Abstract
Objective
To evaluate the impact of a real-time computerized decision support tool in the emergency department that guides medication dosing for the elderly on physician ordering behavior and on adverse drug events (ADEs).
Design
A prospective controlled trial was conducted over 26 weeks. The status of the decision support tool alternated OFF (7/17/06–8/29/06), ON (8/29/06–10/10/06), OFF (10/10/06–11/28/06), and ON (11/28/06–1/16/07) in consecutive blocks during the study period. In patients ≥65 who were ordered certain benzodiazepines, opiates, non-steroidals, or sedative-hypnotics, the computer application either adjusted the dosing or suggested a different medication. Physicians could accept or reject recommendations.
Measurements
The primary outcome compared medication ordering consistent with recommendations during ON versus OFF periods. Secondary outcomes included the admission rate, emergency department length of stay for discharged patients, 10-fold dosing orders, use of a second drug to reverse the original medication, and rate of ADEs using previously validated explicit chart review.
Results
2398 orders were placed for 1407 patients over 1548 visits. The majority (49/53; 92.5%) of recommendations for alternate medications were declined. More orders were consistent with dosing recommendations during ON (403/1283; 31.4%) than OFF (256/1115; 23%) periods (p≤0.0001). 673 (43%) visits were reviewed for ADEs. The rate of ADEs was lower during ON (8/237; 3.4%) compared with OFF (31/436; 7.1%) periods (p=0.02). The remaining secondary outcomes showed no difference.
Limitations
Single institution study, retrospective chart review for ADEs.
Conclusion
Though overall agreement with recommendations was low, real-time computerized decision support resulted in greater acceptance of medication recommendations. Fewer ADEs were observed when computerized decision support was active.
Keywords: Emergency, computerized decision support, patient safety, decision support, data exchange
Introduction
The elderly represent one of the fastest growing segments of society and account for an increasing number of emergency department (ED) visits and hospital admissions, and one third of all prescribed medications.1–4 Despite these findings, the ED remains an understudied setting for provision of elderly medical care. Older patients are more than twice as likely as younger patients to be treated in the ED for adverse drug events (ADEs), and ADEs account for over 10% of ED visits by older patients.5 6 Many factors combine to increase the potential for medical error and ADEs in the ED, especially for geriatric patients.
Age-related differences in physiology and a high prescription rate make the elderly more susceptible to ADEs.7 It is known that a number of common medications whose standard dosages for otherwise healthy adults are potentially harmful to older patients. Important untoward effects of medications include falls, hip fractures, intracranial hemorrhage, gastrointestinal bleeding, oversedation, and delirium, all of which result in lower quality of life and higher health service utilization.8–16
Patients admitted to the hospital may be continued on medication regimens initiated in the ED. When a problem is discovered, it is often too late, and patients may experience adverse events, resulting in increased cost and length of stay.17 It is important to both the quality and safety of care for elderly patients that medication orders are initiated correctly in the ED. Traditional approaches for improving the appropriateness of medication use in the elderly have focused on avoiding a particular list or group of potentially inappropriate medications (PIMs). Beers was among the first to compile such a list. This list with subsequent modifications remains one of the more well-known guidelines of its type; it is widely used by government agencies and supported by researchers in various settings.18–24 This approach has also been specifically recommended for geriatric care in the ED.25 Application of Beers' criteria in the care of elderly outpatients suggests that nearly one third of certain medications prescribed may be inappropriate.26 27 A recent study estimates that PIMs are administered or prescribed in nearly 19.5 million or 16.8% of ED visits.28 Similar estimates have been reported in Taiwan (20%),29 Brazil (19.6%),30 and Austria (12.5%).31 Data from one national study of ADEs in the ED suggest that simply avoiding PIMs from the Beers list may be insufficient in preventing ADEs.6 This study found that nearly 50% of all ADEs in the ED were attributable to three classes of medication (antiplatelets/anticoagulants, digoxin, and diabetic medications), and among the leading drugs in each of these classes, only one is considered a PIM. Conversely, these medications are generally considered critical for patients taking them. The ADEs related to these medications appear to be due to the narrow therapeutic windows of these medications, suggesting that the key for avoiding ADEs in many cases may be in improving dosing and frequency in prescribing rather than simply avoidance of PIMs.
Newer approaches to medication recommendations for elderly patients include consideration of alternate dosages and frequencies as well consideration of comorbidities and other patient factors in medication selection and dosing.32 Because few clinicians are expert in geriatric pharmacology, and medication orders in the ED often cannot be delayed for consultation, real-time decision support about drug selection and dosing would potentially be valuable to both clinicians and patients. Computerized physician order entry (CPOE) without added computerized decision support (CDS) has been shown to decrease serious medication errors, near misses or potential ADEs, and preventable ADEs.33 Computerized decision support improves physician performance and clinical practice in roughly two-thirds of trials.34 35 When combined, CPOE and CDS have been shown to improve quality of care in both the inpatient36–39 and the outpatient settings,40 41 and specifically in elderly inpatients.42 Taken together this suggests that computerized advice on ordering medications in the elderly ED patient might be an important additional preventive strategy.43 Accordingly, CDS was among the highest priority recommendations made in a proposed research agendas for geriatric emergency medicine.44 45
This study represents the first report we are aware of using a real-time computerized decision support tool to enhance safe medication ordering for geriatric emergency patients, and represents a novel approach to guidance in the ED setting, with provision of recommendations for alternate medications, dosing, and frequency, relative to outright avoidance of PIMs. The use of CDS with CPOE has been studied for various applications in the ED setting but not for medication orders in the ED. We identified only a single study related to medication safety in geriatric emergency patients, which was directed at avoiding PIMs for prescriptions written at discharge from the ED.46 We implemented age-adjusted dosing and guided medication selection integrated with an existing computerized order entry system. We hypothesized that CDS for medication ordering in elderly ED patients would result in a higher proportion of drug selections and dosing that were consistent with recommendations, and might decrease the incidence of ADEs or potential surrogate markers for ADEs among admitted patients.
