Abstract
Gastric cancer is the most common cancer worldwide. The proportion of early gastric cancer (EGC) cases at diagnosis has increased because of the use of mass screening endoscopy in older adults. Endoscopic mucosal resection has become the standard treatment for EGC in cases with standard indications because of its low risk of lymph node metastasis. A new endoscopic method, endoscopic submucosal dissection, has recently become available. This method allows en bloc resection without limitation of the size of the lesion. The goal of this article is to review the history and methods of endoscopic treatment with EGC, the conventional and extended indications, the therapeutic outcomes, and the complication rates.
Keywords: Early gastric cancer, Endoscopic submucosal dissection
INTRODUCTION
The incidence of gastric cancer has been decreasing for several decades. Gastric cancer has become a relatively rare cancer in North America and in most of Northern and Western Europe.1 However, gastric cancer remains the most common cancer worldwide with approximately 870,000 new cases and 650,000 deaths per year.2 In Korea, the age-adjusted annual incidence of gastric cancer per 100,000 persons is 62.8 for men and 25.7 for women.3 The proportion of early gastric cancer (EGC) at diagnosis is increasing due to the use of mass screening endoscopy. In the past, the standard treatment of gastric cancer was surgical resection; however, the endoscopic treatment has increased due to advances in the instruments available and clinician experience.4 Endoscopic mucosal resection (EMR) has been used as the first line treatment for EGC without evidence of lymph node (LN) metastasis. Evidence supports that EMR for EGC, without LN metastasis, has similar efficacy compared to open surgery.5 Furthermore, as life expectancy has been extended because of improved quality of life and better treatment of chronic disease, many patients have one or more chronic diseases. For patients with co-morbidities, EMR is an alternative method without the same risk as open surgery due to the reduced invasiveness. A recent study demonstrated that there was no difference in the complication rate between high risk and low risk patients.6 Thus, EMR may be a good alternative treatment choice for patients with co-morbid diseases. The goal of this article is to describe the history and the methods of endoscopic treatment of EGC, the indications, the points to consider with regard to extended indications, the therapeutic outcomes and complications.
HISTORY OF TECHNICAL DEVELOPMENT: FROM EMR TO ESD
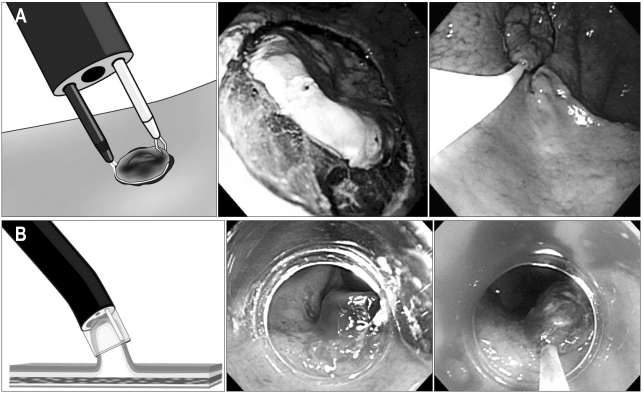
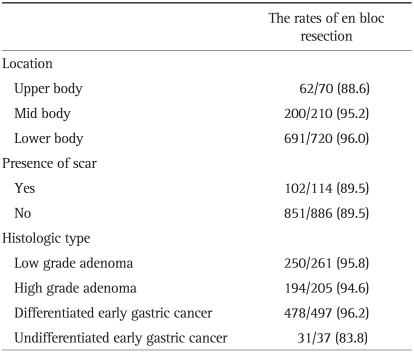
In 1974, the first attempt of endoscopic treatment for polypoid type gastric cancer was reported in Japan.7 In 1984, the strip biopsy was described as a development of the endoscopic snare polypectomy;8 this technique used a double channel endoscope. After submucosal injection around the lesion, a grasper was used to lift the lesion through one channel, and then a snare that was inserted through the other channel was used to resect the lesion. In 1988, another EMR technique (EMR after circumferential pre-cutting, EMR-P) using hypertonic saline mixed with diluted epinephrine solution was introduced;9 cutting around the lesion with a needle knife is done after hypertonic saline injection into the submucosal layer, and then the lesion is removed by a snare (Fig. 1). In 1992, the EMR using transparent cap (EMR-C) technique, was developed and used for early esophageal cancer and EGC.10 This technique uses a transparent hood that is connected to the tip of a standard endoscope. After the submucosal injection, the lesion is sucked into the cap while a specialized crescent-shaped snare which is located at the tip of the cap is closed.
