Abstract
Background
15% to 20% of patients with metastatic colorectal cancer show isolated peritoneal carcinomatosis with significat clinical relevance. Their prognosis is poor, with a reported mean survival of less than one year. Moreover, there is a lack of knowledge regarding treatment optimally tailored to individual patients.
Methods
To identify and characterize the current treatment options for this condition, we reviewed pertinent literature retrieved by a PubMed search on the terms “peritoneal carcinomatosis,” “colorectal cancer,” “treatment,” “hyperthermic intraperitoneal chemotherapy,” “cytoreductive surgery,” and “humans.”
Results
Most patients with peritoneal carcinomatosis are treated with systemic chemotherapy in addition to best supportive care. Some undergo surgical or interventional treatment, such as ostomy or stent placement for intestinal obstruction. About one-third are candidates for multimodal treatment, consisting of surgical cytoreduction with intraoperative hyperthermic intraperitoneal chemotherapy. The main selection criteria are limited tumor mass (peritoneal cancer index <20), absence of organ metastases, feasibility of complete macroscopic cytoreduction (absence of disseminated small bowel disease), and approval of the interdisciplinary tumor board. Patients selected in this way have a 5-year survival rate of 30% to 50% after multimodal treatment.
Conclusion
The treatment of peritoneal carcinomatosis is usually considered to be purely palliative, in view of the extensive spread of the disease. However, for a subgroup of patients multimodal treatment can be provided with curative intent. The available survival data support the provision of multimodal strategies, including cytoprotective surgery, intraoperative intraperitoneal and systemic chemotherapy, for patients with peritoneal carcinomatosis in specialized centers. This type of treatment should be incorporated into treatment algorithms and guidelines.
Once a gastrointestinal malignancy has reached the stage of peritoneal carcinosis, this is usually considered the final stage of a metastatic cancer. Multiple peritoneal metastases are commonly treated palliatively. However, in the last two decades views on peritoneal carcinosis, its etiology, treatment options and multidisciplinary therapy planning have changed considerably. In addition to symptomatic treatment like pain management, systemic and local therapies are now available. These can be palliative or curative but require a well tailored treatment plan. The following treatment approaches for colorectal cancer (CRC) will be discussed as an example.
Incidence
Peritoneal carcinosis has long been considered a consequence of systemic dissemination of the cancer via the bloodstream. There is now increasing evidence for a (mainly) local etiology whereby the primary tumor spontaneously disseminates into the peritoneal cavity, particularly in cases where there is damage to the primary tumor’s capsule.
In 15–20% of all patients diagnosed with metastatic disease for the first time, peritoneal carcinosis is the only manifestation (regional lymph nodes excluded). If other organs already have metastases, peritoneal carcinosis is very likely to coexist. The percentage of patients with established peritoneal carcinosis increases to up to 30% in the presence of local metastases such as liver metastases.
Symptoms and diagnosis
The symptoms of peritoneal carcinosis are non-specific. Indirect signs such as bowel obstruction or ascites can be suggestive of peritoneal carcinosis but are not disease specific. If ascites is present, cytology from aspirated fluid can help to establish the diagnosis (Table 1).
Table 1. Incidence of peritoneal carcinosis with different tumors.
| Tumor | Incidence of peritoneal carcinosis |
| Colorectal Cancer | 15–20% if isolated 20–30% with other localizations e.g liver |
| Stomach Cancer | 10–20% when first diagnosed, 30–40% for T3N + primary tumor, 40–50% with recurrence |
| Ovarian Cancer | 60–80% at first diagnosis, 55–75% with recurrence |
| Pancreatic cancer, cholangiocellular carcinoma | 5–10% |
| Cancer of unknown primary (CUP syndrome), breast cancer, renal cell carcinoma, retroperitoneal sarcoma | <5% |
Imaging is by computerized tomography (CT) of the abdomen with intravenous, oral or rectal contrast; by magnetic resonance imaging; or positron emission tomography (PET-CT) (3). In many cases diagnostic laparoscopy can be helpful to gain more detailed information on the intra-peritoneal dissemination of the tumor or histological confirmation of the suspicion of peritoneal carcinosis (4).
