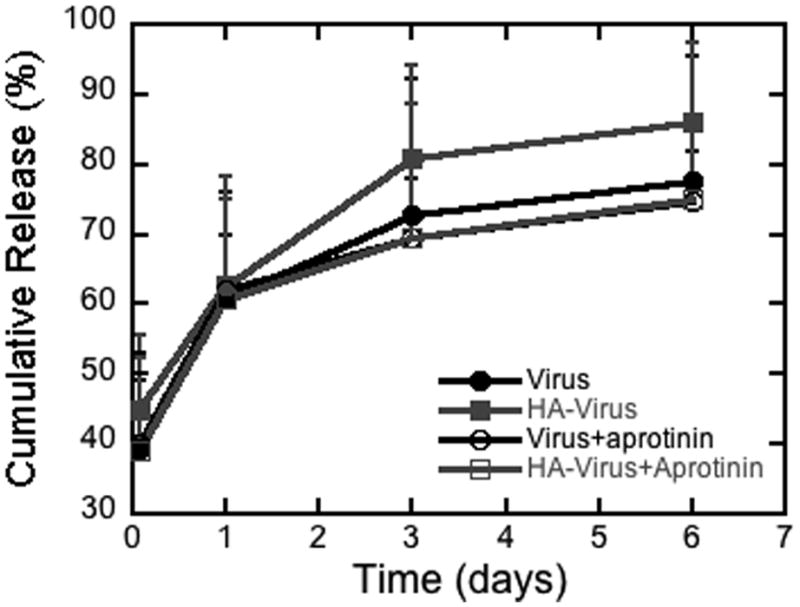
A) Cumulative release of lentivirus from fibrin gels formed with multiple thrombin concentrations. B) Cumulative release of virus from fibrin gels containing HA. Virus or HA/Virus complexes were encapsulated in fibrin and released in media alone or media containing aprotinin (0.1 mg/mL).
We subsequently characterized the activity of the virus encapsulated within fibrin with and without HA. Encapsulation within fibrin did not reduce the activity of the virus at 24 and 48 hours relative to unencapsulated virus (Figure 5). For the virus associated with HA, the virus activity was initially increased approximately 20-fold, while at 24 and 48 hours the virus activity was similar to the unencapsulated virus. The initial increase in activity in the presence of HA is consistent with previous reports of these nanoparticles for virus delivery [17]. However, the decline in activity to control values at 24 and 48 hours may result from displacement of the virus from HA that occurs from the binding of fibrin.
Figure 5. Virus activity in fibrin hydrogels.
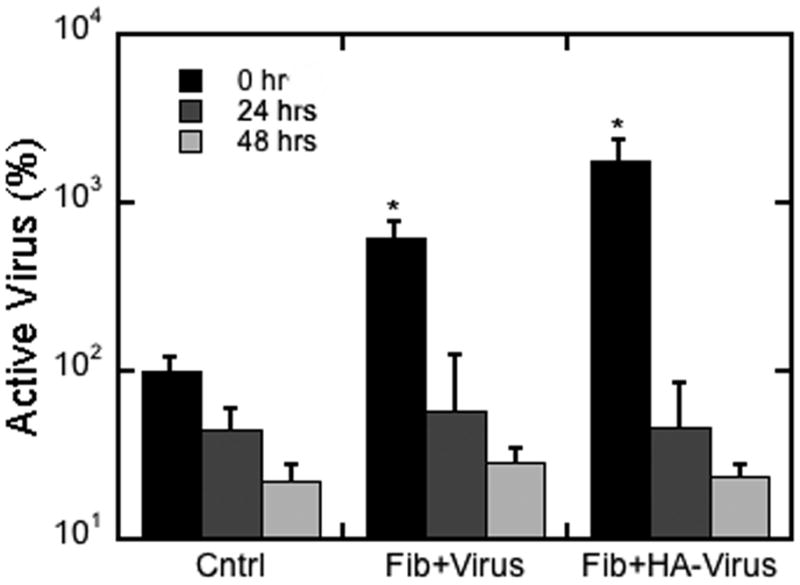
Viral activity was determined at 0, 24, and 48 hours for hydrogels formed with lentivirus with and without HA. The control condition was unencapsulated virus. The symbol * indicates statistical significance relative to control group at p < 0.05.
