Abstract
Neisseria gonorrhoeae is a common bacterial sexually transmitted infection. Like all Gram-negative bacteria, the outer membrane of the gonococcus is rich in endotoxin, a known ligand for Toll-like receptor (TLR)-4. However, the role of endotoxin and its cognate receptor TLR4 in the mucosal response to acute gonococcal infection in the genital tract of women is unclear. To test this, we examined the course of infection following vaginal inoculation of Neisseria gonorrhoeae in mice carrying the Lpsd mutation in Tlr4, which renders them unresponsive to endotoxin. While there was no difference in the duration of colonization, the Lpsd mice had a significantly higher peak bacterial burden which coincided with a massive polymorphonuclear cell influx and the concomitant upregulation of a subset of inflammatory cytokine and chemokine markers. Notably, infected Lpsd mice showed a decrease in IL-17, suggesting Th17 responses are more dependent on TLR4 signaling in vivo. Defective PMN-mediated and complement-independent serum killing of gonococci in Lpsd mice was also observed and may account for the increased bacterial burden. This is the first in vivo evidence that TLR4-regulated factors modulate the early inflammatory response to gonococcal infection in the female reproductive tract and control bacterial replication.
Keywords: Toll-like receptors, TLR4, Neisseria gonorrhoeae, cytokines, mouse models
INTRODUCTION
Neisseria gonorrhoeae is the second most commonly reported notifiable disease in the United States, and infections due to gonorrhea are associated with the development of pelvic inflammatory disease (PID) in women, which can lead to tubal infertility and chronic pelvic pain 1. In addition, gonococcal infections have been reported to enhance HIV transmission in co-infected individuals 2. Recently released statistics from the Centers for Disease Control (CDC) for 2009 show a record low 301,174 cases of gonorrhea were reported in the United States that year, reflecting about 111 cases per 100,000 people 1. However, while overall rates were decreased from previous years, significant disparities remain with regard to gender and race. In this report the CDC noted 71% of all cases of gonorrhea occurred in African Americans, and African American women age 15 through 19 showed the highest rates of 2,613.8 cases per 100,000 people.
Gonorrhea is almost exclusively transmitted by close sexual contact, and can occur at several mucosal surfaces, including the urethra, cervix, rectum and pharynx. Early events in the establishment of infection involve interactions between gonococci and epithelial cells, which lead to colonization of the mucosal surface, release of local inflammatory mediators, and the recruitment of professional immune cells. The current model for gonococcal pathogenesis in women suggests that N. gonorrhoeae is unable to invade the squamous epithelium of the vagina and ectocervix, but rather colonizes and transmigrates across the columnar epithelium of the uterine endocervix 3. In women, infection with gonorrhea usually remains localized to the lower reproductive tract, where it can induce an inflammatory cervical exudate containing polymorphonuclear cells (PMN) with intracellular gonococci. However, like many sexually transmitted infections in women, gonococcal cervicitis is often described as subclinical or asymptomatic since patients rarely report subjective evidence of increased vaginal discharge or pelvic pain. This can lead to continued transmission between sexual partners and delayed treatment 4. The latter is believed to contribute to the development of PID in a subset of women. PID is a syndrome associated with an acute infection of the upper genital tract structures, including the uterus, oviducts, and ovaries, which can then lead to the complications of tubal infertility and ectopic pregnancy 5, 6.
While in vitro studies have implicated the involvement of several innate immune receptors and pathways in the induction of inflammatory signals during gonococcal infection, none have been able to adequately address the complex interaction of various cell types at the mucosal surface during an in vivo infection in women. To better address this aspect of gonococcal pathogenesis, we developed a female mouse genital tract infection model that mimics several aspects of human infection 7. The model, although limited by a number of host restrictions, has been used successfully to study several aspects of host-pathogen interactions during gonococcal infections. In this study, we used our model to evaluate the role of endotoxin, a key virulence factor for all Gram-negative bacteria, in the pathogenesis of lower genital tract infection with N. gonorrhoeae. Endotoxin is a potent proinflammatory trigger by virtue of its ability to engage the Toll-like receptor (TLR)-4 receptor complex, which is expressed on the surface of a variety of cells including monocytes, macrophages, dendritic cells and PMNs [reviewed in 8]. We examined the course of infection and production of proinflammatory cytokines in mice that are sufficient or deficient in TLR4 signaling by comparing BALB/c mice and BALB/c mice carrying the Lpsd mutation in Tlr4 9. Although there was no difference in the rate of clearance of infection between wild type and Lpsd mutant mice, the colonization load and a number of acute inflammatory markers were significantly lower in the wild type mice. The TLR4 mutant mice displayed an intense inflammatory cytokine and chemokine response in vivo, with a massive PMN influx and a significantly higher peak bacterial burden. The only cytokines that appeared to be down regulated in vivo were IL-17 and related family members, including IL-6, IL-21, and IL-22, suggesting that Th17-mediated responses were dependent on TLR4 signaling events. Interestingly, the induction of proinflammatory cytokines in vitro was largely dependent on TLR4 function, suggesting a more complex interplay of cell types and endogenous flora occurs in vivo. Finally, we observed defective PMN-mediat e d and complement-independent serum killing of gonococci in the TLR4 mutant mice, suggesting a role for TLR4 in mediating antibacterial responses. These data suggest that a TLR4-regulated factor provides a barrier to gonococcal colonization, and that TLR4 plays a negative role in regulating inflammation during gonococcal cervicitis.
RESULTS
TLR4 mutant mice have a higher colonization load of N. gonorrhoeae following vaginal inoculation
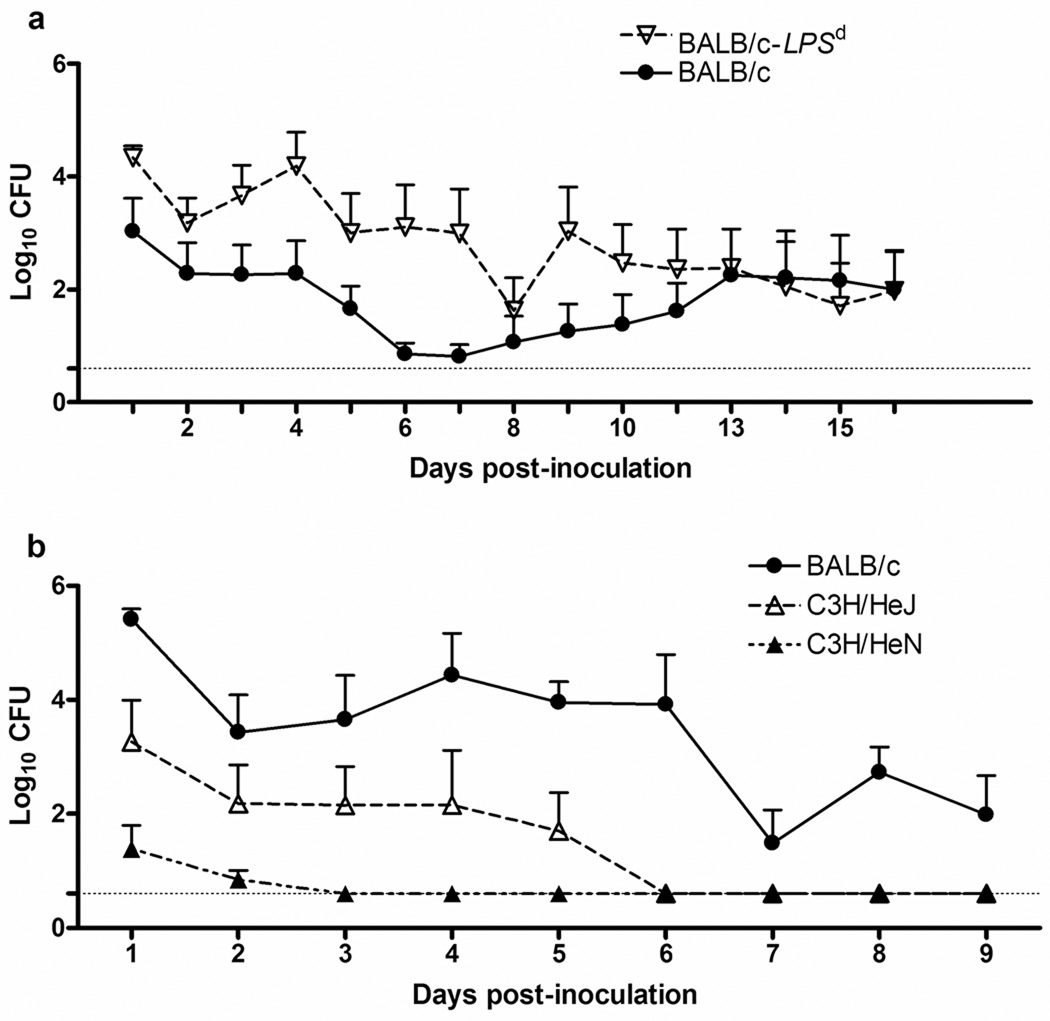
Female BALB/c mice exhibit a significant localized PMN influx in response to gonococcal infection in the lower reproductive tract, and support high levels of colonization over the course of infection 10, 11. In order to determine the importance of TLR4 signaling during experimental murine infection, we took advantage of the C.C3-tlr4LPS-d/J mouse strain, in which C3H/HeJ mice were back bred onto the BALB/c background to create a congenic BALB/c mouse strain that contains a segment of chromosome 4 which includes the Lpsd allele of Tlr4 9. Thus, these mice, which we will refer to as BALB/c-Lpsd, express the Lpsd mutation in Tlr4 12 and, like the C3H/HeJ parental strain, are unresponsive to LPS. We found no difference in the duration of gonococcal colonization in BALB/c mice (mean, 7.1days; range 0–16) versus the BALB/c-Lpsd mice (mean, 9.3 days; range 2–16). However, as shown in Fig. 1a, a significant difference in the bacterial burden was observed between days 1–10 of infection, with ~1–2 logs more bacteria recovered from the BALB/c-Lpsd mice compared to BALB/c mice. This result suggests that TLR4 is required for control of bacterial replication during infection in the lower reproductive tract of female mice.