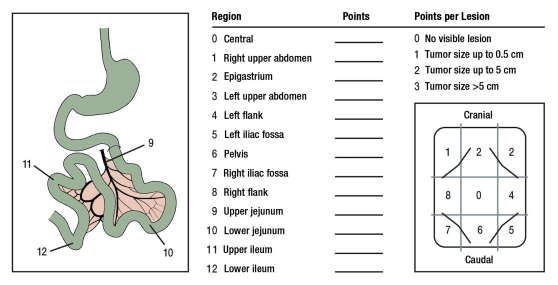
The exact extent of the peritoneal carcinosis can only be determined precisely during surgery. The peritoneal cancer index (PCI, Washington Cancer Institute) is usually used to quantify this (Figure 1). The scores are between 0 and a maximum of 39 and can be determined prior to surgery with the help of the CT results (5).
Figure 1.
Peritoneal Cancer Index (modified following Sugarbaker et al. 1995 [5])
Multimodal therapy with intent to cure
In advanced disease, symptomatic supportive therapy and systemic chemotherapy are the treatments of choice. Other options are palliative surgery, such as a stoma for bowel obstruction. It remains unclear to what extent systemic therapy significantly improves prognosis in isolated peritoneal carcinosis (e1).
As with isolated liver metastases, the combination of ablation therapy (usually surgical) and systemic chemotherapy can be curative, or control disease progression in the long term even if metastases are present. However, only about a third of all patients with peritoneal carcinosis would be suitable for this kind of therapy (e2). The surgical treatment of liver metastases from colorectal cancer has already been accepted as a therapeutic standard even though the published evidence for this is weaker than the evidence for the treatment of isolated peritoneal metastases (e3).
Multimodal therapy combines cytoreductive surgery (CRS) with hyperthermic intra-peritoneal chemotherapy (HIPEC) and systemic combination (SC) therapy. To improve the prognosis, the complete resection of all macroscopic visible tumor mass (complete macroscopic cytoreduction) is paramount. Remaining microscopic tumor material or remaining tumor cells should be eradicated immediately after resection and during the same surgery with the help of HIPEC.
For this combined therapy to be successful, careful case selection is essential. The criteria are: isolated and limited peritoneal carcinosis without extra-abdominal metastases and a Peritoneal Cancer Index (PCI) <20 and the absence of cancer of the small bowel. Under these conditions, complete macroscopic surgical cytoreduction is feasible. An assumed benefit of the use of the combined therapy strategy under inclusion of additional patient-related factors like biological age, co-morbidity and estimated risk of perioperative complications, is an absolute necessity in order to proceed. Multidisciplinary tumor board agreement is needed to pursue the combined treatment strategy. The above criteria for patient selection were agreed at a conference of the Peritoneal Surface Malignancy Group International (PSOGI) for colorectal cancer (6).
Surgical cytoreduction by parietal and visceral peritonectomy
The aim of cytoreductive surgery is complete macroscopic cytoreduction. Different kinds of parietal and visceral peritonectomy are used (7). These can be:
Omentectomy, if needed with splenectomy
Cholecystectomy and resection of the greater omentum
Peritonectomy right upper abdomen
Peritonectomy left upper abdomen
Pelvic peritonectomy with removal of the rectosigmoid junction, if necessary with hysterectomy
Distal stomach resection.
In addition, further organ removal such the removal of the small bowel, liver, pancreas or parts of the diaphragm, adnexa(e), uterus or the removal of parts of the bladder might be necessary. The extent of the intervention can vary a great deal, ranging from isolated right hemicolectomy, omentectomy and partial parietal peritonectomy to multivisceral resection. Such interventions can take many hours.
Hyperthermic intraperitoneal chemotherapy
HIPEC is done after resection. This can be performed in principle either as an open or closed procedure. The therapeutic aim is to achieve a high local concentration of chemotherapy in the peritoneal cavity and absorption of the substance into the superficial cell layers and therefore cause only minimal systemic toxic side effects from the cytostatic medication. The additional hyperthermia increases the therapeutic effect of the chemotherapy by improving penetration of the tissue. Extensive experimental studies also show the direct cytotoxic effect of hyperthermia (8).