Methods
Study setting
The study took place in an urban, academic, tertiary care ED with 54 000 annual ED visits and approximately 10 000 visits by patients aged 65 or older. The ED exclusively uses a computerized ED order entry system (EDOE) that is integrated with the hospital computing system for all medication orders. The hospital is a primary site for a 4-year emergency medicine residency. Off-service residents also rotate through the ED. Orders were placed by resident and attending physicians. The health system IRB reviewed and approved this study. Their assessment was that neither physician nor patient consent was required. Use of this decision support tool was already in place in the inpatient setting at the time of this study of its use in the ED. Its use in various settings was planned as part of a ‘Signature Initiative’ for the health system to share decision support tools across platforms. We studied the tool as part of the roll-out of the application, which remained on after the OFF and ON periods (which were determined a priori for this study). Physicians were aware of what they would see when alternate medications/doses were recommended.
Patient population
The main analysis included all persons aged 65 years or older who had an order for a medication in the knowledge base during the study period. The study excluded patient orders in which qualifying medication orders were subsequently cancelled (78) and any orders with missing data (21). New medication orders in the ED were the intended target for the CDS provided.
Study design
The study was a prospective controlled trial that took place over four consecutive periods consisting of control or ‘OFF’ periods (usual computerized order entry, periods 1 and 3) alternating with intervention or ‘ON’ periods (computerized order entry plus decision support system; periods 2 and 4. The first two periods were comprised of 6-week blocks (7/17/6–8/29/6 and 8/29/6–10/10/6) and the second two periods were comprised of 7-week blocks (10/10/6–11/28/6; 11/28/6–1/16/07). Dates for the periods overlap as the time for changeover in the status of the CDS tool was 07:00. This study design of alternating study and control periods was intended to explore whether any noted changes appeared attributable to learning as opposed to the active status of the application. Patient visits were assigned to the group (intervention or control) based on the day of admission to the treatment area of the ED.
Intervention
When ordering a medication in EDOE, after choosing the name of a drug from a coded list, the order entry application provides a list of potential doses; one is offered as the most commonly used, or ‘default,’ dose. This dose is selected unless the user chooses another dose on the drop-down list offered by the application or selects ‘other’ and types in a value. With few exceptions, all medications have a frequency default setting of ‘×1,’ with other frequencies entered by selecting from a menu or choosing ‘other’ and typing in a value. These defaults are intended to offer general guidance and to be appropriate for most clinical circumstances in which the drug is used.
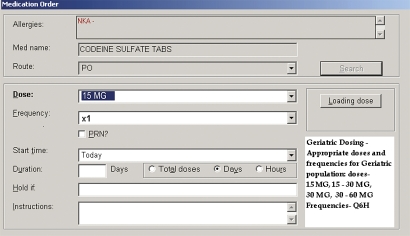
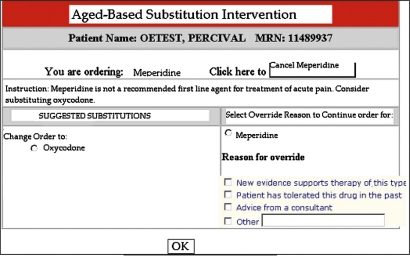
When the application detects an age greater than or equal to 65 and any medication in the study medication knowledge base is ordered, the application potentially modifies one or more of the following parameters: medication selection, default dosage, or default frequency. The adjusted values are displayed in a manner indistinguishable from routine ordering apart from notification to the user that adjustments had been made based on age (figure 1). The clinician can choose to accept or override recommendations. Both structured and free text field responses were available for use in providing a reason for overrides, which were required only when declining alternate medication recommendations (figure 2). Structured field options included ‘New evidence supports therapy of this type,’ ‘Patient has tolerated this drug in the past,’ ‘Advice from a consultant,’ and ‘Other’ with a free text box. During control periods, the application was inactive and only the unadjusted parameters were displayed. The intervention provided no additional education relating to prescribing for older adults, Beers' criteria, or the objectives of the study before or during the study period.
Figure 1.
Ordering screen showing age-adjusted dosage options with highlighted recommended default dosing.
Figure 2.
Ordering screen showing age-based medication substitution recommendation as well as option for override.
Data acquisition
The application automatically stored order information into a computerized database that included administrative and demographic data. This included data tracking when the application made a recommendation, the final order made by the ordering physician, and reasons for overriding a recommendation. With the exception of the chart review component of the study evaluating ADE rate, we obtained all data through queries of this database.
Knowledge base
The knowledge base used was developed for a previous study in our institution42 and was modified in part from Beers' criteria. An expert panel including a geriatrician, a general psychiatrist, a pharmacist, two general internists, and an anesthesiologist specializing in pain management developed modifications, including dosage and frequency. The original developers of the knowledge base intended to use this tool for the inpatient setting. As such, no emergency physician was included on the panel. While unfortunate, this is no different from other studies that have applied Beers' original or modified criteria to the ED, which have to date also never included an emergency physician. After reviewing the relevant literature, the expert panel convened and reviewed all medications in the hospital's drug formulary and selected those medications that are known to cause adverse events in the geriatric adults. Four classes of medications were targeted for the intervention: benzodiazepines (BZDs), opioids, sedative-hypnotics/neuroleptics, and non-steroidal anti-inflammatory drugs.