FIG. 1. TLR4 controls gonococcal colonization load in both susceptible BALB/c mice and resistant C3H mice.
Mice were inoculated intravaginally with strain FA1090 or PBS as described in the text. The recovery of gonococci from wild type or congenic mutant mice that are homozygous for the tlr4LPS-d/J gene (BALB/c-Lpsd) was determined and is expressed as log10 CFU per 100 µl of vaginal swab suspension (± Standard Error). (a) BALB/c (TLR4 wt; n=8) and BALB/c-Lpsd (TLR4 mutant; n=8); (b) C3H/HeN (TLR4 wt; n=7), C3H/HeJ (TLR4 mutant; n=5), and BALB/c mice (n=6). In each experiment, mice were inoculated intravaginally with 1 × 106 (Fig 1A) or 1 × 107 CFU (Fig. 1B) of N. gonorrhoeae strain FA1090 or PBS, and bacterial recovery was determined at the indicated times. Limit of detection for the assay is Log10 0.6 CFU, and is indicated by the dashed line. *, p ≤ 0.007 (panel A) and p ≤ 0.026 (panel B), as determined by a repeated measures ANOVA. These results are representative of two independent experiments with 4–6 mice per group in repeat experiments.
We recently reported that, in contrast to BALB/c mice, mice of the C3H/HeN strain are resistant to gonococcal colonization 10. We were therefore curious if loss of TLR4-mediated signaling would have any effect on this strain that, otherwise, appeared unable to support gonococcal infection. To test this possibility, we compared the colonization loads of C3H/HeN mice, which are wild type for TLR4, with C3H/HeJ mice, which express the Lpsd mutation in Tlr4 12. A group of BALB/c mice was inoculated in parallel. As expected, C3H/HeN mice were resistant to infection with N. gonorrhoeae (mean duration of recovery, 0.7 day; range 0–2) compared to BALB/c mice (mean duration of recovery, 7.5 days; range 2–9), confirming our previously published data 10. In contrast, the C3H/HeJ mice exhibited a longer duration of infection (2.3 days; range 0–5) and significantly higher colonization load compared to the C3H/HeN mice, although they did not reach the same level of colonization as the BALB/c mice (Fig. 1b). While the basis of resistance in the C3H/HeN mouse strain remains unknown, these data suggest that the innate resistance to gonococcal colonization of this inbred mouse strain can, at least partially, be overcome by the loss of TLR4-mediated signaling events.
TLR4 mutant mice generate an exaggerated inflammatory response to gonococcal infection in vivo
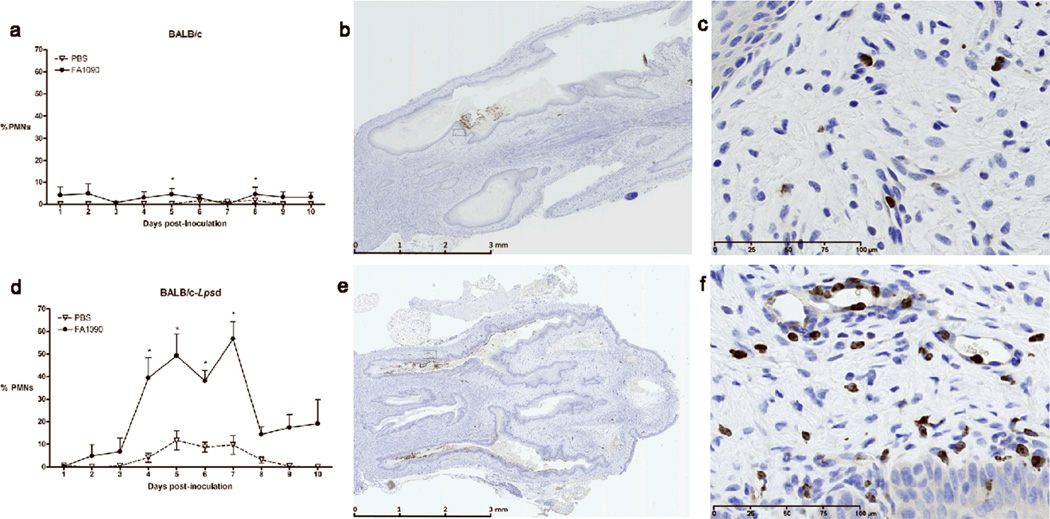
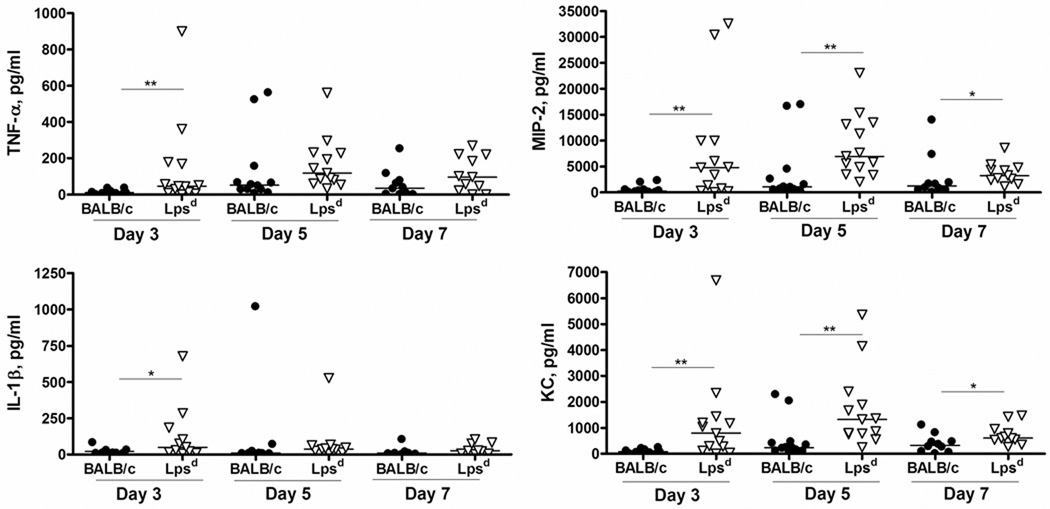
BALB/c mice upregulate vaginal proinflammatory cytokines and chemokines in response to infection with N. gonorrhoeae, the expression of which peaks on day 5 and coincides with a concomitant PMN influx. We hypothesized that the inability to control early gonococcal replication might reflect inadequate proinflammatory signaling in the absence of TLR4 function, and therefore examined the vaginal milieu for evidence of inflammation by looking for PMNs in vaginal smears and measuring the levels of some classic proinflammatory cytokines and chemokines, specifically IL-1β, TNF-α, KC and MIP-2, in vaginal washes from infected and uninfected mice of each background. To our surprise, we detected significantly higher percentages of vaginal PMNs on days 4–7 of infection in the BALB/c-Lpsd mice compared to wild type BALB/c mice, with as many as 60% PMNs detected in the TLR4 mutant mice that were colonized with high numbers of N. gonorrhoeae (Fig. 2a,d). Higher numbers of PMNs were also detected within genital tract tissues and within the vaginal lumen from TLR4-mutant mice using a granulocyte-specific stain (Fig. 2b–c, e–f). Coincident with the PMN influx, we observed upregulation of IL-1β, TNF-α, KC and MIP-2 in the vaginal washes of infected mice over time, however, the BALB/c-Lpsd mice had significantly higher levels compared the the BALB/c control mice (Fig. 3). This difference was most pronounced for the potent neutrophil chemoattractants MIP-2 and KC, which remained elevated relative to control mice even out to 7 days. Of note, we found that at baseline, uninfected control BALB/c-Lpsd mice had higher levels of proinflammatory cytokines and chemokines compared to uninfected control BALB/c mice, with levels of IL-1β and TNF-α (p ≤ 0.02, day 3) and KC [days 1 (p = 0.01) and 3 (p = 0.04)] significantly different between these two groups (data not shown). These data suggest that the TLR4 mutation might have an effect on basal secretion of proinflammatory mediators that could also affect the threshold of activation by bacterial ligands.
FIG. 2. TLR4 mutant mice have an exaggerated PMN influx in response to gonococcal infection.