Fig. 1.
Standard endoscopic mucosal dissection (EMR) techniques. (A) EMR after circumferential pre-cutting (EMR-P). (B) Cap-fitted endoscopy (EMR-C).
After this, the EMR with ligation (EMR-L) was introduced.11,12 This technique uses a standard endoscopic variceal ligation device to capture the lesion. The EMR-C and the EMR-L are simple and effective methods for small cancers; however, it is unsuitable for lesions larger than 20 mm. Usefulness for en bloc resections is limited.
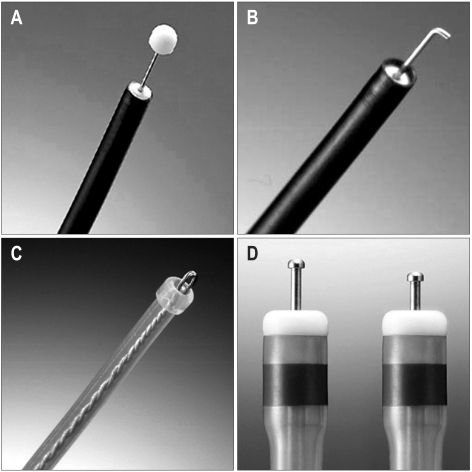
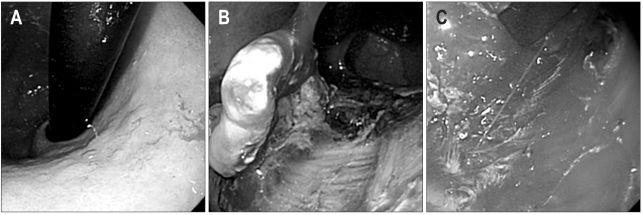
In late 1990s, endoscopic submucosal dissection (ESD) method was developed and endoscopic treatment for EGC has been extended to lesions larger than 20 mm in size; after indigocarmine spray, marking around the lesion is done by various knives. Circumferential mucosal pre-cutting is performed using a standard needle knife after saline mixed with diluted epinephrine (1:100,000) and indigocarmine injection into the submucosal layer. Then, submucosal layer under the lesion is dissected with lateral movement using various knives (Fig. 2). Initially, ESD used an insulation-tipped (IT) diathermic knife for the submucosal dissection.13,14 A variety of other endoscopic knives such as needle knife, hook knife, flex knife, triangle tip knife, flush knife, and splash knife and IT-2 knife have been developed and used (Fig. 3).
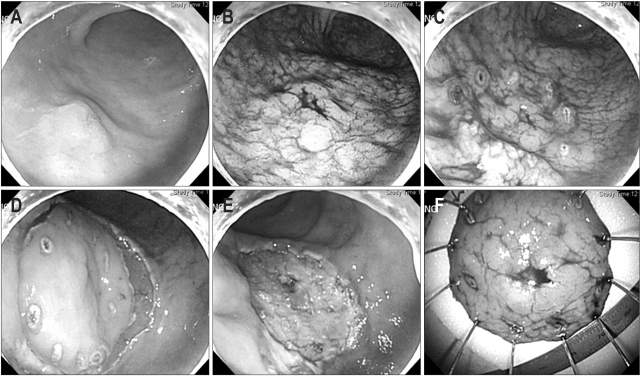
Fig. 2.