The knowledge base included alternate dosing suggestions for 72 medications and medication substitutions for 35 others, guided by published literature and drug monographs. In all, this applied to 85 unique medication/route combinations. The expert panel determined optimal adjustments in dose list, default dose amount, and default frequency for each medication. Specific recommendations included offering shorter-acting in place of longer-acting BZDs, oxycodone (PO) or morphine (IV) in place of meperidine, pentazocine, or propoxyphene, offering haloperidol in place of chlorpromazine or fluphenazine, and recommending ibuprofen in place of non-steroidal anti-inflammatory drugs such as ketorolac, ketoprofen, and others, and COX-2 inhibitors in patients over age 65. Due to technical reasons, orders for oxycodone/acetaminophen were not flagged as study medications in the knowledge base and so no decision support was provided for this medication during the study periods.
Outcome measures
The main outcome measure evaluated the effects of the intervention on physician ordering behavior. Specifically, this regarded (i) agreement with suggested recommendations for medication substitutions, and (ii) agreement with recommended dosage substitutions for drug selection and initial dosing, broken down by drug class. For dosage adjustments, we considered a medication order entry selection to be in agreement with recommendations if the dose did not exceed the parameters set forth for the preferred dose in the knowledge base. Because of potential complexities in analyzing decision support received for repeated orders of the same medication, we focused only on the initial order for a particular medication in an individual patient.
Secondary outcome measures included ED length of stay (EDLOS), admission rate, 10-fold dosing orders, the number of ‘rescue’ antidote drugs administered in the study periods (naloxone and flumazenil), and the relative proportion of ADEs detected during ON and OFF periods. We included these measures to serve as potential surrogate markers of ADEs to broaden capture of potential events. For example, if otherwise missed as an ADE in chart review, a case of oversedation or altered mental status may have resulted in a prolonged EDLOS, an admission, or the need for administration of a reversal agent. Because many factors affect EDLOS and admission rate, differences in these measures are difficult to interpret and associations require appropriate qualification. However, their inclusion was intended to help obtain a full picture beyond the primary outcomes assessed. The number of ‘greater than 10-fold orders’ was calculated by identifying orders where either the initial or the total dose was at least 10-fold higher than the recommended daily dose. A 10-fold cut-off was chosen because it has been reported in other populations to be an important indicator of error.47
ADE review
For each period, we reviewed a random sample of charts for the presence of ADEs. Resource limitations precluded a large chart review on the scale generally required to detect differences in ADE rates, so our initial sample size calculation aimed to detect a large reduction in ADEs of 50%, anticipating that this portion of the study would be underpowered but nevertheless important. Assuming a background ADE rate of 6%, we estimated that we would need to review 1151 charts. Chart review consisted of an initial screening for potential adverse drug events (PADEs) based in part on certain triggers, including orders for medications written in excess of recommended dosages and ADEs. This review was performed by trained research nurses with specific experience in ADE screening using explicit review and standardized forms validated for use in prior studies.48 An ADE was defined as an injury caused by a drug.49 Determination of the presence of an ADE required that it occurred after administration of a study medication in the ED and within 24 h of its administration. In this review we did not attempt to further identify preventability or severity of the events. Two physicians blinded to the study period (RG and HL) performed a secondary review to confirm the presence of ADEs with any disagreements to be arbitrated by a third physician (DB). We compared ADE rates among ON and OFF periods and present summary details. The nursing portion of the review proceeded with random sampling by study period with the secondary physician review occurring after study completion.
Statistical analysis
Continuous data were presented as mean (SD) and analyzed with t-tests. Categorical data were presented as proportions and analyzed using the χ2 test. In comparing patient cohort characteristics, because the unit of analysis was patient visit and not unique patient, we used a repeated measures analysis for the categorical data. Data were compared either between intervention and control or between the four study periods. Fisher's exact test was used to compare relative ADE rates because of the small number of outcomes. All reported p values were based on two-tailed tests of statistical significance. All analytical programming was conducted using SAS version 9.2.
Results
After exclusions, there were 2398 orders among 1407 patients. Fifty-one of these patients made at least one visit in both a study and control period, for a total of 1548 visits. There were 551 orders for study medications in period 1, 600 in period 2, 564 in period 3, and 683 in period 4, and 21 orders during the study period for rescue medications. There were no significant differences in the number of patients or the number of orders during ON versus OFF periods, and no differences in cohort demographics (table 1). Orders written during the study period included 38 of the 72 medications in the knowledge base.
Table 1.
Patient characteristics
| Control (n=668) | Intervention (n=739) | p Value | |
| Age | 75 (7.2) | 74 (7.4) | 0.23 |
| % Female | 60% | 61% | 0.86 |
| Race | |||
| White | 69% | 70% | 0.37* |
| African-American | 15% | 16% | |
| Hispanic | 12% | 10% | |
| Other | 4% | 4% | |
| Disposition | |||
| Admitted | 50% | 53% | 0.30* |
| ED observation unit | 26% | 26% | |
| Discharged | 21% | 17% | |
| Other | 3% | 3% | |
p Value presented as white versus non-white and admitted versus not admitted.
ED, emergency department.