Mice were inoculated intravaginally with strain FA1090 or PBS as described in the text, and the vaginal PMN influx was measured by determining the percent of PMNs among 100 vaginal cells in stained vaginal smears. (a,d) PMN count in vaginal smears from infected and uninfected mice. PMN influx in vaginal smears is shown for infected vs. uninfected mice over time for (a) BALB/c and (d) BALB/c-Lpsd mice. Significance was calculated using an unpaired t-test. *, There was a significant difference between infected vs. uninfected strains, with p ≤ 0.05. These results are representative of two independent experiments. (b–c, e–f) Immunohistochemical analysis of PMN influx into the lower genital tract. Genital tract tissue was extracted from infected BALB/c and BALB/c-Lpsd mice mutant mice on day 7 of infection and stained with Gr-1-specific antibodies to detect PMNs, as described in the text. Shown above are representative images for infected (b,c) BALB/c and (e,f) BALB/c-Lpsd mice. Original magnification: 100 × (b, e) and 400X (c, f).
FIG. 3. TLR4 mutant mice upregulate inflammatory cytokines and chemokines in response to gonococcal infection.
Mice were infected as described in the text, and vaginal washes were obtained over time for assay of IL-1β, TNF-α, MIP-2 and KC by custom multiplex assay. Shown above are data pooled from three independent experiments. Each data point represents an individual mouse; n=10–12 for BALB/c, n=11–13 for BALB/c-Lpsd. The horizontal line represents the median value for each time point. Statistics were calculated using a Mann-Whitney U-test, with p values as follows: *, p< 0.05; **, p< 0.01.
Cells from TLR4 mutant mice are hyporesponsive to N. gonorrhoeae when stimulated in vitro
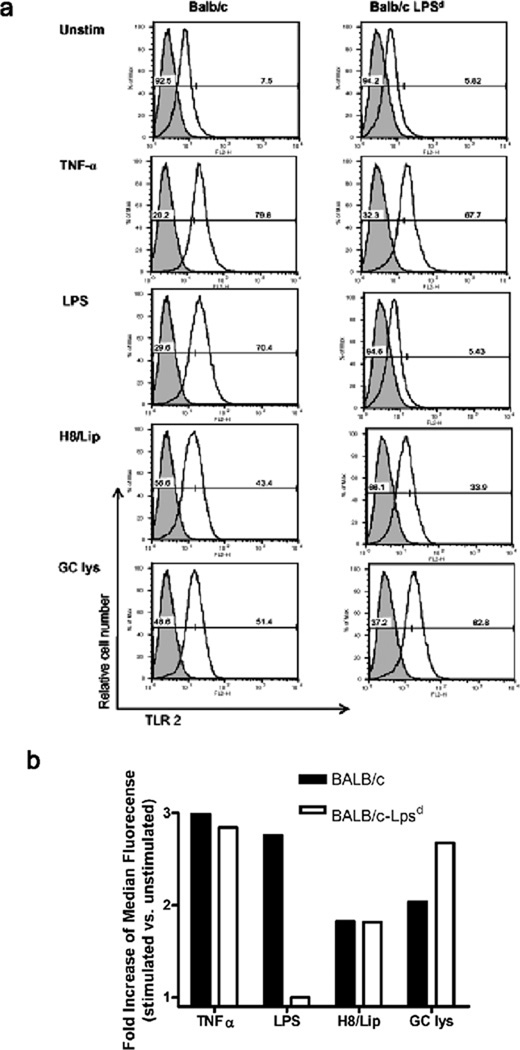
One possible explanation for the elevated inflammatory markers in the BALB/c-Lpsd infected mice was that they were hyperresponsive to the TLR4-independent bacterial ligands encountered during infection, such as bacterial lipoproteins and porin, that activate TLR2. In order to determine if the BALB/c-Lpsd mice had altered expression of TLR2 relative to the wild type BALB/c mice, we looked for evidence of TLR2 on the surface of bone marrow derived macrophages (BMDM) by flow cytometry, and tested whether protein expression was altered in response to treatment with N. gonorrhoeae or other TLR ligands. As shown in Fig. 4, we found that both strains of mice expressed similar levels of TLR2 on the surface, which were upregulated in response to exposure to TNF-α, the synthetic triacylated gonococcal lipopeptide H.8/Lip 13 and to lysates of N. gonorrhoeae FA1090. Predictably, only the BALB/c strain upregulated TLR2 in response to LPS, while BALB/c-Lpsd did not. These data suggest that TLR2 expression in the BALB/c-Lpsd strain is not significantly altered compared to the BALB/c mice.
FIG. 4. Expression of surface TLR2 does not differ between BALB/c and TLR4 mutant mouse strains.
BMDM were derived from BALB/c and BALB/c-Lpsd mice as described in the text. Cells were stained for surface expression of TLR2 either at rest or after 24 hours treatment with the agents noted above. (a) Shown above are FACS histograms for demonstrating staining using either the specific anti-TLR2 mAb (black line) or a matched isotype control (gray shading). The vertical axis represents the relative cell number, while the horizontal axis represents the intensity of fluorescence in the FL2 channel. The percentage of cells that are either negative or positive for TLR2 is shown for each histogram. (b) The bottom graph shows the median fluorescence as calculated from the histograms for each condition. Treatments were as follows: unstim, unstimulated cells; TNF-α, 40 ng/ml; LPS, 100 ng/ml; H8/Lip, 1 µg/ml; GC lys (N. gonorrhoeae FA1090 crude lysates) MOI 10:1. These histograms are representative of two independent experiments.
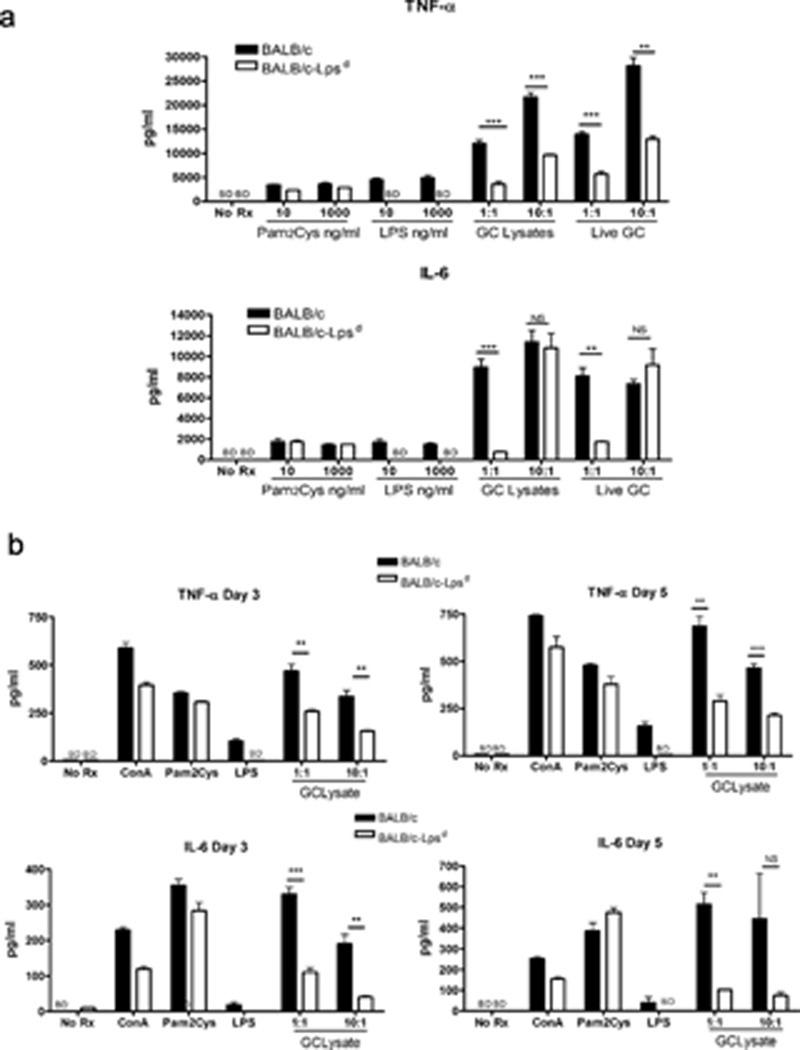
In order to determine if cells derived from BALB/c and BALB/c-Lpsd mice responded similarly to TLR ligands and N. gonorrhoeae, we next examined the response of BMDM (Fig. 5a) and splenic mononuclear cells (Fig. 5b) in vitro. We found that cells from both mouse strains responded similarly to a TLR2 ligand, the synthetic lipopeptide Pam2-Cys-SK4 in terms of upregulation of TNF-α and IL-6, while cells from the BALB/c-Lpsd only were unresponsive to the TLR4 ligand, LPS. However, when exposed to live N. gonorrhoeae or crude gonococcal lysates, cytokine induction was reduced in the BALB/c-Lpsd mice compared to the BALB/c mice, although not to baseline. These results confirm that the response to N. gonorrhoeae, at least by mononuclear cells, is primarily driven by TLR4 but that TLR4-independent cell activation can occur. This finding is similar to what we previously reported with the related pathogen N. meningitidis 14. We conclude from these in vitro studies that cells derived from mice carrying the Lpsd mutation in TLR4 are not hyperresponsive to TLR2 ligands or N. gonorrhoeae in vitro.