The endoscopic submucosal dissection (ESD) procedure. (A) On the anterior wall of the antrum, a 1.2-cm type IIa early gastric cancer is found. (B) Indigocarmine is sprayed along the extent of tumor to aid visualization. (C) Marking outside the lesion. (D) After injection of saline mixed with diluted epinephrine (1:100,000) and indigocarmine into the submucosal layer, circumferential mucosal pre-cutting is performed using a standard needle knife. (E) After dissection of the submucosal layer, a post-ESD induced ulcer is seen. (F) Fixation of the tissue specimen.
Fig. 3.
Different types of knives used for endoscopic submucosal dissection. (A) Insulation-tipped diathermic knife. (B) Hook knife. (C) Flex knife. (D) Dual knife.
Recently, ESD has evolved in endoscopic surgery. New instruments (magnetic anchor-guided ESD, springs, multitask devices, and double endoscopic intraluminal surgery) have been introduced and have begun to play a role in enabling excellent visualization of the submucosal layer.15-17 These techniques have reduced some technical problems involved in the ESD procedure.
INDICATIONS FOR ENDOSCOPIC RESECTION FOR EGC
1. Conventional indications
The most important indications for endoscopic treatment of EGC are determined by considering the risk of LN metastasis and technical problems and whether to resect the tumor en bloc. The conventional criteria for endoscopic resection of EGC which was proposed by Japanese group are: 1) differentiated adenocarcinoma, 2) intramucosal cancer, 3) size of the lesion less than 20 mm, 4) without any endoscopic findings of ulceration,4,18 5) no LN involvement or metastasis by computed tomography. Lesions that meet all of the above mentioned criteria should be considered for en bloc resection by conventional EMR methods due to the low risk of LN metastasis.
2. Extended indications
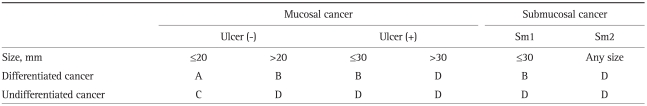
Recently, based on surgical data, extended indications for EMR have been proposed (Table 1). After an analysis of the results of 5,265 patients who underwent gastrectomy with LN dissection, Gotoda et al.4,19 and An et al.20 reported the lesion that meets these criteria has no or minimal risk of LN metastasis: 1) no size limitation for intramucosal differentiated cancers without ulceration that have no lymphovascular invasion, 2) less than 3 cm in diameter for ulcerated differentiated intramucosal cancers without lymphovascular invasion, 3) less than 3 cm in diameter for differentiated cancers (extension into the submucosal for less than 500 micrometers) without lymphovascular invasion, 4) less than 2 cm in diameter for undifferentiated intramucosal cancers without ulceration. Currently, the extended criteria for ESD are in use in Japan.
Table 1.
Criteria for Expanded Endoscopic Resection in Patients with Early Gastric Cancer
Data from Soetikno et al. J Clin Oncol 2005;23:4490-4498.53
A, classic indications; B, expanded indications; C, surgery, but need for further consideration; D, surgery.
However, there are several issues to consider with the extended indications. The first issue is the risk of LN metastasis. In a Korean study, 855 patients who underwent gastrectomy with LN dissection for EGC were analyzed; LN metastasis was identified in 4.7% (20/427) of mucosal cancers and 22.2% (95/428) of submucosal cancers. In the mucosal cancers that were less than 10 mm in size, LN metastasis was detected in 2 cases.21 In addition, among 2,173 patients who underwent gastrectomy with LNs dissection, LN metastasis was found in 4.5% (50/1,108) of mucosal cancers. Among the mucosal cancers, there was LN metastasis in 2 differentiated cancers without ulceration that were 21 to 40 mm in size, and 1 differentiated cancer with ulceration, 21 to 30 mm in size (tumor size was not described).22 Although these results reflect only a small portion of mucosal cancers, the possibility of LN metastasis should be considered when the extended criteria are used for the treatment of EGC.