Recommendations for alternate medications
There were 62 orders during control periods and 53 orders during study periods for non-recommended medications, where the decision support tool suggested an alternate medication. Among the orders for non-recommended medications during the ON periods, physicians declined the recommendation in 92.5% (49/53) of suggestions. Among these 115 non-recommended medication orders, the most common medications were diazepam (67%), clonazepam (10%) and indomethacin (7%). In 28 cases (51%), the reason given for overriding the alternate recommendation was the structured selection ‘patient has tolerated this medication in the past.’ A total of 24 additional free text responses were entered as reasons for overrides. These included some iteration of the following: reason for use (eg, back pain) given (7), physician preference (6), one time or low dose, should tolerate/will monitor (4), consultant recommendation (2), allergy or ‘had problems with (this medication) in the past’ (2), ‘this automated warning is not relevant or appropriate’ (1), ‘home medication’ (1), and ‘OK’ (1).
Recommendations for alternate dosing and/or frequency
Overall, a greater number of initial orders during the ON periods were consistent with recommendations (403/1283; 31%) than during the OFF periods (256/1115; 23%) periods (p≤0.001; table 2). However, overall agreement with recommendations was low for ON periods: 403/1283 (31%; 95% CI 29% to 34%) versus OFF periods: 256/1115 (23%; 95% CI 21% to 26%). There did appear to be a trend between the active status of the application and ordering behavior (table 2). There was an insufficient number of orders for sedative-hypnotics to analyze inter-period effects for this class.
Table 2.
Acceptance rate by overall and individual periods, showing number and percentage of study medication orders consistent with recommended dose
| Period 1 | Period 2 | Period 3 | Period 4 | OFF periods | ON periods | χ2 | |
| OFF | ON | OFF | ON | (1 and 3) | (2 and 4) | ||
| Benzodiazepines | |||||||
| N | 22/109 | 29/134 | 34/121 | 53/154 | 56/230 | 82/288 | 0.29 |
| % | 20% | 22% | 28% | 34% | 24% | 29% | |
| Non-steroidal anti-inflammatory drugs | |||||||
| N | 11/80 | 10/72 | 6/78 | 17/70 | 17/158 | 27/142 | 0.04 |
| % | 14% | 14% | 8% | 24% | 11% | 19% | |
| Opiates | |||||||
| N | 91/343 | 127/377 | 85/326 | 159/423 | 176/669 | 286/800 | <0.001 |
| % | 27% | 34% | 26% | 38% | 26% | 36% | |
| Sedative-hypnotics | |||||||
| N | 0/19 | 3/17 | 7/39 | 5/36 | 7/58 | 8/53 | 0.64 |
| % | 0% | 18% | 18% | 14% | 12% | 15% | |
| Total | |||||||
| N | 124/551 | 169/600 | 132/564 | 234/683 | 256/1115 | 403/1283 | <0.001 |
| % | 23% | 28% | 23% | 34% | 23% | 31% | |
Secondary outcome measures
No significant differences were observed in admission rate, reversal drug administration, number of 10-fold orders, or ED length of stay among non-admitted patients (tables 1 and 3). Among the 38 10-fold orders, haloperidol accounted for the majority (23), written almost entirely in the dosage of 5 mg, which is exactly 10 times the preferred dose in the knowledge base. This was followed by hydromorphone (6) and BZDs (5).
Table 3.
Secondary outcome measures
| OFF | ON | p Value | |
| Mean EDLOS (SE) for discharged patients (h) | 5.8 (0.27) | 5.6 (0.30) | 0.71 |
| Admission rate (%) | 50 | 53 | 0.15 |
| Rescue drugs administered | 11 | 10 | 0.59 |
| 10-fold dosing orders | 21 | 17 | 0.87 |
p Value from χ2 test to evaluate for a difference across four study periods.
EDLOS, emergency department length of stay.
ADE review
We reviewed charts for 673/1548 (44%) patient visits distributed as 314, 141, 122, and 96, respectively across the four study periods, with a non-uniform distribution of sampling that resulted from resource limitations (table 4). There were 39 ADEs identified, distributed as 8/237 (3%; 95% CI 1% to 6%) during ON periods and 31/436 (7%; 95% CI 5% to 9%) during OFF periods (p=0.02). Among the 39 ADEs, 10 occurred among patients with orders in agreement with recommendations while 29 occurred among patients whose orders were not in agreement with recommendations (table 4).
Table 4.
Adverse drug events (ADEs) identified by period and broken down by agreement with recommended dose (yes/no)
| Period 1 | Period 2 | Period 3 | Period 4 | OFF periods | ON periods | |
| Charts reviewed | 314 | 141 | 122 | 96 | 436 | 237 |
| ADEs (#) | 21 (7%) | 1 (7%) | 10 (7%) | 7 (6%) | 31 (7%) | 8 (3%) |
| Agreement with recommended dose? | ||||||
| Yes | 3 | 1 | 4 | 2 | 7 | 3 |
| No | 18 | 0 | 6 | 5 | 24 | 5 |
The leading types of ADEs identified include altered mental status (27) (this included delirium, somnolence, lethargy, combativeness, confusion, unsteady gait), nausea/vomiting (11), hypotension (4), and respiratory depression (1). These do not sum to 39, as a number of ADEs included more than one sign/symptom listed.