To apply HIPEC, multiple drainage tubes are placed in different areas of the abdomen at the end of surgery. A roller pump with a heat exchanger is used and the temperature is controlled. The ideal temperature is 42–43ºC and the perfusion should last for 30–120 minutes, depending on which protocol is used. The choice of chemotherapy and perfusion protocol depends on the tumor (9). Mitomycin C is the most commonly used cytostatic agent to date. Oxaliplatin and irinotecan, which are standard medications in systemic therapy, are increasingly used for HIPEC (Table 2).
Table 2. Pharmacokinetic data and characteristics of cytostatic drugs for intra peritoneal use in colorectal cancer with peritoneal carcinosis.
| Cytostatic | Dosage intraperitoneal (mg/m2) | Combination with IV 5-FU | Depth of Penetration (mm) | Synergy with hyperthermia |
| Oxaliplatin | 200–460 | possible | 1–2 | present |
| Cisplatin | 50–250 | no data available | 1–3 | present |
| Mitomycin | 20–35 | possible | 2 | present |
| 5-FU | 400–650 | not applicable | 0.2 | not present |
5-FU = 5-fluorouracil; modified according to Ceelen et al. (1)
Post-operative mortality, morbidity and quality of life after CRS and HIPEC
The data for morbidity and mortality post-CRS and HIPEC which have so far been collected are similar to those of other extensive visceral surgical interventions and are generally between 0% and 6% (in one series even 17%). The mortality rate in centers where already more than 100 patients have been treated is less than 3% (10).
Post-operative morbidity rates quoted in the literature are between 25% and 41%. The most common complications are: anastomotic leakage, bowel perforation, pancreatitis, abscess or bleeding. A common problem is the delayed oral nutrition because of paralytic ileus. Complications associated with chemotherapy can arise from the cytostatic drugs used for HIPEC. These are principally neutropenia (average toxicity rate grade III/IV of 5.6%, range 0–28) or renal failure (average toxicity rate grade III/IV of 1.7%, range 0–7) (10, e4). The risks of post-operative thrombosis, pulmonary embolism and pneumonia are also increased.
As the combined therapy is complex and requires a learning curve, the foundation of specialist centers is paramount. In a study of data from 323 consecutive interventions at the Netherlands Cancer Institute the learning curve with respect to mortality and morbidity only reached a plateau after 130 interventions (11). Acceptable results can only be achieved in centers specialized in the treatment of peritoneal carcinosis.
The few available systematic studies of post-operative quality of life show – depending on the extent of the surgery and the rate of peri- and post-operative complications – a deterioration in quality of life in the first few weeks following surgery. However, most patients achieve the same level of quality of life as before the operation during the first four months following surgery. Some patients’ quality of life can be significantly improved long term (12).
Perioperative systemic chemotherapy
In the absence of prospective studies, the analysis of the French register shows that adjuvant systemic chemotherapy correlates with a better prognosis for the patient; after complete tri-modal therapy, survival of 34 months and a five year survival of over 30% were reported. Additional adjuvant systemic chemotherapy after CRS and HIPEC improves the prognosis (hazard ratio 0.6) in multivariate studies (13).
Currently, prospective studies are looking at the value of neo-adjuvant chemotherapy prior to CRS and HIPEC. As part of the COMBATAC study, a national multicenter one arm phase II study, patients with peritoneal carcinosis associated with cancer of the appendix or colon received three months neo-adjuvant and adjuvant combination chemotherapy plus cetuximab in combination with CRS and HIPEC. The COMBATAC study is a joint study of the Surgical Oncology Working Group of the German Society for General and Visceral Surgery (Deutsche Gesellschaft für Allgemein- und Viszeralchirurgie, DGAV) with the Medical Oncology Working Group (Arbeitsgemeinschaft Internistische Onkologie, AIO) of the German Cancer Society (14).
Prognosis after multimodal therapy
Multiple studies on multimodal therapy are available (Table 3).