In vitro expression
The in vitro expression profile of fibrin gels loaded with lentivirus in the absence and presence of HA was subsequently investigated. Cells were encapsulated within the hydrogels and gene expression increased from day 5 to day 14 for all conditions (Figure 6). Hydrogels formed with fibrin alone had significantly increased expression at day 5 relative to the hydrogels formed with HA. However, at day 14, the expression levels between conditions were similar. The decreased expression at day 5 may be due to the slower degradation of the hydrogels that limit cell migration and gene expression [23], and we subsequently investigated the impact of aprotinin on the levels of gene expression. Gene expression in the presence of aprotinin was similar to that obtained with HA at both day 5 and 14, suggesting that gene expression at the early times was limited by the degradation of the matrix.
Figure 6. In vitro infection.
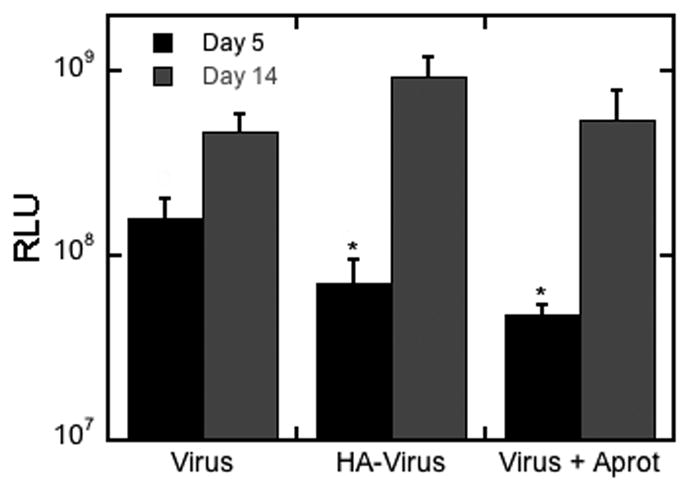
Cells were seeded on top of fibrin gels containing lentivirus. Transgene expression was compared for different conditions (fibrin with lentivirus, fibrin with lentivirus in the presence of aprotinin (a protease inhibitor), fibrin with lentivirus and HA) at day 5 and 14 of incubation. Limiting degradation rate lowers transgene expression relative to non-limited degradation after 5 days. The same trend is seen for HA/virus complexes in fibrin. The symbol * indicates statistical significance relative to control group (fibrin with lentivirus) at p < 0.05.
In vivo gene delivery
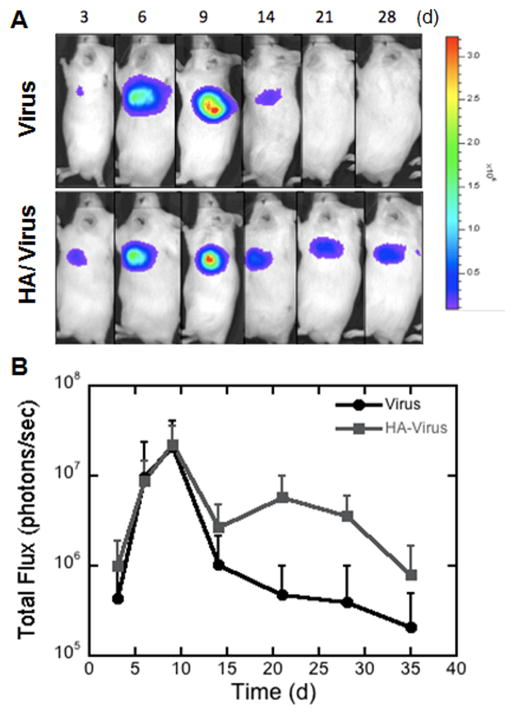
The ability of the hydrogels to promote gene delivery in vivo was subsequently investigated. Fibrin gels encapsulating lentivirus or HA/virus complexes were implanted subcutaneously into a mouse, and luciferase levels were determined by bioluminescence imaging (Figure 7A). Between days 3 and 14, expression was similar for both lentivirus or lentivirus-HA delivery from fibrin hydrogels (Figure 7B). Expression levels increase steadily from day 3 to day 9, with maximal expression at day 9. For lentivirus alone, expression declines from this day 9 maximum through day 14 and declines further through day 35. For lentivirus-HA, expression declines from the day 9 maximum to day 14, remained steady through day 30, and declined through day 35. Expression levels were significantly different between the two conditions at day 21 (p<0.05).
Figure 7. In Vivo Expression Profile.
Fibrin hydrogels (Th25/Fn25) containing either virus alone or HA/Virus were transplanted subcutaneously in a mouse model and the expression profile was followed for 28 days. A) Representative images of the in vivo expression at multiple days. B) Luminescence levels were determined from images captured by in vivo imaging (n = 6). The symbol * indicates statistical significance relative to control group at p < 0.05.