Discussion
Responding to the geriatric ‘demographic imperative’ will represent a major challenge for emergency medicine in the coming decades. Experts have recommended a number of approaches, including structural modifications to EDs, surveillance schemes, protocols, specialized personnel, and computerized decision support to bring evidence-based diagnostics and therapies to bear upon the unique challenges in caring for these patients.50
We found that providing age-related medication computerized decision support at the point of order entry resulted in increasing the proportion of medication orders consistent with recommendations. In addition, the ADE rate was lower in the intervention group, although several secondary outcomes did not change. Findings were primarily driven by opiates, representing approximately two-thirds of all potentially inappropriate orders. Findings for other medication categories were mixed, some with a potential learning (BZDs, sedative-hypnotics) and one inter-period potential reversal effect (sedative-hypnotic).
Our study design was based loosely on a similar inpatient study conducted in our institution, which obtained somewhat different results. That study found an improvement in the prescription of the recommended total daily dose, a reduction in the incidence of 10-fold dosing orders, and in the prescription of non-recommended drugs, and was associated with a lower in-hospital fall rate, but showed no reduction in hospital length of stay. Because the inpatient study had different primary and secondary outcome measures and used a different approach for the detection of ADEs reviewed, these are not directly comparable. Because patient length of stay in the ED is measured in hours rather than days, and since the majority of orders are for a frequency of ‘×1,’ the primary outcome measures focused on the initial order for a medication rather than a total daily dose received (as was the emphasis in the inpatient study). We restricted our analysis of agreement with recommendations to initial orders for a medication, excluding subsequent orders for the same drug. Such multiple orders were infrequent, and their inclusion would have complicated analysis and made explanation cumbersome without contributing to the analysis.
Our design, consisting of alternating OFF and ON periods of computerized decision support was intended to assess whether changes in ordering behavior across study periods were sustained or rather corresponded to the active status of the decision support tool. This would indicate whether potential changes were due to real time effects of decision support versus learning that took place after the initial activation period. Since residents rotate through the ED, and likely write the majority of medication orders (though this detail was not captured), this may have been unnecessary. In order to avoid problems associated with multiple testing, we did not perform a statistical test for each inter-period interval. However, there was a significant change across periods and an apparent trend present.
The decision support tool resulted in increased agreement of medication orders with recommendations, although overall agreement with recommendations was low, and the majority of medication substitution recommendations made were declined. This is likely the result of a number of factors. The first of these is that it is notoriously difficult to change physician behavior. Although computer interventions have fared well among various strategies for impacting behavior, this phenomenon continues to be a challenging area in research and quality improvement efforts.
We believe that perhaps a more important factor may be that the medication knowledge base used in our study was not designed specifically for the ED setting. Medication and dosing recommendations that are inconsistent with an emergency physician's experience regarding efficacy and safety in the acute setting, may lead to rejection of recommended changes. While it is unclear whether or to what extent input by emergency physicians would have changed recommendations for initial drug doses, especially for this first trial in the ED, it is possible that the low to moderate compliance rates might indicate general disagreement with some of these recommendations.
Emergency patients are often of higher acuity and in higher levels of pain than those in an outpatient clinic or perhaps the medical floor, and may in particular require higher doses of analgesia than required in other settings. In addition, since emergency physicians (EPs) are physically present in the treatment area when medications are ordered and administered to patients, it may be that the feedback EPs receive on the efficacy and the dangers of commonly used medications and dosages helps avoid the more frequent pitfalls in prescribing. In this light, it is not surprising that the most common reason given for overriding recommendations for an alternate medication was that the patient had not had problems with the drug previously.
These findings are also consistent with those of a recent study of the impact of CDS on prescribing behavior for outpatient prescriptions from the ED.46 The knowledge base used in the present study shares similarities with the Beers' list, but as previously noted also includes recommendations for alternate dose, frequency, and total daily dose. It is encouraging that only 38 of the 85 unique medication/dosing recommendations were ordered in the ED. Limited applicability of existing medication recommendations for older adults to the emergency setting is not unique to our study, and has been previously discussed in the emergency medicine literature.25 51 It is worth noting that the development of Beers' criteria was consensus-based and that the original expert panel and subsequent updates have not included EP input. Recently, alternate criteria such as STOPP (Screening Tool of Older Persons' potentially inappropriate Prescriptions) have been proposed and studied.52 Further guideline development for use in the ED would likely benefit from site-specific adjustment.
We were interested in testing whether our intervention would result in fewer adverse events, recognizing that our study was potentially underpowered to detect this. We did find a decrease in ADEs during the periods when the CDS tool was active and that among the ADEs detected, the majority were related to medication orders not in agreement with recommendations. However, because the observed agreement rate with recommendations was low even during ON periods, this may limit the conclusions that can be drawn from this comparison.
As previously noted, because differences in ADE rates can be difficult to demonstrate, we looked at a number of secondary outcome measures intended as surrogates for adverse events, such as increased admission rate, EDLOS for discharged patients, use of rescue reversal drugs, and 10-fold drug orders. We did not find differences in these secondary outcome measures.
While the majority of recommendations from the knowledge base used in this study, in Beers' criteria, and other criteria proposed have focused on preventing PIMs or dosages thought to be associated with delirium, sedation, and other untoward outcomes, it bears consideration that there may also be risks and problems associated with under-treatment. For example, it is possible that recommendations erring toward subtherapeutic dosing of sedative-hypnotics in the setting of agitated delirium might result in an increased risk of self-harm or need for physical restraint use. Similarly, under-dosage of analgesics may result in under-treatment of pain and suffering. While the CDS knowledge base considered 5 mg of haloperidol to be a ‘10-fold order’ that might warrant review, many emergency physicians using this medication for acute agitation in the elderly might find 0.5 mg to be an ineffectively low starting dose. This reflects some of the limitations of the knowledge base which might be very appropriate for other settings or conditions but bear adjustment.