Table 3. Main studies examining multimodal therapy for colorectal cancer with peritoneal carcinosis.
| Author, year | Study | Level of evidence | Therapy(treatment arm vs control arm) | Number of patients (n) | Median survival (months) or survival rate |
| Verwaal et al. 2003 (15), 2008 (16) | prospective randomized | 1b | SC vs. CRS + HIPEC+SC | 105 | 12 vs. 22 |
| Elias et al. 2009 (17) | retrospective, matched-pair | 3b | SC vs. CRS + HIPEC | 48 | 23 vs. 63 |
| Franko 2010 et al. (18) | retrospective, matched-pair | 3b | SC vs. CRS + EPIC | 105 | 16 vs. 34 |
| Matheme et al. 2004 (19) | historic comparison group | 3b | CRS + EPIC vs. debulking + SC | 18 | 32 vs. 14 |
| Glehen et al. 2004 (20) | Multinational register | 2b | CRS + HIPEC | 506 | 19 |
| Elias et al. 2010 (21) | French register | 4 | CRS + HIPEC + SC | 523 | 30 |
| Cao et al. 2009 (22) | systematic meta-analysis | 2a | CRS + HIPEC + SC | >2000 (47 studies) | 15 to 60 |
| Elias et al. 2010 (13) | retrospective comparative study | 2b | CRS + HIPEC + SC colon vs. rectum | 368 | 5 years: 30% vs. 38% |
| Elias 2004 et al. (e5) | prospective randomized (not completed) | 2b | CRS + SC vs. CRS + EPIC | 35 | 2 years: 60% vs. 60% |
| Esquivel et al. 2007 (6) | consensus statement | 3a | SC + CRS + HIPEC | – | – |
| Glehen et al. 2010 (e6) | retrospective multi-center | 2b | CRS + HIPEC + EPIC | 1106 | 30 |
SC: systemic chemotherapy; EPIC: early post-operative intraperitoneal chemotherapy; CRS: cytoreductive surgery (peritonectomy); HIPEC: hyperthermic intraperitoneal chemotherapy
Verwaal et al. conducted a prospective randomized multicenter study on a group of patients with colorectal cancer which had metastasized into the peritoneum. The study compared the use of combined treatment with CRS, HIPEC and additional systemic chemotherapy (5-fluorouracil [5-FU]/folinic acid) to systemic chemotherapy alone (control group). Median overall survival in the therapy group was 22.2 months compared to 12.6 months in the control group. In patients with complete macroscopic cytoreduction (completeness of cytoreduction score [CCR] 0/1 [= remaining tumor <2.5 mm]; 82% of patients) median survival was 48 months and the five-year survival rate was 45% (median progression-free survival time 24 months). However, these results have limited contemporary relevance as this, the only available complete phase III study, used from today’s point of view an old fashioned and less effective systemic chemotherapy (with 5-FU/folinic acid alone) (15, 16).
In a matched pair study from the US, Franko et al. were able to show that the prognosis for patients in the control group after systemic combination chemotherapy alone was better than in the above study but much worse than after multimodal therapy (18).
Elias et al. published results of a phase II study with 48 patients with colorectal carcinoma metastasized into the peritoneum. Median survival was 63 months (after complete macroscopic cytoreduction and HIPEC with oxaliplatin) compared to 24 months with chemotherapy alone, also in a case control study (17).
An international register published by Glehen et al. was able to show in 506 patients from 28 international centers that the probability of survival following CRS and HIPEC was 30% after five years (20).
National registers, like the ones in France and the Netherlands, show similar results in cohorts of more than 500 patients. After complete cytoreduction with CRS and HIPEC the survival probability was also 30% after five years (21). In other observational studies of colorectal carcinoma with peritoneal carcinosis median survival was between 28 and 60 months after complete macroscopic cytoreduction and HIPEC (22) (Figure 2).
Figure 2.
Overview of the most important survival curves (the control groups correlate with systemic therapy, the others with multimodal treatment) (15– 18, 20, 21); HIPEC, hyperthermic intraperitoneal chemotherapy
In addition, a “second look” laporotomy can be performed after adjuvant systemic chemotherapy for patients who initially have non-metastatic disease and a high risk of developing peritoneal carcinosis like those with T3/T4 tumor stage or ruptured tumor capsule. At this laparotomy, up to 50% of patients will already have a diagnosis of peritoneal carcinosis (23).