The second issue is the histological discrepancy before and after endoscopic resection of gastric adenoma and EGC. Park et al.23 reported that the discrepancy rate between the histology of the endoscopic biopsy and the resected specimen was 40.6% for the gastric adenoma and 23.7% for the EGC. Recently, of the 293 lesions diagnosed as low grade gastric adenoma by forcep biopsy, 51 (18.7%). Histology was upgraded after endoscopic resection. They reported that absence of whitish discoloration was associated with significant factor influencing histologic discrepancies (p=0.001; odds ratio, 5.29; confidence interval, 1.95 to 14.37).24 In another study of low grade gastric adenoma diagnosed by forcep biopsy, 272 lesions (89.2%) were finally diagnosed as low grade adenoma and 33 lesions (10.8%) were diagnosed as having high grade foci, including 1 intramucosal carcinoma. they demonstrated that lesion size >1 cm on endoscopy and finding of tubulovillous or villous histology on forcep biopsy specimens were independent risk factor for histologic discrepancy before and after endoscopic resection of low grade adenoma.25 Thus, therapeutic endoscopists have to keep in mind about the possibility of histological discrepancy before and after endoscopic resection.
The third issue is the differences in the pathological diagnosis of gastric adenocarcinoma and premalignant lesions between East and West: 1) The different concept of gastric adenoma and dysplasia. In Western countries, gastric dysplasia is divided into flat/depressed dysplasia and elevated dysplasia. Only an elevated dysplasia is called adenoma. In Eastern countries (mainly Japan and including Korea), the terms "dysplasia" and "adenoma" are both used for non-invasive neoplastic lesion regardless of tumor shape (protruded, flat, depressed, etc.).26 2)Difference in diagnostic criteria for gastric carcinoma. Western pathologists consider invasion into the lamina propria of the mucosa mandatory for the diagnosis of carcinoma, whereas nuclear and structural features are much important for the Japanese pathologists. Western pathologists diagnose as low- or high-grade dysplasia in cases diagnosed as well differentiated carcinoma by Japanese pathologists.27 3) Japanese classification of differentiated adenocarcinoma with high grade atypia and low grade atypia. Japanese pathologists have divided differentiated gastrointestinal adenocarcinoma into low-grade atypia and high-grade atypia.28 Western and Korean pathologists, generally do not use the Japanese sub-classification of gastric adenocarcinoma. As mentioned above, standardization of terms and diagnostic criteria to reduce the discrepancy in diagnosis between the East and West are needed.
The fourth issue is the histological heterogeneity in submucosal invasive differentiated type gastric adenocarcinoma. Histologically, gastric carcinoma is generally classified into differentiated and undifferentiated type or intestinal and diffuse type. These types are based on morphological features and histological backgrounds.29,30 Several reports showed that a large portion of gastric cancers had histological mixed cancers (differentiated and undifferentiated type).31,32 Mita and Shimoda33 reported that the rate of LN metastasis was significantly higher in differentiated submucosal cancer with histological heterogeneity (combined differentiated type, with poorly differentiated component) than in that without histological heterogeneity (27% vs 7%, p<0.001). It is recommended to be applied to the differentiated submucosal cancer without histological heterogeneity when endoscopic resection in differentiated submucosal cancer is considered. Thus, we have to focus on the histological mixed cancer due to increased risk of LN metastasis in case with submucosal invasion.
The fifth issue is the endoscopic treatment of undifferentiated cancer; the risk of LN metastasis is significantly increased in these cases, due to lymphovascular invasion.34 Recently, 310 undifferentiated cancers without ulceration were analyzed in Japan. There was no LN metastasis in the tumors without lymphovascular invasion and the sizes were less than 20 mm.35 In Korean research, one study demonstrated that poorly differentiated mucosal cancer or minimal submucosal infiltration (≤500 µm) was reported as acceptable for curative endoscopic resection due to the low risk of lymphovascular invasion.36 Another study reported that signet ring cell EGC was more appropriate for endoscopic treatment than poorly differentiated EGC because the latter was significantly associated with ulcers, submucosal invasion, and lymphovascular invasion with reference to signet ring cell EGC. Additionally, youth was viewed as an independent risk factor for LN metastasis only in poorly differentiated EGC;37 however, the results require further study confirmation due to the small sample size.