The decision-support tool and knowledge base could clearly be improved further. As noted, recommendations err on the side of conservative treatment, generally meaning alternate medications or lower dosages. Finding an appropriate range makes sense and recommendations that are sensitive to specific indications and to mental status would be ideal. It would also seem important to be able to assess the additive effects of medications of different types and among different classes. For example, if a patient were ordered a BZD and two different narcotic medications, the ordering physician would receive the same decision support as for a patient just receiving one of these three drugs. Future efforts could target such additional complexities and address errors of prescribing omission in the elderly,53 and might include some educational intervention in addition to the CDS. Most immediately, future work should include derivation of an ED-specific evidence-based knowledge base for elderly medication dosing. Decision support using this knowledge base could then be re-examined to investigate its impact on elderly ED patients.
Limitations
This study has a number of limitations. The chart review portion of this study included a retrospective review. Although the previously validated screening methodology used was directed at minimizing potential hindsight bias, the inherent limitations of retrospective review with incomplete documentation cannot be completely eliminated. Emergency physicians and emergency medicine nurses are reported to miss >70% of patients with cognitive dysfunction compared to prospective data collection using validated structured screening tools.54 Chart abstraction that relies in part on documentation of delirium, for example, would potentially miss this as well. The effect of this would be to underestimate the number of ADEs manifested as confusion. We did not perform an assessment of inter-rater reliability among the nurse reviewers. However, prior internal assessments of inter-rater reliability for the same nurse reviewers using the same process has been ≥94%. Lack of follow-up on discharged ED patients who may have sustained ADEs manifested at home and who may have gone to other facilities for subsequent treatment also present limitations.
Due to technical reasons, unfortunately, orders for oxycodone/acetaminophen were not flagged as drugs in the knowledge base and so there was no decision support provided for this medication during the study periods. This was discovered too late in the study to make adjustments. Also due to technical issues in reactivating the status of the CDS after the second OFF period, the last two periods of the study were 1 week longer than the first 2 weeks. This was distributed evenly for study and control periods and we do not think it impacts the findings of the study.
The methodology in the chart review included identification of orders as PADEs if they were above the recommended dosage or frequency. Because the secondary reviews for ADEs was performed for those charts flagged as having PADEs, the likely effect of this would be to enrich the denominator of charts undergoing secondary review with those that were non-compliant, potentially biasing the results. Because of this, the significant differences observed in ADEs between compliant and non-compliant orders must be qualified accordingly, potentially over-estimating the impact of the decision support tool.
We did not collect data regarding physician demographics during the study, and thus cannot report these data. Although we collected information on reasons for declining medication substitution recommendations, we did not collect this data for when suggested dosage recommendations were declined. This was in part because this information was not included for collection in the CDS tool for the inpatient study our intervention was based on, and in part related to desires to make the CDS as least disruptive as possible. We also did not routinely collect data on patients' home medications as part of this study. Our restriction of the evaluation for ADEs to a 24 h period might conceivably limit our ability to differentiate between ADEs caused by medication ordered in the ED versus chronic medications that were previously described. Our study examined medication orders rather than medication administration. The medication administration record was not computerized and this impaired the feasibility of using this for our review. However, we do not expect this record to differ from the data obtained from our ordering data.
The study was conducted in a single institution. Because the intervention was tested at a teaching hospital where house officers write the majority of medication orders, the results may not be generalizable to other non-teaching hospital settings. We did not conduct an assessment of physician acceptability for the intervention. While the low overall compliance with the intervention may speak to acceptability of the details of the recommendations, this does not assess the process of the decision support.
Although the decision support provided includes recommendations for alternate frequency of dosing, as the large majority of medication orders in the ED have the default frequency of ‘×1’ this was not applicable. For a small number of orders frequency recommendations may have been provided. However, as the number of these was very small we did not evaluate changes in frequency as an outcome measure.
Finally, consideration of all adults ≥65 years as one population could be problematic. Confounding variables such as occult cognitive dysfunction, functional status, frailty, fall-risk, transportation deprivation, economic constraints, and insecure social safety nets may all influence the incidence of ADEs for a given medication and would ideally be considered for incorporation into future trials assessing ADE interventions in older ED populations.
Conclusions
In summary, the use of a real-time computerized decision support tool for guided medication dosing in elderly emergency patients resulted in improved agreement with recommended ordering behavior among emergency physicians. Fewer ADEs were observed when computerized decision support was active. This association, however, must be qualified by low overall agreement rates with CDS recommendations. Future directions in this area might focus on ED-specific adjustment of dosing recommendations and larger studies powered to detect and confirm expected differences in ADEs.
Footnotes
Funding: This study was supported by the Esther B. Kahn Foundation.
Competing interests: None.
Ethics approval: This study was approved by Partners Healthcare IRB.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Administration on Aging Profile of Older Americans: 2010. http://www.aoa.gov/aoaroot/aging_statistics/Profile/2010/2.aspx (accessed Jul 2011).