FIG. 5. Cells derived from TLR4 mutant mice are hyporesponsive to stimulation with N. gonorrhoeae.
(a) BMDM or (b) splenic mononuclear cells were derived from BALB/c and BALB/c-Lpsd mice as described in the text, and treated with the indicated agents. Supernatant was collected at 24 hours for (a) and at the indicated time points for (b), and assayed for TNF-α or IL-6 by ELISA. Pam2Cys, lipopeptide Pam2-Cys-Ser-Lys4; GC lys, N. gonorrhoeae FA1090 crude lysates. Significance was calculated using an unpaired t-test, with p values as follows: **, p<0.01; ***, p<0.001; NS, not significant; BD, below detection. These data are representative of two independent experiments.
Serum and PMNs from TLR4 mutant mice are defective in killing N. gonorrhoeae in vitro
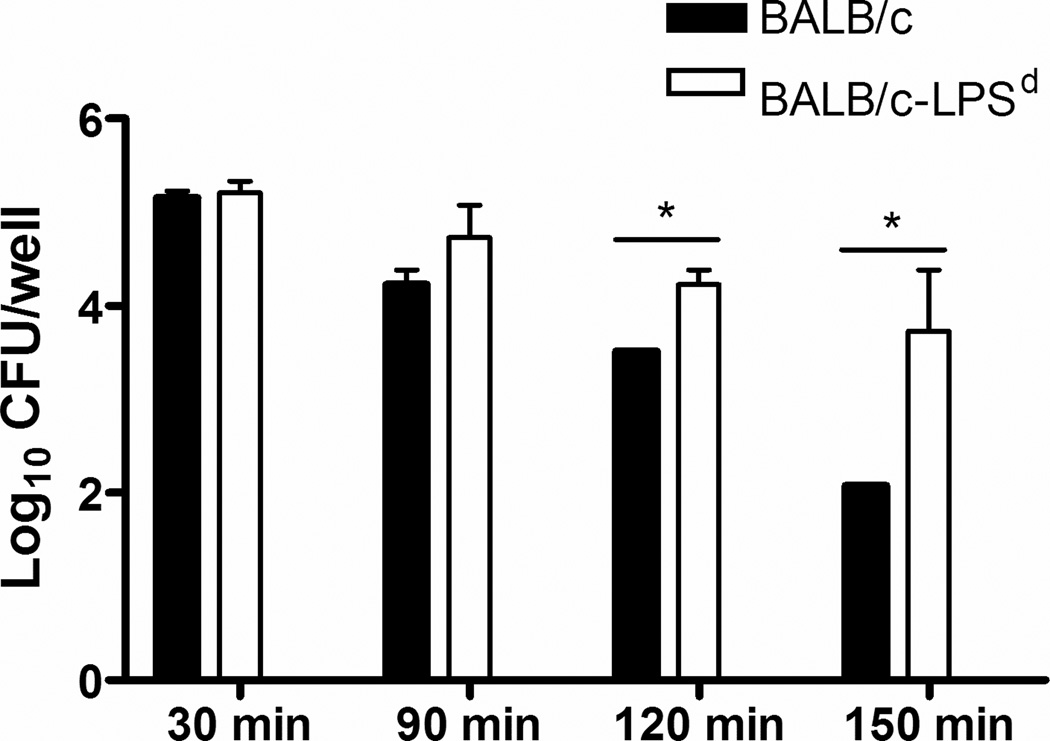
The inability of BALB/c-Lpsd mice to control infection in the presence of a marked inflammatory response was intriguing, and we therefore compared the capacity of serum and PMNs from normal BALB/c and BALB/c-Lpsd mice to kill N. gonorrhoeae. For serum killing assays, we used heat-inactivated (HI) sera in order to examine complement-independent factors. Incubation of FA1090 bacteria with HI-serum from BALB/c mice at a final concentration of 20% resulted in a 1–2 log decrease in recovery after 120 and 150 min incubation, compared to the number of gonococci recovered from HI-serum from BALB/c-Lpsd mice (Fig. 6).
FIG. 6. Heat-stable killing activity of mouse serum is greater in wild type mice compared to Lpsd mutant mice.
The number of viable bacteria was determined following incubation with HI-serum from BALB/c versus BALB/c-Lpsd mice at 30, 90, 120 and 150 min post inoculation. HI-serum from BALB/c mice killed FA1090 bacteria more efficiently when compared to HI-serum from BALB/c-Lpsd. Both sera had killing activity when compared to the buffer control. Log10 CFU recovered from no serum controls at any time point ranged from 5.07–5.54 (data not shown). * p < 0.05, unpaired t-test. These data are representative of four independent experiments.
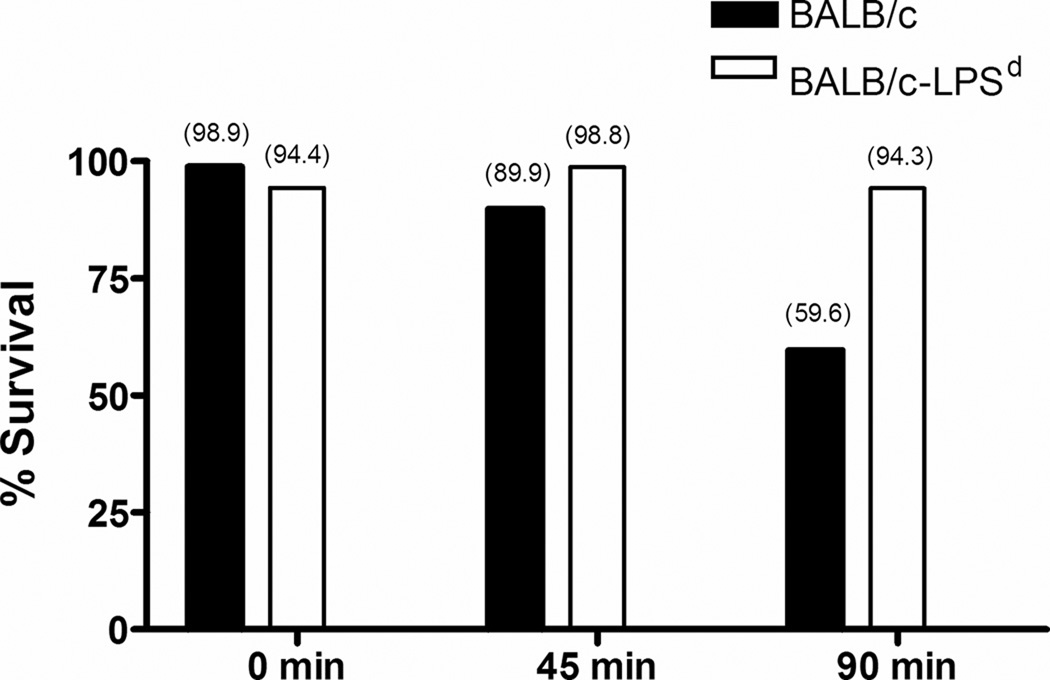
To test the relative capacity of TLR4-sufficient and -deficient PMNs to kill bacteria, gonococci were serum-opsonized and incubated with PMNs from BALB/c versus BALB/c-Lpsd mice, as described in the Methods. Although the percentage of serum used in these assays (10%) was lower than that used in the serum killing assays (20%) and shown to not significantly kill N. gonorrhoeae at 90 min, serum from normal BALB/c mice was used to opsonize gonococci for phagocytic uptake by both normal and TLR4-defective PMNs to control the potential differences in the killing activity of sera from the two mouse strains at this lower concentration. Recovery of N. gonorrhoeae following incubation with PMNs from BALB/c mice was 40% reduced relative to the HI-NMS control (Fig. 7). In contrast, PMNs from BALB/c-Lpsd mice, which were tested in parallel, showed no evidence of being able to kill N. gonorrhoeae under at the same time point. We conclude that both cellular and heat stable soluble factors may contribute to the inability of BALB/c-Lpsd mice to clear infection despite the induction of an intense inflammatory response.
FIG. 7. PMNs from BALB/c-Lpsd mice are defective for killing N. gonorrhoeae.
Gonococci were opsonized with 10% serum from BALB/c mice and then incubated with PMNs from normal and BALB/c-Lpsd mice. The number of gonococci recovered following 0, 45 and 90 min incubation is expressed as the percentage of bacteria recovered from PMNs incubated with bacteria that were pre-incubated in HI-serum. Absolute values are provided in parentheses above the bars. These data are representative of two independent experiments, where reduced recovery of N. gonorrhoeae occurred after 90 min incubation with BALB/c PMNs only (59.6% and 52.9% for each experiment).