For the expansion of the criteria for endoscopic submucosal treatment of EGC, confirmation of no difference in the long term survival data between endoscopic treatment and conventional surgery is needed. Thus, large, prospective studies of ESD are needed to address such issues.
TREATMENT OUTCOMES
The disease-specific 5- and 10-year survival rates of EMR are both 99% for differentiated mucosal cancers of less than 2 cm in size.5 Currently, EMR for EGC is the standard initial treatment for EGC in patients that meet the conventional indications.38 In Japan, the EMR outcome for EGC, the rates of en bloc resection, complete resection, recurrence rate, and disease free survival have been reported to be 75.8%, 73.9%, 1.9%, and 99.1%, respectively. For the Korean multicenter study of EMR for EGC, 514 EGCs in 506 patients were treated by EMR. The most commonly used techniques have been circumferential precutting followed by snare resection (EMR-P, 52.3%). The rate of en bloc resection and complete resection were 71.8% and 77.6%, respectively. Tumor size was associated with the rate of complete resection. The rate of complete resection for lesions with diameter of 3 cm or less was 80.2%, whereas the rate for lesions larger than 3 cm was 56.4%. For completely resected mucosal cancers, the median duration of follow-up was 23.5 months (range, 5 to 70 months). In this group, the rate of local recurrence, perforation, and bleeding were 6%, 0.6%, and 13.8%, respectively.39
The risk of local recurrence after EMR depends on the number of resected specimens. In Japan, the recurrence rate after EMR was 2% to 37%.40 When the number of resected specimens was up to 2 samples, the recurrence rate was within 10%. However, it increased to above 20% when the resected specimens included three samples. In other study, significant factor of local recurrence after EMR was a macroscopic finding, especially in case with ulcer finding, the rate of recurrence was 50% which was higher than for other types.39
The introduction of ESD could increase the en bloc resection rate. The outcomes of ESD showed ≥95% en bloc resection rate and about a 90% complete resection rate in several studies.41,42 In a Korean multicenter study of ESD, the rate of en bloc resection and complete resection was reported to be 95.3% and 87.7%, respectively. The rates of delayed bleeding, significant bleeding, and perforation were 15.6%, 0.6%, and 1.2%, respectively. the rates of en bloc resection was significantly associated with the location of the lesions, presence of a scar, and histologic type (Table 2).43 Recently, the outcome of patients with EGC which fulfilled the expanded indication was reported in Japan.44 In this study, ESD was performed for patients with EGC (mucosal cancer without ulcer findings irrespective of tumor size; mucosal cancer with ulcer findings ≤3 cm in size). A total of 559 EGC lesions were enrolled and the median follow-up period was 30 months (range, 6 to 89 months). The rate of en bloc resection and curative resection was 94.9% and 94.7%, respectively. The 5-year overall and disease-specific survival rates were 97.1% and 100%, respectively. The survival outcome of ESD in expanded indication was likely to be excellent, though long term survival outcome remains to be established by a large prospective study.
Table 2.
Factors for En Bloc Resection of ESD for Early Gastric Neoplasms
Data from Chung et al. Gastrointest Endosc 2009;69:1228-1235.43
Data are presented as number (%).