- 2.Shelton PS, Fritsch MA, Scott MA. Assessing medication appropriateness in the elderly: a review of available measures. Drugs Aging 2000;16:437–50 [DOI] [PubMed] [Google Scholar]
- 3.McCaig LF, Stussman BJ. National Hospital Ambulatory Medical Care Survey: 1996 emergency department summary. Adv Data 1997;293:1–20 [PubMed] [Google Scholar]
- 4.Nawar EW, Niska RW, Xu J. National Hospital Ambulatory Medical Care Survey: 2005 emergency department summary. Adv Data 2007;386:1–32 [PubMed] [Google Scholar]
- 5.Hohl CM, Dankoff J, Colacone A, et al. Polypharmacy, adverse drug-related events, and potential adverse drug interactions in elderly patients presenting to an emergency department. Ann Emerg Med 2001;38:666–71 [DOI] [PubMed] [Google Scholar]
- 6.Budnitz DS, Pollock DA, Weidenbach KN, et al. National surveillance of emergency department visits for outpatient adverse drug events. JAMA 2006;296:1858–66 [DOI] [PubMed] [Google Scholar]
- 7.Meredith S, Feldman P, Frey D, et al. Possible medication errors in home healthcare patients. J Am Geriatr Soc 2001;49:719–24 [DOI] [PubMed] [Google Scholar]
- 8.Herings RM, Stricker BH, de Boer A, et al. Benzodiazepines and the risk of falling leading to femur fractures: dosage more important than elimination half-life. Arch Intern Med 1995;155:1801–7 [PubMed] [Google Scholar]
- 9.Liu B, Anderson G, Mittmann N, et al. Use of selective serotonin reuptake inhibitors or tricyclic antidepressants and risk of hip fractures in elderly people. Lancet 1998;351:1303–7 [DOI] [PubMed] [Google Scholar]
- 10.Ray WA, Griffin MR, Downey W. Benzodiazepines of long and short elimination half-life and the risk of hip fracture. JAMA 1989;262:3303–7 [PubMed] [Google Scholar]
- 11.Thapa PB, Gideon P, Cost TW, et al. Antidepressants and the risk of falls among nursing home residents. N Engl J Med 1998;339:875–82 [DOI] [PubMed] [Google Scholar]
- 12.Gray SL, Lai KV, Larson EB. Drug-induced cognition disorders in the elderly: incidence, prevention and management. Drug Saf 1999;21:101–22 [DOI] [PubMed] [Google Scholar]
- 13.Chin MH, Wang LC, Jin L, et al. Appropriateness of medication selection for older persons in an urban academic emergency department. Acad Emerg Med 1999;6:1232–42 [DOI] [PubMed] [Google Scholar]
- 14.Fu AZ, Liu GG, Christensen DB. Inappropriate medication use and health outcomes in the elderly. J Am Geriatr Soc 2004;52:1934–9 [DOI] [PubMed] [Google Scholar]
- 15.Fick DM, Waller JL, Maclean JR, et al. Potentially inappropriate medication use in a medicare managed care population: association with higher costs and utilization. J Manag Care Pharm 2001;7:407–13 [Google Scholar]
- 16.Lau DT, Kasper JD, Potter DE, et al. Hospitalization and death associated with potentially inappropriate medication prescriptions among elderly nursing home residents. Arch Intern Med 2005;165:68–74 [DOI] [PubMed] [Google Scholar]
- 17.Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA 1997;277:307–11 [PubMed] [Google Scholar]
- 18.Beers MH, Ouslander JG. Risk factors in geriatric drug prescribing: a practical guide to avoiding problems. Drugs 1989;37:105–12 [DOI] [PubMed] [Google Scholar]
- 19.Beers MH, Ouslander JG, Rollingher I, et al. Explicit criteria for determining inappropriate medication use in nursing homes. Arch Intern Med 1991;151:1825–32 [PubMed] [Google Scholar]
- 20.Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly. Arch Intern Med 1997;157:1531–6 [PubMed] [Google Scholar]
- 21.Aparasu RR, Mort JR. Inappropriate prescribing for the elderly: beers criteria-based review. Ann Pharmacother 2000;34:338–46 [DOI] [PubMed] [Google Scholar]
- 22.Jano E, Aparasu RR. Healthcare outcomes associated with beers' criteria: a systematic review. Ann Pharmacother 2007;41:438–47 [DOI] [PubMed] [Google Scholar]
- 23.Chutka DS, Takahashi PY, Hoel RW. Inappropriate medications for elderly patients. Mayo Clin Proc 2004;79:122–39 [DOI] [PubMed] [Google Scholar]
- 24.Zhan C, Sangl J, Bierman A, et al. Potentially inappropriate medication use in the community-dwelling elderly: findings from the 1996 Medical Expenditure Panel Survey. JAMA 2001;286:2823–9 [DOI] [PubMed] [Google Scholar]
- 25.Hustey FM. Beers criteria and the ED: an adequate standard for inappropriate prescribing? Am J Emerg Med 2008;26:695–6 [DOI] [PubMed] [Google Scholar]
- 26.Mort JR, Aparasu RR. Prescribing potentially inappropriate psychotropic medications in the ambulatory elderly. Arch Intern Med 2000;160:2825–31 [DOI] [PubMed] [Google Scholar]
- 27.Caterino JM, Emond JA, Camargo CA. Inappropriate medication administration to the acutely ill elderly: a nationwide emergency department study, 1992–2000. J Am Geratr Soc 2004;52:1847–55 [DOI] [PubMed] [Google Scholar]
- 28.Muerer WJ, Potti TA, Kerber KA, et al. Potentially inappropriate medication utilization in the emergency department visits by older adults: analysis from a nationally representative sample. Acad Emerg Med 2010;17:231–7 [DOI] [PubMed] [Google Scholar]
- 29.Chen YC, Hwang SJ, Lai HY, et al. Potentially inappropriate medication for emergency department visits by elderly patients in Taiwan. Pharmacoepidemiol Drug Saf 2009;18:53–61 [DOI] [PubMed] [Google Scholar]