Th17 responses are upregulated in wild type BALB/c mice but not in Lpsd mutants
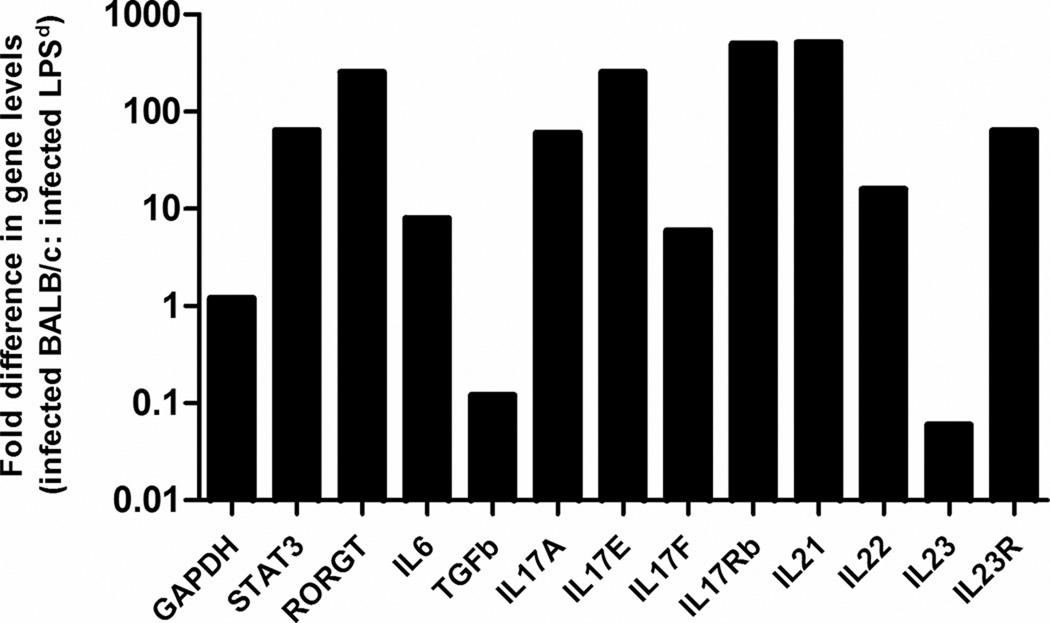
We previously reported that IL-17 responses are induced and are protective during experimental murine gonococcal infection 15. We therefore examined cytokines, receptors and transcription factors known to be critical for the induction of a Th17 response to determine whether IL-17 responses were absent or less pronounced in TLR4 mutant mice. As shown in Fig. 8, transcripts for RORGT, STAT3, IL-6, IL-17A, IL-17E, IL-17F, IL-17 RB, IL-21 and IL-22 were highly upregulated in infected wild type BALB/c mice compared to BALB/c-Lpsd mice by relative real-time PCR, suggesting that Th17 responses were more dependent on TLR4 signaling in vivo. Thus, while many of the classic inflammatory markers were elevated in the TLR4 mutant mouse, those involved in the IL-17 response were higher in wild type mice. This result is consistent with the ability of wild type mice to better control gonococcal replication and is also suggestive that Th17 responses were more dependent on TLR4 signaling.
FIG. 8. Th17 type cytokine production is impaired in the TLR4 mutant mice.
Relative expression of GAPDH, RORGT, STAT3, IL-6, IL-17A, IL-17E, IL-17F, IL-17 RB, IL-21, IL-22, IL-23A and IL-23R following intravaginal inoculation with FA1090. Total RNA was extracted from vaginal washes and subjected to relative real-time PCR, as described in the text. A relative change of 2-fold or greater between infected BALB/c vs. infected BALB/c-Lpsd mice was considered significant. These results are representative of two independent experiments.
DISCUSSION
The role of Neisseria endotoxin in the pathogenesis of meningococcal sepsis is generally accepted, and endotoxin levels in the blood stream correlate with clinical presentation, activation of complement, coagulopathy and plasma TNF-α and IL-6 levels 16–20. In contrast, the role of endotoxin in the pathogenesis of gonococcal infection, which is most often a localized mucosal infection, is less clear. Several reports with cultured human cells that suggest that the columnar epithelium of the endocervix is deficient in expression of the endotoxin receptors TLR4 and MD2, leading us to hypothesize that epithelial cell responses to gonococci are primarily driven by TLR2, which recognizes bacterial lipoproteins and neisserial porin 21, 22. However, there is the caveat that in vivo there could be alterations in TLR4 levels as a result of hormonal influences and endogenous flora 21, 23, 24. Moreover, TLR4 signaling could play a role in the pathogenesis of gonococcal cervicitis independently of epithelial cells, since professional phagocytic cells, including neutrophils, macrophages, and dendritic cells, are present in the lumen as well as the submucosal space of the lower genital tract, and they express the TLR4 receptor complex and are exquisitely sensitive to endotoxin. Thus, an in vivo model is required to test the role of LOS and TLR4 signaling during gonococcal infection in order to examine the cooperation between multiple cell types and the panel of receptors they express.
Our approach, using the BALB/c mouse strain expressing the Lpsd mutation in TLR4, allowed us the opportunity to test the role of gonococcal endotoxin during lower reproductive tract infection in a susceptible surrogate female host. We found a significantly higher colonization load in the TLR4 mutant mice compared to the wild type mice, which correlated with significantly higher PMN influx. As the in vitro data demonstrate, N. gonorrhoeae is capable of inducing TNF-α and IL-6 even in the absence of TLR4 signaling. This is likely a result of signaling via TLR2 and possibly other innate immune receptors, and may therefore explain the ability of the TLR4 mutant mice to upregulate inflammatory cytokines and chemokines in the lower genital tract. However, while both mouse strains mounted an inflammatory cytokine response to gonococcal infection, the infected BALB/c-Lpsd mice developed a markedly exaggerated chemokine and PMN response when compared to the BALB/c control mice. One possible explanation for this observation rests with the increased bacterial burden that we observed within the same time frame. The presence of more bacteria in the genital tract may drive a more exaggerated induction of the potent neutrophil chemoattractants, MIP-2 and KC, leading to a more pronounced PMN influx. In fact, the timing of the PMN peak coincided with the increased bacterial colonization that we observed, which is consistent with excessive bacterial replication driving the induction of proinflammatory mediators via TLR4-independent pathways. Of note, while IFN-γ has been reported to suppress both MIP-2 and mouse KC secretion 25, 26, we found no detectable levels of IFN-γ in either mouse strain following inoculation with N. gonorrhoeae. Thus, the explanation for the increased chemoattractants is not a result of differential IFN-γ production in the two genetic backgrounds. Additional factors may have contributed to the massive number of PMNs detected in the genital tract in the TLR4 mutant mice. For example, we cannot exclude an additional role for TLR4 signaling in either the induction of PMN apoptosis or the clearance of apoptotic PMNs by activated macrophages.
The more interesting question then becomes, why did the TLR4 mutant strain fail to control early bacterial replication, especially in light of the massive PMN influx that we observed? The role of PMNs in controlling gonococcal replication and clearance is a topic of debate in the field of gonococcal pathogenesis. The hallmark of gonorrhea is the appearance of Gram-negative diplococci within PMNs in genital secretions 27–29, but it remains controversial if these intracellular bacteria are replicating or in the process of being killed. One of the earliest reports to promote the idea that gonococci are rapidly killed within PMNs was by Watt and colleagues 30, and numerous subsequent studies supported this model 31–33. However, Casey et al. later reported that while the majority of intracellular gonococci within PMNs are killed by O2-independent antimicrobial systems, a small subset of intracellular bacteria, approximately 2%, are able to survive and replicate 34. Similar results were found by Simons 35 and Criss 36, although the percent of surviving bacteria differs from study to study. Our observation that PMNs from TLR4 mutant mice are less able to kill gonococci over time suggests that TLR4 activation is required for PMNs to limit bacterial replication. Moreover, our data that heat-inactivated serum from TLR4 mutant mice is also defective in bacterial killing suggests that there are additional complement-independent antibacterial factors found in serum that are dependent on TLR4 expression.
Reports on the innate immune responses to other infectious processes have similarly revealed an important role for TLR4 signaling in controlling bacterial burden. For example, Montminy et al. demonstrated that the absence of TLR4 activation by the hypoacylated lipid A of Yersinia pestis directly contributed to mortality 37. When Y. pestis was engineered to produce a more biologically active hexaacylated form of lipid A, the pathogen was completely avirulent via the subcutaneous route, demonstrating that evasion of TLR4-dependent innate immune activation by the pathogen was a virulence mechanism. Similarly, TLR4 mutant mice were found to be incapable of controlling or eradicating Gram-negative respiratory infections secondary to Klebsiella pneumoniae 38, Haemophilus influenzae 39, and Pasteurella pneumotropica 40, 41. In the same manner, we would argue that early activation of TLR4-dependent responses during the mucosal challenge of mice with N. gonorrhoeae is an important protective mechanism by the host. Evidence that removal of this essential host defense mechanism was able to partially compensate for the innate resistance of the C3H/HeN mouse strain to gonococcal colonization lends further support to this model.