In some countries such as France, multimodal therapy of peritoneal carcinosis for selected patients with isolated peritoneal carcinosis from colorectal carcinoma is already recommended as standard treatment in the national guidelines. This does not mean that every patient can be treated but that the indication for multimodal therapy should be evaluated in each patient (21).
Cytoreductive surgery followed by hyperthermic intra-peritoneal chemotherapy can be given to patients with isolated and limited peritoneal carcinosis without extra-abdominal metastases and a PCI<20. The condition for this is complete macroscopic cytoreduction and an assumed benefit for the patient, as agreed by the multidisciplinary tumor board. The therapy should be given in a specialized center and preferably within the framework of a study.
Palliative therapy
Palliative surgical therapy
Most patients with manifest peritoneal carcinosis will in practice first receive palliative therapy options, in particular supporting gut motility, ensuring adequate nutrition through parenteral or enteral nutrition, adequate analgesia and psychological support (24).
Surgical intervention should be considered early for established stenosis and/or bleeding. With the help of (sometimes laparoscopic) colostomy or ileostomy or intestointestinal bypass, symptoms can be relieved with improved quality of life. If an area of stenosis is small and well defined, endoluminal stents can be created endoscopically or, as a last resort, a percutaneous endoscopic gastrostomy can be considered.
Palliative systemic chemotherapy
There is no doubt that systemic therapy with combination chemotherapy plus the use of monoclonal antibodies has significantly improved prognosis for patients with metastasized colorectal carcinoma. This therapy is therefore the standard treatment for metastasized disease. However, the prognosis in peritoneal carcinosis is frequently poor. Verwaal et al. analyzed as part of the above mentioned prospective randomized study the effectiveness of systemic chemotherapy alone in the control group. The median overall survival of patients treated only with 5-FU/folinic acid was worse than the median overall survival of patients treated with combined therapy but was nevertheless 12.6 months (4) and was therefore comparable to other therapy results with 5-FU/folinic acid. In a recently published study, Elias et al. report a median survival of 24 months after single systemic combination chemotherapy (3).
Intraperitoneal Immunotherapy
The intraperitoneal application of catumaxomab is a new treatment option. This tri-functional antibody which is active against the EpCAM-antigen of the peritoneal tumor cells has been proven to be effective in the treatment of malignant ascites and is licensed for this indication in Germany (25).
Summary
The treatment of peritoneal carcinosis in patients with colorectal carcinoma has changed in the last few years. The results of palliative systemic chemotherapy and antibody therapy have improved but these therapies can offer no long term control of the disease. For some patients there is an opportunity to achieve long term survival and potentially complete cure by using a multimodal and individual multidisciplinary oncological treatment strategy with cytoreductive surgery, HIPEC and systemic chemotherapy. Of paramount importance for the success of the therapy is patient selection and the treatment of suitable patients at a specialized peritoneal carcinosis center where the therapy can be given with a low mortality rate. It would be reasonable, as is done with liver metastases, to check the indication for multimodal therapy of peritoneal carcinosis for all patients in these centers.
In Germany, multimodal therapy with cytoreductive surgery, HIPEC and chemotherapy is given in about 30 hospitals. Recently the German HIPEC register was introduced and a certification process is being developed by the DGAV. The aim is to ensure the high quality of the treatment and standardization of the process.
The indication must be made by a multidisciplinary specialized tumor board. As the number of specialized bowel cancer centers certified by the German Cancer Society continues to increase more cases of peritoneal carcinosis will be discussed in multidisciplinary tumor conferences. Patients considered suitable for multimodal therapy should then be referred to a center specialized in the treatment of peritoneal carcinosis. Studies like the German COMBATAC study have an important role to play in the evaluation and development of various aspects of therapy and patient selection.
Key Messages.
Up to 15% of patients with metastasized colorectal carcinoma will have isolated peritoneal carcinosis. This makes it a clinically relevant problem.
Apart from symptomatic treatment, the majority of patients are treated with systemic chemotherapy but there are no prospective studies which have specifically looked at this group.
Selected patients will be given multimodal therapy which consists of surgical cytoreduction and intraoperative hyperthermic intraperitoneal chemotherapy. The intention is to cure. The most important independent prognostic factors are: complete cytoreduction, limited tumor mass (peritoneal cancer index <20), no organ metastases.