The differences in the expression profiles were investigated by immunostaining for the transgene in order to determine distribution of transduced cells. Fibrin gels were loaded with lenti-GFP/HA or lenti-GFP alone. GFP expression was investigated within the hydrogel and in the adjacent tissue. At day 9 after transplantation of the fibrin hydrogel with HA, GFP positive cells were observed within the hydrogel; however, substantially larger numbers of GFP positive cells were observed in tissue surrounding the hydrogel (Figure 8A). This distribution of transduced cells is consistent with cell migration into the hydrogel, along with release of virus into the adjacent tissue. After 21 days, GFP positive staining was present within the hydrogel containing HA-virus, and in the tissue adjacent to the gel (Figure 8B). At day 21, fibrin gels with virus alone (i.e., no HA) had completely degraded and few GFP positive cells were observed (data not shown).
Figure 8. Histology of implanted hydrogels.
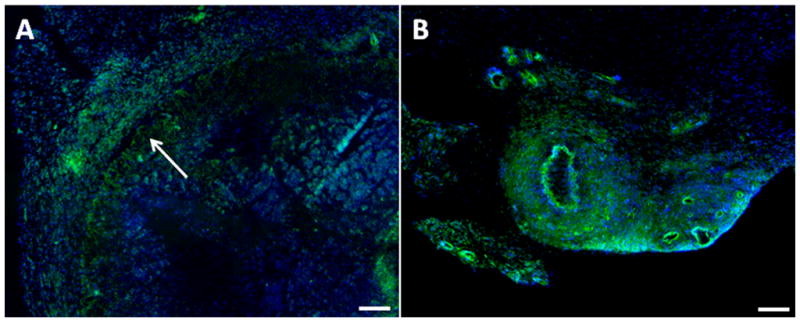
Representative images from tissue samples that were stained with antibodies to GFP (transduced cell) and Hoechst (nuclei) at day 9 (A) and 21 (B) after subcutaneous implantation of HA/virus loaded fibrin hydrogel (A and B). Arrow indicates the border line of hydrogel and the adjacent tissue. Scale bar represents 150 μm.
Discussion
In this manuscript, fibrin was developed as a scaffold for gene delivery to obtain localized transgene expression within the hydrogel that persists for weeks. Vector delivery from hydrogels typically results in transient expression, and often by cells in the surrounding tissue rather than within the injury site. We employed fibrin hydrogels, which are attractive for many applications in regenerative medicine as they support rapid cell ingrowth and are readily degraded by the infiltrating cells; however, strategies to increase their bioactivity could increase their utility. Hydroxyapatite (HA) was investigated as it can interact both with the fibrin hydrogel to add stability, as well as with the lentivirus. HA added to the fibrin hydrogels, which modulated the properties of the hydrogel (degradation rate, rate of cell migration), yet did not significantly impact the vector properties (e.g., activity, release profile). The presence of HA influenced the duration of transgene expression, which persisted beyond day 21 with the incorporation of HA, yet decreased to background levels in the absence of HA. Taken together, these results suggest an impact of the hydrogel properties on the duration of transgene expression, which has not been previously observed for fibrin hydrogels.
The HA incorporated into the hydrogel interacted with fibrin to impact the hydrogel properties, namely matrix degradation and cell migration, which may also influence vector release. Matrices with HA had a slower degradation rate in the presence of collagenase or plasmin when compared to fibrin without HA. Additionally, cells had a reduced migration index within the HA containing fibrin constructs, which is consistent with the reported link between cell migration and degradation rates [23]. This decreased rate of degradation suggests an interaction between the fibrin and the entrapped HA particles. An interaction between HA and fibrin is also supported by the SEM images, which indicate an abnormal pore structure in regions of the gel that contained particles. This dependence of expression on the matrix properties is consistent with previous in vitro studies with fibrin. Fibrin-mediated delivery of lentiviral vectors produced efficient gene transfer in vitro [24], with a dependence on fibrinogen concentration. Low fibrinogen concentrations resulted in rapid degradation and subsequent vector release from the gel, while high concentrations had reduced fibrin degradation and also gene transfer. Fibrin has also been employed for the encapsulation and release of non-viral vectors. A nanoparticle caging technique was developed to concentrate vectors without aggregation within the hydrogel with retention of their activity [25, 26]. Localized expression of VEGF in a choriallantoic membrane assay enhance the number of vessels. Alternatively, fibrin has been processed into a sphere templated scaffold with encapsulation of non-viral vectors that provided expression in vitro for up to 29 days [7].