One additional mechanism behind the important role of TLR4 in controlling gonococcal infection in the mouse model rests with the link between TLR4 and IL-17. Our recent report in Feinen et al. implicates a role for IL-17 and the Th17 axis in bacterial clearance during gonococcal infection in the mouse model, as the duration of colonization was almost doubled in the IL-17 receptor deficient mouse strain compared to control mice 15. Furthermore, it was reported that IL-17 induction by spleen cells from TLR4 mutant C3H/HeJ mice was markedly abrogated in response to N. gonorrhoeae compared to TLR4 wild type mice. Our current data further support a role for TLR4 in the induction of IL-17, as we again observed a reduction in IL-17 and related cytokines in the absence of TLR4 signaling. This finding is consistent with other reports that suggest TLR4 helps to direct the induction of Th-17 cells during infections 42–44.
While our data suggests a role for TLR4 in controlling the acute bacterial burden and regulating the inflammatory response, the effect was most pronounced during the time period that corresponds with the host inflammatory response (days 5–7) and was not seen at later time points. In wild type mice, a 4–5 day period of reduced recovery began on day 5, which was followed by an increase in the number of gonococci recovered. This pattern is typical of of the colonization kinetics of N. gonorrhoeae strain FA1090 in this model 7. Gonococcal colonization of estradiol-treated female mice is cyclical and appears to be hormonally regulated as periods of reduced recovery are not observed in ovariectomized mice 45. As TLR4 has been shown to be hormonally regulated 46, 47, we hypothesize that the protective effect of TLR4-mediated responses may also be cyclical. It is difficult to dissect the reason for clearance of N. gonorrhoeae in the model used here since the need for exogenously delivered estradiol to promote susceptibility to long-term N. gonorrhoeae infection in mice must also be considered. When water-soluble estradiol is used, as in this study, clearance of infection occurs at an average of 10–12 days post-inoculation and coincides with the resumption of the estrous cycle 11, and administration of additional doses of estradiol later in infection sustains colonization (A.E. Jerse, unpublished observation). The reason that estradiol is required for infection is not known, other than it synchronizes mice into a proestrus-like state on the day of bacterial challenge, which is the most susceptible stage for experimental N. gonorrhoeae infection in mice 48 and maintains an estrus-like state based on the presence of predominantly epithelial cells in vaginal smears. Clearance of infection upon resumption of the reproductive cycle may have an immunological basis, however, that involves one or more innate defenses associated with the post-ovulatory stage, which, based on similar rates of clearance of infection in wild type and Lpsd mutant mice, we would predict to be TLR4-independent. Effects mediated by TLR2 and other innate immune receptors, and amplification of the immune response by cytokine feedback mechanisms, thus appears to make the mice fully capable of ultimately eradicating the pathogen.
Another limitation of the model that should be considered when interpreting these data is the host restriction for several receptors utilized by N. gonorrhoeae to adhere to and invade epithelial cells. These receptors include the carcinoembryonic adherence molecules, which are the major class of opacity protein receptors 49 and the human CR3 molecule 50, which while having a high degree of similarity to that of mice, is likely to be host-restricted in that a monoclonal antibody that blocks gonococcal invasion of human cells through this receptor did not stain genital tract tissue from infected mice (unpublished data in collaboration with Drs. Jennifer Edwards and Michael Apicella). The human membrane cofactor protein (CD46) is also host restricted and hypothesized by some to serve as the neisserial pilus receptor 51. In spite of these restrictions, gonococci do adhere to murine epithelial cells via unknown ligand/receptor interactions 7, and because TLR4 is on the surface of cells, gonococcal invasion of epithelial cells through host-restricted pathways would not be required for TLR4-mediated signaling. Therefore the significance of TLR4 signaling for infection as shown here, is unlikely to be altered by the absence of these receptors.
In conclusion, this study reveals a role for TLR4 signaling in the pathogenesis of gonococcal cervicitis. The induction of proinflammatory cytokines and chemokines, as well as similar rates of clearance of the infection over the same period of time regardless of TLR4 expression, speaks to redundancy in the innate immune system whereby alternate receptors are capable of alerting the host to the danger of an invading pathogen. However, the inability of the host to control early bacterial replication during the first week of infection, and the concomitant massive PMN influx, suggests an important role for TLR4 in controlling gonococcal replication during this period, specifically via the antibacterial activity of PMNs and soluble complement-independent serum factors. It remains to be seen what impact TLR4 signaling will have in the upper reproductive tract, where its expression may be higher, and we hope to examine the role of TLR4 in additional experimental systems in future studies.
MATERIALS AND METHODS
Reagents
Ultrapure LPS purified from E. coli serotype O111:B4 was purchased from List Pharmaceuticals (Woburn, MA); the synthetic lipopeptides, Neisseria lipoprotein H8/Lip and Pam2-Cys-Ser-Lys4, were purchased from EMC Microcollections (Tuebingen, Germany).
Bacterial strains and culture conditions
N. gonorrhoeae FA1090 (streptomycin-resistant, serum resistant) is a well characterized serum resistant porB1B strain 52. Bacteria were cultured on GC agar with Kellogg’s supplements and FeNO3 at 37°C under 7% CO2 as described 7. GC agar with vancomycin, colisitin, nystatin, trimethoprim sulfate, and streptomycin (GC-VCNTS agar) was used for mouse infection experiments 7. For in vitro stimulation assays, crude whole cell gonococcal lysates were made by subjecting the bacterial suspensions to a freeze-thaw cycle at −80°C, followed by vigorous vortexing.
Experimental murine infection
Two sets of mouse strains that are congenic except for the Trl4 gene were tested: BALB/cJ (TLR4 wt) and C.C3-tlr4LPS-d/J (TLR4 mutant), from Jackson Laboratories; and C3H/HeN (TLR4 wt) and C3H/HeJ (TLR4 mutant), from National Cancer Institute. For simplicity, the C.C3-tlr4LPS-d/J mouse strain is abbreviated BALB/c-Lpsd. For all experiments, female mice were purchased at 4–6 weeks of age and allowed to acclimate to the animal facility for 10 days. Mice were then treated with water-soluble 17β-estradiol (Sigma) subcutaneously on day −2, day 0 and day 2 and antibiotics (2.4 mg streptomycin sulfate and 0.4 mg vancomycin twice daily via intraperitoneal injection and 0.04 g trimethoprim sulfate per 100 ml drinking water) were administered starting on day −2 through day 10 to promote long-term colonization with N. gonorrhoeae as described 11. For experiments with BALB/c and BALB/c-Lpsd mice, mice were inoculated intravaginally with 1 × 106 colony forming units (CFU) of N. gonorrhoeae strain FA1090, which is a dose that results in infection of 80% of BALB/c mice, or PBS. For experiments with BALB/c, C3H/HeN, and C3H/HeJ mice, a dose of 107 CFU was used. Following inoculation, the number of gonococci recovered from vaginal mucus was quantitatively cultured daily for 10 or 16 days. Vaginal smears were prepared at each time point and stained with a modified Wright stain 7 and the percentage of PMNs per 100 vaginal cells was determined by cytological differentiation under a light microscope. For immunohistochemical staining of tissue for granulocytes, whole genital tracts were harvested from three mice per group on day 7 post-inoculation with N. gonorrhoeae or PBS. Tissues were fixed, sectioned and stained for immunohistochemical analysis by Histoserv, Inc. (Germantown, MD) as described 53 using Anti-Gr1 (1:100) (BD Pharmagen, catalog number 550291) and anti-rat IgG-horse radish peroxidase as the primary and secondary antibodies, respectively. Sections were viewed under a light microscopy and images were scanned using Nanozoomer Digital Pathology (NDP) software (Olympus, USA). All mouse infection experiments were performed at least twice. All protocols were approved by the USUHS and the BUMC Institutional Animal Care and Use Committees.
Chemokine and cytokine measurements
Vaginal washes from wild type and TLR4 mutant mice used in the infection experiments were collected on days 1, 3, 5, and 7 by gently pipetting 50 µl of PBS in and out of the vagina 20 times as described 54. The lavage fluid was then centrifuged at 13,000 × g for 3 min. The supernatant was frozen immediately and stored at −70°C for further analysis. Levels of IL-1β, TNF-α, KC and MIP-2 proteins were measured using a custom Milliplex multiplex assay (Millipore, Billerica, MA). Relative real-time PCR method was used to assess gene expression levels between infected wild type and TLR4-deficient mice as described 10. Briefly, total RNA was extracted from pooled vaginal samples using Qiagen mini RNAeasy isolation kits and 100–500 ng of total extracted RNA were then treated with genomic elimination mixture (SAbiosciences, Frederick, MD). Complementary cDNA was synthesized by adding reverse transcriptase cocktail (SABiosciences) to the DNAse-treated RNA and incubated at 42° C for exactly 15 min, after which the reaction was stopped by heating at 95°C for 5 min. Oligonucleotide primers used to measure Th17 cytokines groups were purchased from SABiosciences. A relative change of 2-fold or greater between infected BALB/c vs. infected BALB/c-Lpsd mice was considered significant.