In these selected patients a five-year survival rate of 30–50% can be achieved.
The published evidence of this treatment justifies its use in specialized centers and it should be incorporated into treatment protocols and guidelines.
Acknowledgments
Translated from the original German by Dr Ute Semrau-Boughton.
Footnotes
Conflict of interest statement
Professor Piso has received payments for lectures from Merck, Fresenius Biotech, and Roche. As part of clinical studies, support was given by Fresenius and Merck. Scientific meetings about peritoneal carcinosis were supported by Thermasolutions, Kardialgut, Rand, and Belmont.
Professor Arnold has received payments for lectures from Merck, Roche, Amgen, and Sanofi-Aventis as well as payments for consultancy work from Roche, Amgen, Sanofi-Aventis, and Fresenius Biotech. As part of clinical studies and scientific projects, support was given by Roche, Merck and Sanofi-Aventis.
References
- 1.Ceelen W, Bracke ME. Peritoneal minimal residual disease in colorectal cancer: mechanism, prevention and treatment. Lancet Oncol. 2009;10:72–79. doi: 10.1016/S1470-2045(08)70335-8. [DOI] [PubMed] [Google Scholar]
- 2.Folprecht G, Köhne C-H, Lutz M. Systemic Chemotherapy in Patients with Peritoneal Carcinomatosis from Colorectal Cancer. Peritoneal Carcinomatosis. 2007:425–440. doi: 10.1007/978-0-387-48993-3_28. [DOI] [PubMed] [Google Scholar]
- 3.Berthelot C, Morel O, Girault S, et al. Use of FDG-PET/CT for peritoneal carcinomatosis before hyperthermic intraperitoneal chemotherapy. Nucl Med Commun. 2011;32:23–29. doi: 10.1097/MNM.0b013e328340e730. [DOI] [PubMed] [Google Scholar]
- 4.Yan TD, Morris DL, Shigeki K, et al. Preoperative investigations in the management of peritoneal surface malignancy with cytoreductive surgery and perioperative intraperitoneal chemotherapy: Expert consensus statement. J Surg Oncol. 2008;98:224–227. doi: 10.1002/jso.21069. [DOI] [PubMed] [Google Scholar]
- 5.Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg. 1995;221:124–132. doi: 10.1097/00000658-199502000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esquivel J, Sticca R, Sugarbaker P, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Society of Surgical Oncology. Ann Surg Oncol. 2007;14:128–133. doi: 10.1245/s10434-006-9185-7. [DOI] [PubMed] [Google Scholar]
- 7.Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42. doi: 10.1097/00000658-199501000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceelen W, Flessner MF. Intraperitoneal therapy for peritoneal tumors: biophysics and clinical evidence. Nat Rev Clin Oncol. 2010;7:108–115. doi: 10.1038/nrclinonc.2009.217. [DOI] [PubMed] [Google Scholar]
- 9.Glehen O, Cotte E, Kusamura S, et al. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol. 2008;98:242–246. doi: 10.1002/jso.21061. [DOI] [PubMed] [Google Scholar]
- 10.Chua TC, Yan TD, Saxena A, et al. Should treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure ? A systematic review of morbidity and mortality. Ann Surg. 2009;249:900–907. doi: 10.1097/SLA.0b013e3181a45d86. [DOI] [PubMed] [Google Scholar]
- 11.Smeenk RM, Verwaal VJ, Zoetmulder FA. Learning curve of combined modality treatment in peritoneal surface disease. Br J Surg. 2007;84:1408–1414. doi: 10.1002/bjs.5863. [DOI] [PubMed] [Google Scholar]
- 12.Glockzin G, Schlitt HJ, Piso P. Peritoneal carcinomatosis: patients selection, perioperative complications and quality of life related to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol. 2009;7 doi: 10.1186/1477-7819-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elias D, Glehen O, Pocard M, et al. A comparative study of complete surgery plus intraperitoneal chemotherapy to treat peritoneal dissemination from colon, rectum, small bowel and nonpseudomyxoma appendix. Ann Surg. 2010;251:896–901. doi: 10.1097/SLA.0b013e3181d9765d. [DOI] [PubMed] [Google Scholar]