Fibrin hydrogels maintain virus activity, yet the interaction between hydrogel and HA may function to displace the vector from the nanoparticles. HA has been previously reported to bind lentivirus, stabilize it against degradation, and enhance gene transfer in vitro and in vivo [17]. The presence of HA enhances the virus activity, consistent with the previous report, only at the initial time point. At later time points, the virus activity is similar to the free virus control. Additionally, lentivirus initially complexed with HA had a similar release profile as free lentivirus entrapped within the hydrogel. For cells seeded onto the hydrogel and subsequent culture in vitro, transgene expression was reduced approximately 45% in the HA condition relative to the free virus at day 5, yet was similar at day 14. This initial decrease in expression likely results from an increased matrix stability, as a similar decrease in expression was observed with aprotinin. Cell migration, which is impacted by the matrix stability, has also been connected to the efficiency of gene delivery [23].
Fibrin hydrogels releasing lentivirus produced localized expression, though HA-containing HA hydrogels extended transgene expression in vivo relative to fibrin alone. The expression profiles through 14 days were similar between fibrin and fibrin with HA, which is consistent with lentivirus delivery from collagen hydrogels [17] Expression is maximal at day 9, and then subsequent declines through day 14. The increase gene expression through day 9 is hypothesized to result from a combination of cell infiltration into the hydrogel and cell proliferation. The decline in gene expression from day 9 to 14 likely results from either clearance of the infected cells by the immune system or cell turnover. After 21 days, fibrin hydrogels containing HA did not have a decline in expression that was observed in the fibrin alone conditions. Interestingly, at day 21, only HA loaded fibrin hydrogels were clearly observed, as hydrogels from fibrin alone were degraded. The nanoparticles entrapped within the fibrin appeared as larger aggregates relative to the earlier tie points. This persistent expression with HA loaded hydrogels may result from the decreased rate of degradation of the hydrogel, which would increase the duration over which macrophages are present at the injury. Alternatively, the aggregated particles may sequester some virus leading to low levels of transduction at later times.
In conclusion, we demonstrate the nanoparticle loaded fibrin hydrogels promoted localized transgene expression that persisted for longer times relative to fibrin alone. The interactions between fibrin and the nanoparticle increased the gel stability, decreased the degradation rate, and the rate of cell migration. Encapsulation within fibrin did not alter the activity of the vector, yet likely resulted in displacement of the vector from the nanoparticle. Fibrin hydrogels have found numerous applications in regenerative medicine, and the localized delivery of lentivirus provides an opportunity to enhance their bioactivity in these applications.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahmed TA, Dare EV, Hincke M. Fibrin: A Versatile Scaffold for Tissue Engineering Applications. Tissue Eng Part B Rev. 2008 doi: 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- 2.Rosso F, Marino G, Giordano A, Barbarisi M, Parmeggiani D, Barbarisi A. Smart materials as scaffolds for tissue engineering. J Cell Physiol. 2005;203:465–470. doi: 10.1002/jcp.20270. [DOI] [PubMed] [Google Scholar]
- 3.Swartz DD, Russell JA, Andreadis ST. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am J Physiol Heart Circ Physiol. 2005;288:H1451–1460. doi: 10.1152/ajpheart.00479.2004. [DOI] [PubMed] [Google Scholar]
- 4.Cesarman-Maus G, Hajjar KA. Molecular mechanisms of fibrinolysis. Br J Haematol. 2005;129:307–321. doi: 10.1111/j.1365-2141.2005.05444.x. [DOI] [PubMed] [Google Scholar]
- 5.De Laporte L, Shea LD. Matrices and scaffolds for DNA delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:292–307. doi: 10.1016/j.addr.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breen A, Strappe P, Kumar A, O’Brien T, Pandit A. Optimization of a fibrin scaffold for sustained release of an adenoviral gene vector. J Biomed Mater Res A. 2006;78:702–708. doi: 10.1002/jbm.a.30735. [DOI] [PubMed] [Google Scholar]