- 30.Farfel JM, Accorsi TA, Franken M, et al. Adverse drug events leading to emergency department visits in elderly: the role of inappropriate prescription. Einstein 2010;8:175–9 http://apps.einstein.br/revista/arquivos/PDF/1473-Einstein_v8n2_p175-9.pdf (accessed 11 Oct 2011). [DOI] [PubMed] [Google Scholar]
- 31.Heininger-Rothbucher D, Daxecker M, Ulmerb H, et al. Problematic drugs in elderly patients presenting to a European emergency room. Eur J Intern Med 2003;14:372–6 [DOI] [PubMed] [Google Scholar]
- 32.Avorn J. Improving drug use in elderly patients: getting to the next level. JAMA 2001;286:2866–8 [DOI] [PubMed] [Google Scholar]
- 33.Bates DW, Leape LL, Cullen DJ, et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA 1998;280:1311–16 [DOI] [PubMed] [Google Scholar]
- 34.Kawamoto K, Houlihan CA, Balas EA, et al. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ 2005;330:765–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garg AX, Adhikari NKJ, McDonald H, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA 2005;293:1223–38 [DOI] [PubMed] [Google Scholar]
- 36.Dexter PR, Perkins S, Overhage JM, et al. A computerized reminder system to increase the use of preventive care for hospitalized patients. N Engl J Med 2001;345:965–70 [DOI] [PubMed] [Google Scholar]
- 37.Dexter PR, Perkins SM, Maharry KS, et al. Inpatient computer-based standing orders vs physician reminders to increase influenza and pneumococcal vaccination rates: a randomized trial. JAMA 2004;292:2366–71 [DOI] [PubMed] [Google Scholar]
- 38.Overhage JM, Tierney WM, Zhou XH, et al. A randomized trial of “corollary orders” to prevent errors of omission. J Am Med Inform Assoc 1997;4:364–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chertow G, Lee J, Kuperman G, et al. Guided medication dosing for inpatients with renal insufficiency. JAMA 2001;286:2839–44 [DOI] [PubMed] [Google Scholar]
- 40.Bates DW, Teich JM, Lee J, et al. The impact of computerized physician order entry on medication error prevention. J Am Med Inform Assoc 1999;6:313–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sequist TD, Gandhi TK, Karson AS, et al. A randomized trial of electronic clinical reminders to improve quality of care for diabetes and coronary artery disease. J Am Med Inform Assoc 2005;12:431–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peterson J, Kuperman GJ, Sheck C, et al. Guided prescription of psychotropic medications for geriatric inpatients. Arch Intern Med 2005;165:802–7 [DOI] [PubMed] [Google Scholar]
- 43.Jha AK, Kuperman GJ, Teich JM, et al. Identifying adverse drug events. J Am Med Inform Assoc 1998;5:305–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilber ST, Gerson LW. A research agenda for geriatric emergency medicine. Acad Emerg Med 2003;10:251–60 [DOI] [PubMed] [Google Scholar]
- 45.Carpenter CR, Gerson LW. Geriatric emergency medicine. In: LoCicero J, Rosenthal RA, Katlic MR, et al., eds. A Supplement to New Frontiers in Geriatrics Research: An Agenda for Surgical and Related Medical Specialties. American Geriatrics Society, 2007:45–71 [Google Scholar]
- 46.Terrell KM, Perkins AJ, Dexter PR, et al. Computerized decision support to reduce potentially inappropriate prescribing to older emergency department patients: a randomized, controlled trial. J Am Geriatr Soc 2009;57:1388–94 [DOI] [PubMed] [Google Scholar]
- 47.Kozer E, Scolnik D, Keays T, et al. Large errors in the dosing of medications for children. N Engl J Med 2002;346:1175–6 [DOI] [PubMed] [Google Scholar]
- 48.Morimoto T, Gandhi TK, Seger AC, et al. Adverse drug events and medication errors: detection and classification methods. Qual Saf Health Care 2004;13:306–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bates DW, Cullen DJ, Laird N. Incidence of adverse drug events and potential adverse Drug Events. JAMA 1995;274:29–34 [PubMed] [Google Scholar]
- 50.Wilber ST, Gerson LW, Terrell KM, et al. Geriatric emergency medicine and the 2006 institute of medicine reports from the committee on the future of emergency care in the U.S. health system. Acad Emerg Med 2006;13:1345–51 [DOI] [PubMed] [Google Scholar]
- 51.Budnitz DS, Shebab N, Kegler SR, et al. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med 2007;147:755–65 [DOI] [PubMed] [Google Scholar]
- 52.Gallagher P, O'Mahony D. STOPP (Screening Tool of Older Persons' potentially inappropriate prescriptions): application to acutely ill elderly patients and comparison with Beers' criteria. Age Ageing 2008;37:1–7 [DOI] [PubMed] [Google Scholar]
- 53.Barry J, Gallagher P, Ryan C, et al. START (screening tool to alert doctors to the right treatment)—an evidence-based screening tool to detect prescribing omissions in elderly patients. Age Ageing 2007;36:632–8 [DOI] [PubMed] [Google Scholar]
- 54.Husty FM, Meldon SW, Simth MD, et al. The effect of mental status screening on the care of elderly emergency department patients. Ann Emerg Med 2003;41:678–84 [DOI] [PubMed] [Google Scholar]