Preparation of bone marrow derived macrophages and splenic mononuclear cells
Bone marrow derived macrophages (BMDM) from BALB/c and BALB/c-Lpsd mice were prepared as follows. Briefly, femurs and tibiae were dissected from female mice aged 6–8 weeks, bone marrows were flushed, and after lysis of red blood cells, cells were cultured in RPMI 1640 supplemented with 10% FBS, 20 µg/ml of gentamicin, 10 µg/ml of ciprofloxacin, and 20% (v/v) of L929 condition medium (containing M-CSF). The cells were incubated at 37°C, 5% CO2 incubator for 7–9 days to allow macrophage differentiation, and removed from ciprofloxacin at least three days prior to infection with N. gonorrhoeae. Differentiated BMDM were plated in 96 well plates (5×104 cells/well) and subsequently stimulated. Supernatants were collected after 24 hours and analyzed for cytokines using commercial ELISA kits for TNF-α (eBioscience; San Diego, CA) and IL-6 (R&D Systems; Minneapolis, MN). Splenic mononuclear cells were prepared as follows. Spleens were removed from mice and spleen cells were prepared. After red blood cell lysis, splenocytes were plated in 24 well plates (1×106 cells/well) in RPMI 1640 supplemented with 10% FBS, 20 µg/ml of gentamicin, 10 µg/ml of ciprofloxacin. Supernatants were collected 3 and 5 days after stimulation and analyzed for cytokines by ELISA as described above.
Serum killing assay
Venous blood was obtained from wild type BALB/c and BALB/c-Lpsd mice by retro-orbital bleeding and the serum separated by centrifugation. Sera were heat-inactivated at 56°C for 30 min. Strain FA1090 was suspended in PBSGCM and passed through 1.2 micron filters to remove bacterial aggregates and adjusted to an optical density of 0.07 at 600 nm and diluted 1:10. Twenty µl (105 CFU) of the bacterial suspension were added to heat-inactivated serum (HI-serum) from BALB/c and BALB/c-Lpsd mice or buffer (no serum control) in complete Hanks balanced salt solution (HBSS buffer with 10 mM glucose, 0.1% gelatin, 1mM CaCl2 and 1mM MgCl2). Final serum concentration was 20% in a final assay volume of 300 µl. After 30, 90, 120 and 150 min of incubation at 37°C, 10 µl of samples were serially diluted in GC broth with 0.05% saponin and cultured on GC agar. The number of colonies from triplicate wells was counted after overnight incubation and the data are expressed as the average log10 CFU/ml.
PMN killing assay
PMNs were elicted from wild type BALB/c and BALB/c-Lpsd mice via peritoneal lavage and the capacity of PMNs to kill N. gonorrhoeae was measured using a modification of the tumbling tube assay as described previously 55. Serum (10%) from normal BALB/c mice (NMS) was used to opsonize the bacteria to control for differences in the killing activity of sera from these two mouse strains. Bacteria that were pre-incubated with HI-NMS, which we previously found does not opsonize gonococcal for PMN uptake 55, 56 were incubated with PMNs in parallel to provide a baseline for loss of bacterial viability during the assay. The number of viable gonococci recovered from each test condition was determined after 0, 45 and 90 min incubation by quantitative culture. The averages of triplicate cultures were calculated and results are expressed as the percent survival [100 × (number of NMS-opsonized CFU recovered at 90 min divided by number of HI-NMS opsonized CFU)].
Flow cytometric analysis
Differentiated BMDM from BALB/c and BALB/c-Lpsd mice were prepared as above. After stimulation under different conditions for 24 hrs, cells were collected and stained with a phycoerythrin (PE) conjugated anti-TLR2 antibody (eBioscience, Inc., San Diego, CA; cat# 12-9021-82) and a biotinylated antibody for the macrophage marker F4/80 (BioLegend, San Diego, CA; cat#122603) plus streptovidin-PE-Cy5 (BD Biosciences, San Jose, CA; cat#554062). Cells were analyzed by flow cytometry using a FACScan microfluorimeter (Becton Dickinson, San Jose, CA). A total of 10,000 events were counted for each condition.
Statistical analysis
The average duration of recovery and colonization load over time were compared between groups by repeated measures analysis of variance (ANOVA) with Bonferroni correction using SPSS software. The influx of PMNs in the infected group on any day was compared to its uninfected group by unpaired t-test. Levels of vaginal cytokines and chemokines as measured by multiplex assay were analyzed using a Mann-Whitney U-test with 95% confidence intervals. For the in vitro stimulation assays, each data point was assayed in triplicate, and graphed as the mean +/− standard error, and compared using an unpaired t-test. GraphPad software was used for all other statistical tests.
Acknowledgments
This work was supported by NIH/NIAID grants R01 AI42053 and U19 AI31496 (to A.E.J.), and R01 AI46613 and U19 AI084048 (to R.R.I.).
Footnotes
DISCLOSURE: The authors declare no conflict of interest with this work.
REFERENCES
- 1.CDC. Atlanta: U.S. Department of Health and Human Services; 2010. [Google Scholar]
- 2.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards JL, Apicella MA. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin Microbiol Rev. 2004;17:965–981. doi: 10.1128/CMR.17.4.965-981.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hook EW, Handsfield HH. In: Sexually Transmitted Diseases. Holmes KK, et al., editors. New York: McGraw-Hill Companies, Inc; 1999. pp. 451–466. [Google Scholar]
- 5.Cates W, Jr, Rolfs RT, Jr, Aral SO. Sexually transmitted diseases, pelvic inflammatory disease, and infertility: an epidemiologic update. Epidemiol Rev. 1990;12:199–220. doi: 10.1093/oxfordjournals.epirev.a036054. [DOI] [PubMed] [Google Scholar]
- 6.Westrom L. Incidence, prevalence, and trends of acute pelvic inflammatory disease and its consequences in industrialized countries. Am J Obstet Gynecol. 1980;138:880–892. doi: 10.1016/0002-9378(80)91077-7. [DOI] [PubMed] [Google Scholar]
- 7.Jerse AE. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect Immun. 1999;67:5699–5708. doi: 10.1128/iai.67.11.5699-5708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palsson-McDermott EM, O'Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogel SN, et al. Construction of a BALB/c congenic mouse, C.C3H-Lpsd, that expresses the Lpsd allele: analysis of chromosome 4 markers surrounding the Lps gene. Infect Immun. 1994;62:4454–4459. doi: 10.1128/iai.62.10.4454-4459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Packiam M, Veit SJ, Anderson DJ, Ingalls RR, Jerse AE. Mouse strain-dependent differences in susceptibility to Neisseria gonorrhoeae infection and induction of innate immune responses. Infect Immun. 2010;78:433–440. doi: 10.1128/IAI.00711-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song W, et al. Local and humoral immune responses against primary and repeat Neisseria gonorrhoeae genital tract infections of 17beta-estradiol-treated mice. Vaccine. 2008;26:5741–5751. doi: 10.1016/j.vaccine.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poltorak A, et al. Genetic and physical mapping of the Lps locus: Identification of the Toll-4 receptor as a candidate gene in the critical region. Blood Cells, Molecules, and Diseases. 1998;24:340–355. doi: 10.1006/bcmd.1998.0201. [DOI] [PubMed] [Google Scholar]
- 13.Fisette PL, Ram S, Andersen JM, Guo W, Ingalls RR. The Lip lipoprotein from Neisseria gonorrhoeae stimulates cytokine release and NF-kB activation in epithelial cells in a TLR2-dependent manner. J Biol Chem. 2003;278:46252–46260. doi: 10.1074/jbc.M306587200. [DOI] [PubMed] [Google Scholar]
- 14.Ingalls RR, Lien E, Golenbock DT. Membrane-Associated Proteins of a Lipopolysaccharide-Deficient Mutant of Neisseria meningitidis Activate the Inflammatory Response through Toll-Like Receptor 2. Infect Immun. 2001;69:2230–2236. doi: 10.1128/IAI.69.4.2230-2236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feinen B, Jerse AE, Gaffen SL, Russell MW. Critical role of Th17 responses in a murine model of Neisseria gonorrhoeae genital infection. Mucosal Immunol. 2010;3:312–321. doi: 10.1038/mi.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandtzaeg P, Joo GB, Brusletto B, Kierulf P. Plasminogen activator inhibitor 1 and 2, alpha-2-antiplasmin, plasminogen, and endotoxin levels in systemic meningococcal disease. Thromb Res. 1990;57:271–278. doi: 10.1016/0049-3848(90)90326-8. [DOI] [PubMed] [Google Scholar]
- 17.Brandtzaeg P, et al. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J Infect Dis. 1989;159:195–204. doi: 10.1093/infdis/159.2.195. [DOI] [PubMed] [Google Scholar]
- 18.Brandtzaeg P, Mollnes TE, Kierulf P. Complement activation and endotoxin levels in systemic meningococcal disease. J Infect Dis. 1989;160:58–65. doi: 10.1093/infdis/160.1.58. [DOI] [PubMed] [Google Scholar]