- 7.Saul JM, Linnes MP, Ratner BD, Giachelli CM, Pun SH. Delivery of non-viral gene carriers from sphere-templated fibrin scaffolds for sustained transgene expression. Biomaterials. 2007;28:4705–4716. doi: 10.1016/j.biomaterials.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 8.des Rieux A, Shikanov A, Shea LD. Fibrin hydrogels for non-viral vector delivery in vitro. J Control Release. 2009;136:148–154. doi: 10.1016/j.jconrel.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lei P, Padmashali RM, Andreadis ST. Cell-controlled and spatially arrayed gene delivery from fibrin hydrogels. Biomaterials. 2009;30:3790–3799. doi: 10.1016/j.biomaterials.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breen AM, Dockery P, O’Brien T, Pandit AS. The use of therapeutic gene eNOS delivered via a fibrin scaffold enhances wound healing in a compromised wound model. Biomaterials. 2008;29:3143–3151. doi: 10.1016/j.biomaterials.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 11.Breen A, Dockery P, O’Brien T, Pandit A. Fibrin scaffold promotes adenoviral gene transfer and controlled vector delivery. J Biomed Mater Res A. 2009;89:876–884. doi: 10.1002/jbm.a.32039. [DOI] [PubMed] [Google Scholar]
- 12.Fukunaka Y, Iwanaga K, Morimoto K, Kakemi M, Tabata Y. Controlled release of plasmid DNA from cationized gelatin hydrogels based on hydrogel degradation. J Control Release. 2002;80:333–343. doi: 10.1016/s0168-3659(02)00026-3. [DOI] [PubMed] [Google Scholar]
- 13.Kushibiki T, Tomoshige R, Fukunaka Y, Kakemi M, Tabata Y. In vivo release and gene expression of plasmid DNA by hydrogels of gelatin with different cationization extents. J Control Release. 2003;90:207–216. doi: 10.1016/s0168-3659(03)00197-4. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Lee IL, Lim WS, Chia SM, Yu H, Leong KW, Mao HQ. Evaluation of collagen and methylated collagen as gene carriers. Int J Pharm. 2004;279:115–126. doi: 10.1016/j.ijpharm.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Lee PY, Li Z, Huang L. Thermosensitive hydrogel as a Tgf-beta1 gene delivery vehicle enhances diabetic wound healing. Pharm Res. 2003;20:1995–2000. doi: 10.1023/b:pham.0000008048.58777.da. [DOI] [PubMed] [Google Scholar]
- 16.Meilander-Lin NJ, Cheung PJ, Wilson DL, Bellamkonda RV. Sustained in vivo gene delivery from agarose hydrogel prolongs nonviral gene expression in skin. Tissue Eng. 2005;11:546–555. doi: 10.1089/ten.2005.11.546. [DOI] [PubMed] [Google Scholar]
- 17.Shin S, Shea LD. Lentivirus immobilization to nanoparticles for enhanced and localized delivery from hydrogels. Mol Ther. 18:700–706. doi: 10.1038/mt.2009.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin S, Tuinstra HM, Salvay DM, Shea LD. Phosphatidylserine immobilization of lentivirus for localized gene transfer. Biomaterials. 31:4353–4359. doi: 10.1016/j.biomaterials.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gobin AS, West JL. Cell migration through defined, synthetic ECM analogs. FASEB J. 2002;16:751–753. doi: 10.1096/fj.01-0759fje. [DOI] [PubMed] [Google Scholar]
- 20.Fisher KE, Pop A, Koh W, Anthis NJ, Saunders WB, Davis GE. Tumor cell invasion of collagen matrices requires coordinate lipid agonist-induced G-protein and membrane-type matrix metalloproteinase-1-dependent signaling. Mol Cancer. 2006;5:69. doi: 10.1186/1476-4598-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makowski GS, Ramsby ML. Differential effect of calcium phosphate and calcium pyrophosphate on binding of matrix metalloproteinases to fibrin: comparison to a fibrin-binding protease from inflammatory joint fluids. Clin Exp Immunol. 2004;136:176–187. doi: 10.1111/j.1365-2249.2004.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah GA, Nair CH, Dhall DP. Physiological studies on fibrin network structure. Thromb Res. 1985;40:181–188. doi: 10.1016/0049-3848(85)90328-7. [DOI] [PubMed] [Google Scholar]
- 23.Shepard JA, Huang A, Shikanov A, Shea LD. Balancing cell migration with matrix degradation enhances gene delivery to cells cultured three-dimensionally within hydrogels. J Control Release. 146:128–135. doi: 10.1016/j.jconrel.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raut SD, Lei P, Padmashali RM, Andreadis ST. Fibrin-mediated lentivirus gene transfer: implications for lentivirus microarrays. J Control Release. 2010;144:213–220. doi: 10.1016/j.jconrel.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei Y, Rahim M, Ng Q, Segura T. Hyaluronic acid and fibrin hydrogels with concentrated DNA/PEI polyplexes for local gene delivery. J Control Release. 2011 doi: 10.1016/j.jconrel.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tokatlian T, Shrum CT, Kadoya WM, Segura T. Protease degradable tethers for controlled and cell-mediated release of nanoparticles in 2- and 3-dimensions. Biomaterials. 2010;31:8072–8080. doi: 10.1016/j.biomaterials.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]