A phase II study of ESD with extended indications for EGC has been started in Korea to evaluate the safety and efficacy of ESD for EGC. This study will collect data on the 5-year survival rate, overall survival in cases without ulcerated lesions, overall survival in cases with ulcerated lesions, the recurrence-free survival, 5-year recurrence-free survival, adverse events and serious adverse events.45
COMPLICATIONS ASSOCIATED WITH ENDOSCOPIC RESECTION
The common complications of ESD are pain, bleeding, and perforation. The pain after endoscopic resection is generally mild and easily controlled by proton pump inhibitors and opioids. Bleeding is the most common complication and is divided into immediate and delayed bleeding. Immediate bleeding during the procedure frequently occurs with the resection of tumors located in the upper third of the stomach because the blood supply is good and many large vessels are located in the body of the stomach (Fig. 4). The rate of bleeding after ESD has been reported to range from 1.8% to 15.6% in Korea.43,46,47 Delayed bleeding is defined as hematemesis or melena at 0 to 30 days after the procedure. In a Korean multicenter study of ESD, delayed bleeding was related to the location (upper portion) and size of the tumor (>40 mm), recurrent lesions, and the macroscopic type (flat lesion).43
Fig. 4.
Bleeding during the endoscopic submucosal dissection procedure. (A) Type IIb early gastric cancer on the high body. (B) Dissection of the submucosal layer. (C) Arterial bleeding from the submucosal layer.
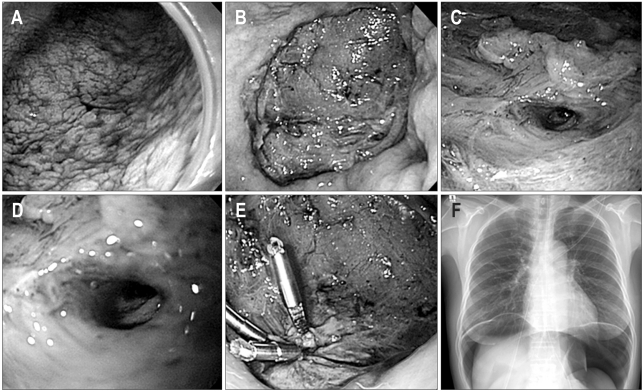
Perforation is less common compared to bleeding. The perforation risk is about 1% to 4% during an ESD procedure.48 Perforations are divided into micro-perforation and frank perforation by the endoscopic findings, as well as immediate and delayed perforations based on the time of occurrence. Generally, a perforation of the stomach is conservatively managed without surgery after closing it with endoclips; this is because the stomach during endoscopic resection is considered clean due to fasting and gastric acid, which have antibacterial effects (Fig. 5).49,50 If a severe pneumoperitoneum develops due to a perforation, decompression of the pneumoperitoneum must be performed using a 14-G puncture needle to prevent the deterioration of breathing and/or neurogenic shock.51
Fig. 5.
Perforation during the endoscopic submucosal dissection (ESD) procedure. (A) Type IIb early gastric cancer on the lower body. (B) Ulcer after ESD procedure. (C, D) Frank perforation of the ulcer bed. (E) Endoscopic closure with clips. (F) Free air on the chest X-ray.
Minor complications that occur with ESD include stricture and aspiration pneumonia. Strictures frequently occur at the cardia and pylorus, especially with lesions around 1 cm at the gastroesophageal junction and pylorus, 3/4 circumferential resection of the lumen and/or longitudinal dissection of more than 5 cm in size; they are successfully treated by balloon dilatation.52 The endoscopist should explain the possibility of a stricture to patients with risk factors before performing the ESD. In patients of advanced age, aspiration pneumonia can occur after an ESD; frequent removal of gastric fluid helps prevent pneumonia and avoid over distention during the ESD.
CONCLUSIONS
EMR is currently considered the standard initial treatment for EGC in cases that meet the conventional indications. This procedure provides patients with a good quality of life and there is also a cost benefit for EGC treatment compared to conventional surgery. The improvement of endoscopic skills with advanced and endoscopic instruments has resulted in a new technique, ESD. ESD is better than the EMR for the removal of large lesions. However, the technical difficulty requires a long learning period and the technical invasiveness increases the risk of bleeding and perforation. Recently, new technical approaches (magnetic anchor-guided ESD, springs, multitask devices, and double endoscopic intraluminal surgery) have evolved in ESD and are making ESD a more efficient and less time-consuming procedure.