- 19.Waage A, Brandtzaeg P, Halstensen A, Kierulf P, Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989;169:333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waage A, et al. Local production of tumor necrosis factor alpha, interleukin 1, and interleukin 6 in meningococcal meningitis. Relation to the inflammatory response. J Exp Med. 1989;170:1859–1867. doi: 10.1084/jem.170.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fichorova RN, Cronin AO, Lien E, Anderson DJ, Ingalls RR. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of Toll-like receptor 4-mediated signaling. J Immunol. 2002;168:2424–2432. doi: 10.4049/jimmunol.168.5.2424. [DOI] [PubMed] [Google Scholar]
- 22.Massari P, et al. Cutting edge: immune stimulation by neisserial porins is toll-like receptor 2 and MyD88 dependent. J Immunol. 2002;168:1533–1537. doi: 10.4049/jimmunol.168.4.1533. [DOI] [PubMed] [Google Scholar]
- 23.Herbst-Kralovetz MM, et al. Quantification and comparison of toll-like receptor expression and responsiveness in primary and immortalized human female lower genital tract epithelia. Am J Reprod Immunol. 2008;59:212–224. doi: 10.1111/j.1600-0897.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 24.Pioli PA, et al. Differential expression of Toll-like receptors 2 and 4 in tissues of the human female reproductive tract. Infect Immun. 2004;72:5799–5806. doi: 10.1128/IAI.72.10.5799-5806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohmori Y, Hamilton TA. IFN-gamma selectively inhibits lipopolysaccharide-inducible JE/monocyte chemoattractant protein-1 and KC/GRO/melanoma growthstimulating activity gene expression in mouse peritoneal macrophages. J Immunol. 1994;153:2204–2212. [PubMed] [Google Scholar]
- 26.Tran EH, Prince EN, Owens T. IFN-gamma shapes immune invasion of the central nervous system via regulation of chemokines. J Immunol. 2000;164:2759–2768. doi: 10.4049/jimmunol.164.5.2759. [DOI] [PubMed] [Google Scholar]
- 27.Apicella MA, et al. Modification by sialic acid of Neisseria gonorrhoeae lipooligosaccharide epitope expression in human urethral exudates: an immunoelectron microscopic analysis. J Infect Dis. 1990;162:506–512. doi: 10.1093/infdis/162.2.506. [DOI] [PubMed] [Google Scholar]
- 28.Farzadegan H, Roth IL. Scanning electron microscopy and freeze-etching of gonorrhoeal urethral exudate. Br J Vener Dis. 1975;51:83–91. doi: 10.1136/sti.51.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ovcinnikov NM, Delektorskij VV. Electron microscope studies of gonococci in the urethral secretions of patients with gonorrhoea. Br J Vener Dis. 1971;47:419–439. doi: 10.1136/sti.47.6.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watt PJ. The fate of gonococci in polymorphonuclear leucocytes. J Med Microbiol. 1970;3:501–509. doi: 10.1099/00222615-3-3-501. [DOI] [PubMed] [Google Scholar]
- 31.Gibbs DL, Roberts RB. The interaction in vitro between human polymorphonuclear leukocytes and Neisseria gonorrhoeae cultivated in the chick embryo. J Exp Med. 1975;141:155–171. doi: 10.1084/jem.141.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rest RF, Fischer SH, Ingham ZZ, Jones JF. Interactions of Neisseria gonorrhoeae with human neutrophils: effects of serum and gonococcal opacity on phagocyte killing and chemiluminescence. Infect Immun. 1982;36:737–744. doi: 10.1128/iai.36.2.737-744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas DW, Hill JC, Tyeryar FJ., Jr Interaction of gonococci with phagocytic leukocytes from men and mice. Infect Immun. 1973;8:98–104. doi: 10.1128/iai.8.1.98-104.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casey SG, Shafer WM, Spitznagel JK. Neisseria gonorrhoeae survive intraleukocytic oxygen-independent antimicrobial capacities of anaerobic and aerobic granulocytes in the presence of pyocin lethal for extracellular gonococci. Infect Immun. 1986;52:384–389. doi: 10.1128/iai.52.2.384-389.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simons MP, Nauseef WM, Apicella MA. Interactions of Neisseria gonorrhoeae with adherent polymorphonuclear leukocytes. Infect Immun. 2005;73:1971–1977. doi: 10.1128/IAI.73.4.1971-1977.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Criss AK, Katz BZ, Seifert HS. Resistance of Neisseria gonorrhoeae to non-oxidative killing by adherent human polymorphonuclear leucocytes. Cell Microbiol. 2009;11:1074–1087. doi: 10.1111/j.1462-5822.2009.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montminy SW, et al. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol. 2006;7:1066–1073. doi: 10.1038/ni1386. [DOI] [PubMed] [Google Scholar]
- 38.Branger J, et al. Role of Toll-like receptor 4 in gram-positive and gram-negative pneumonia in mice. Infect Immun. 2004;72:788–794. doi: 10.1128/IAI.72.2.788-794.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, et al. Toll-like receptor 4 mediates innate immune responses to Haemophilus influenzae infection in mouse lung. J Immunol. 2002;168:810–815. doi: 10.4049/jimmunol.168.2.810. [DOI] [PubMed] [Google Scholar]
- 40.Chapes SK, Mosier DA, Wright AD, Hart ML. MHCII, Tlr4 and Nramp1 genes control host pulmonary resistance against the opportunistic bacterium Pasteurella pneumotropica. J Leukoc Biol. 2001;69:381–386. [PubMed] [Google Scholar]
- 41.Hart ML, Mosier DA, Chapes SK. Toll-like receptor 4-positive macrophages protect mice from Pasteurella pneumotropica-induced pneumonia. Infect Immun. 2003;71:663–670. doi: 10.1128/IAI.71.2.663-670.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhan U, et al. Cooperative interactions between TLR4 and TLR9 regulate interleukin 23 and 17 production in a murine model of gram negative bacterial pneumonia. PLoS One. 2010;5:e9896. doi: 10.1371/journal.pone.0009896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Higgins SC, Jarnicki AG, Lavelle EC, Mills KH. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J Immunol. 2006;177:7980–7989. doi: 10.4049/jimmunol.177.11.7980. [DOI] [PubMed] [Google Scholar]
- 44.Jordan JM, Woods ME, Olano J, Walker DH. The absence of Toll-like receptor 4 signaling in C3H/HeJ mice predisposes them to overwhelming rickettsial infection and decreased protective Th1 responses. Infect Immun. 2008;76:3717–3724. doi: 10.1128/IAI.00311-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cole JG, Fulcher NB, Jerse AE. Opacity Proteins Increase Neisseria gonorrhoeae Fitness in the Female Genital Tract Due to a Factor under Ovarian Control. Infect Immun. 2010;78:1629–1641. doi: 10.1128/IAI.00996-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirata T, et al. Expression of toll-like receptors 2, 3, 4, and 9 genes in the human endometrium during the menstrual cycle. J Reprod Immunol. 2007;74:53–60. doi: 10.1016/j.jri.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Yao XD, Fernandez S, Kelly MM, Kaushic C, Rosenthal KL. Expression of Toll-like receptors in murine vaginal epithelium is affected by the estrous cycle and stromal cells. J Reprod Immunol. 2007;75:106–119. doi: 10.1016/j.jri.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 48.Dalal SJ, Estep JS, Valentin-Bon IE, Jerse AE. Standardization of the Whitten Effect to induce susceptibility to Neisseria gonorrhoeae in female mice. Contemp Top Lab Anim Sci. 2001;40:13–17. [PubMed] [Google Scholar]
- 49.Eades-Perner AM, et al. Mice transgenic for the human carcinoembryonic antigen gene maintain its spatiotemporal expression pattern. Cancer Res. 1994;54:4169–4176. [PubMed] [Google Scholar]
- 50.Edwards JL, et al. A co-operative interaction between Neisseria gonorrhoeae and complement receptor 3 mediates infection of primary cervical epithelial cells. Cell Microbiol. 2002;4:571–584. doi: 10.1046/j.1462-5822.2002.t01-1-00215.x. [DOI] [PubMed] [Google Scholar]
- 51.Kallstrom H, Liszewski MK, Atkinson JP, Jonsson AB. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol Microbiol. 1997;25:639–647. doi: 10.1046/j.1365-2958.1997.4841857.x. [DOI] [PubMed] [Google Scholar]
- 52.Cohen MS, et al. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J Infect Dis. 1994;169:532–537. doi: 10.1093/infdis/169.3.532. [DOI] [PubMed] [Google Scholar]
- 53.Vonck RA, Darville T, O'Connell CM, Jerse AE. Chlamydial infection increases gonococcal colonization in a novel murine coinfection model. Infect Immun. 2011;79:1566–1577. doi: 10.1128/IAI.01155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cone RA, et al. Vaginal microbicides: detecting toxicities in vivo that paradoxically increase pathogen transmission. BMC Infect Dis. 2006;6:90. doi: 10.1186/1471-2334-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu H, Jerse AE. Alpha-2,3-sialyltransferase enhances Neisseria gonorrhoeae survival during experimental murine genital tract infection. Infect Immun. 2006;74:4094–4103. doi: 10.1128/IAI.00433-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soler-Garcia AA, Jerse AE. Neisseria gonorrhoeae catalase is not required for experimental genital tract infection despite the induction of a localized neutrophil response. Infect Immun. 2007;75:2225–2233. doi: 10.1128/IAI.01513-06. [DOI] [PMC free article] [PubMed] [Google Scholar]