- 14.Glockzin G, Rochon J, Arnold D, Lang SA, Klebl F, Koller M, Schlitt HJ, Piso P. Additive Therapiemaßnahmen bei ausgewählten Patienten mit peritoneal metastasiertem Kolon- und Appendixkarzinom. Journal Onkologie. 2010;8:412–414. [Google Scholar]
- 15.Verwaal V, van Ruth S, de Bree, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2003;21:3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 16.Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;5:2426–2432. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 17.Elias D, Lefevre JH, Chevalier J, et al. Complete Cytoreductive Surgery Plus Intraperitoneal Chemohyperthermia With Oxaliplatin for Peritoneal Carcinomatosis of Colorectal Origin. J Clin Oncol. 2009;27:681–685. doi: 10.1200/JCO.2008.19.7160. [DOI] [PubMed] [Google Scholar]
- 18.Franko J, Ibrahim Z, Gusani, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer. 2010;116:3756–3762. doi: 10.1002/cncr.25116. [DOI] [PubMed] [Google Scholar]
- 19.Matheme H, Hanssons J, Berglund A, et al. Improved survival in patients with peritoneal metastases from colorectal cancer: a preliminary study. Br J Cancer. 2004;90:403–407. doi: 10.1038/sj.bjc.6601586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22:3284–3292. doi: 10.1200/JCO.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Elias D, Gilly F, Quenet F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperaive intraperitoneal chemotherapy: retrospective anaylsis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28:63–68. doi: 10.1200/JCO.2009.23.9285. [DOI] [PubMed] [Google Scholar]
- 22.Cao C, Yan TD, Black D, et al. A systematic review and meta-analysis of cytoreductive surgery with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2009;16:2152–2165. doi: 10.1245/s10434-009-0487-4. [DOI] [PubMed] [Google Scholar]
- 23.Elias D, Goere D, Pietrantonio D, et al. Results of systematic second-look surgery in patients at high-risk of developing colorectal peritoneal carcinomatosis. Ann Surg. 2008;247:445–450. doi: 10.1097/SLA.0b013e31815f0113. [DOI] [PubMed] [Google Scholar]
- 24.Glockzin G, Piso P. Palliative Viszeralchirurgie. Onkologe. 2007;13:625–631. [Google Scholar]
- 25.Heiss MM, Mrawa P, Koralewski P, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phas II/III trial. Int J Cancer. 2010;127:2209–2221. doi: 10.1002/ijc.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e1.Pelz JOW, Chua TC, Esquivel J, et al. Evaluation of best supportive care and systemic chemotherapy as treatment stratified according to the retrospective Peritoneal Surface Disease Severity Score for peritoneal carcinomatosis of colorectal origin. BMC Cancer. 2010;10:689–701. doi: 10.1186/1471-2407-10-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e2.Moran BJ. Decision-making and technical factors account for the learning curve in complex surgery. J Pub Health. 2006;28:375–378. doi: 10.1093/pubmed/fdl048. [DOI] [PubMed] [Google Scholar]
- e3.Schmiegel W, Pox C, Reinacher-Schick A, et al. S3 Guidelines for Colorectal Carcinoma. Z Gastroenterol. 2010;48:65–136. doi: 10.1055/s-0028-1109936. [DOI] [PubMed] [Google Scholar]
- e4.Roviello F, Caruso S, Marrelli D, et al. Treatment of peritoneal carcinomatosis with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: state of the art and future developments. Surg Oncol. 2010;20:e38–e54. doi: 10.1016/j.suronc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- e5.Elias D, Delperro JR, Sideris L, et al. Treatment of peritoneal carcinomatosis from colorectal cancer: impact of complete cytoreductive surgery and difficulties in conducting randomized studies. Ann Surg Oncol. 2004;11:518–521. doi: 10.1245/ASO.2004.09.008. [DOI] [PubMed] [Google Scholar]
- e6.Glehen O, Gilly FN, Bereder JM, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1290 patients. Cancer. 2010;15:5608–5618. doi: 10.1002/cncr.25356. [DOI] [PubMed] [Google Scholar]