Additional studies of ESD are needed to evaluate the extended indications with regard to efficacy, standard diagnosis and terminology and long term outcome. ESD may become a standard procedure for patients with EGC in cases that meet the extended indications; however, confirmation of the long term outcomes and appropriate indications for the procedure are needed.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Bertuccio P, Chatenoud L, Levi F, et al. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009;125:666–673. doi: 10.1002/ijc.24290. [DOI] [PubMed] [Google Scholar]
- 2.Lau M, Le A, El-Serag HB. Noncardia gastric adenocarcinoma remains an important and deadly cancer in the United States: secular trends in incidence and survival. Am J Gastroenterol. 2006;101:2485–2492. doi: 10.1111/j.1572-0241.2006.00778.x. [DOI] [PubMed] [Google Scholar]
- 3.Kang KJ, Lee JH. Characteristics of gastric cancer in Korea - with an emphasis on the increase of the early gastric cancer (EGC) J Korean Med Assoc. 2010;53:283–289. [Google Scholar]
- 4.Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–225. doi: 10.1007/pl00011720. [DOI] [PubMed] [Google Scholar]
- 5.Uedo N, Iishi H, Tatsuta M, et al. Longterm outcomes after endoscopic mucosal resection for early gastric cancer. Gastric Cancer. 2006;9:88–92. doi: 10.1007/s10120-005-0357-0. [DOI] [PubMed] [Google Scholar]
- 6.Kim BJ, Chang TH, Kim JJ, et al. Efficacy and safety of endoscopic submucosal dissection for early gastric cancer in patients with comorbid diseases. Gut Liver. 2010;4:186–191. doi: 10.5009/gnl.2010.4.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oguro Y, Fukutomi H, Suzuki S, et al. Experience of polypectomy for elevated early gastric cancer. Prog Dig Endosc. 1974;5:77–80. [Google Scholar]
- 8.Tada M, Shimada M, Murakami F, et al. Development of strip-off biopsy. Gastroenterol Endosc. 1984;26:833–839. [Google Scholar]
- 9.Hirao M, Masuda K, Asanuma T, et al. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264–269. doi: 10.1016/s0016-5107(88)71327-9. [DOI] [PubMed] [Google Scholar]
- 10.Inoue H, Takeshita K, Hori H, Muraoka Y, Yoneshima H, Endo M. Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc. 1993;39:58–62. doi: 10.1016/s0016-5107(93)70012-7. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki Y, Hiraishi H, Kanke K, et al. Treatment of gastric tumors by endoscopic mucosal resection with a ligating device. Gastrointest Endosc. 1999;49:192–199. doi: 10.1016/s0016-5107(99)70485-2. [DOI] [PubMed] [Google Scholar]
- 12.Kim HS, Lee DK, Baik SK, et al. Endoscopic mucosal resection with a ligation device for early gastric cancer and precancerous lesions: comparison of its therapeutic efficacy with surgical resection. Yonsei Med J. 2000;41:577–583. doi: 10.3349/ymj.2000.41.5.577. [DOI] [PubMed] [Google Scholar]
- 13.Gotoda T, Kondo H, Ono H, et al. A new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: report of two cases. Gastrointest Endosc. 1999;50:560–563. doi: 10.1016/s0016-5107(99)70084-2. [DOI] [PubMed] [Google Scholar]
- 14.Ohkuwa M, Hosokawa K, Boku N, Ohtu A, Tajiri H, Yoshida S. New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy. 2001;33:221–226. doi: 10.1055/s-2001-12805. [DOI] [PubMed] [Google Scholar]
- 15.Gotoda T, Oda I, Tamakawa K, Ueda H, Kobayashi T, Kakizoe T. Prospective clinical trial of magnetic-anchor-guided endoscopic submucosal dissection for large early gastric cancer (with videos) Gastrointest Endosc. 2009;69:10–15. doi: 10.1016/j.gie.2008.03.1127. [DOI] [PubMed] [Google Scholar]
- 16.Sakurazawa N, Kato S, Miyashita M, et al. An innovative technique for endoscopic submucosal dissection of early gastric cancer using a new spring device. Endoscopy. 2009;41:929–933. doi: 10.1055/s-0029-1215191. [DOI] [PubMed] [Google Scholar]
- 17.Mochiki E, Yanai M, Toyomasu Y, et al. Clinical outcomes of double endoscopic intralumenal surgery for early gastric cancer. Surg Endosc. 2010;24:631–636. doi: 10.1007/s00464-009-0666-1. [DOI] [PubMed] [Google Scholar]
- 18.Japanese Gastric Cancer Association. Gastric cancer treatment guideline. 2ne ed. Kyoto: Japanese Gastric Cancer Association; 2004. [Google Scholar]
- 19.Gotoda T, Sasako M, Ono H, Katai H, Sano T, Shimoda T. Evaluation of the necessity for gastrectomy with lymph node dissection for patients with submucosal invasive gastric cancer. Br J Surg. 2001;88:444–449. doi: 10.1046/j.1365-2168.2001.01725.x. [DOI] [PubMed] [Google Scholar]
- 20.An JY, Baik YH, Choi MG, Noh JH, Sohn TS, Kim S. Predictive factors for lymph node metastasis in early gastric cancer with submucosal invasion: analysis of a single institutional experience. Ann Surg. 2007;246:749–753. doi: 10.1097/SLA.0b013e31811f3fb7. [DOI] [PubMed] [Google Scholar]
- 21.Kim WS, Kim BS, Chung BS, et al. Clinical analysis for lymph node metastasis as a guide to modified surgery for early gastric cancer. J Korean Surg Soc. 1998;54:47–55. [Google Scholar]
- 22.Kwak CS, Lee HK, Cho SJ, et al. Analysis of clinicopathological factors associated with lymph node metastasis in early gastric cancer review of 2,137 cases. J Korean Cancer Assoc. 2000;32:674–681. [Google Scholar]
- 23.Park EH, Kang KT, Kim BH, et al. The histologic discrepancy before and after endoscopic submucosal dissection of gastric adenoma and early gastric cancer. Korean J Gastrointest Endosc. 2007;34:125–131. [Google Scholar]
- 24.Kim YJ, Park JC, Kim JH, et al. Histologic diagnosis based on forceps biopsy is not adequate for determining endoscopic treatment of gastric adenomatous lesions. Endoscopy. 2010;42:620–626. doi: 10.1055/s-0030-1255524. [DOI] [PubMed] [Google Scholar]
- 25.Min BH, Kim KM, Kim ER, et al. Endoscopic and histopathological characteristics suggesting the presence of gastric mucosal high grade neoplasia foci in cases initially diagnosed as gastric mucosal low grade neoplasia by forceps biopsy in Korea. J Gastroenterol. 2011;46:17–24. doi: 10.1007/s00535-010-0289-2. [DOI] [PubMed] [Google Scholar]
- 26.Schlemper RJ, Kato Y, Stolte M. Review of histological classifications of gastrointestinal epithelial neoplasia: differences in diagnosis of early carcinomas between Japanese and Western pathologists. J Gastroenterol. 2001;36:445–456. doi: 10.1007/s005350170067. [DOI] [PubMed] [Google Scholar]
- 27.Schlemper RJ, Itabashi M, Kato Y, et al. Differences in diagnostic criteria for gastric carcinoma between Japanese and western pathologists. Lancet. 1997;349:1725–1729. doi: 10.1016/S0140-6736(96)12249-2. [DOI] [PubMed] [Google Scholar]
- 28.Shimoda T, Nimura S, Sekine S, Nakanish Y. Progress of gastric research and its clinical implication. Stomach Intest. 2003;38:43–56. [Google Scholar]
- 29.Nakamura K, Sugano H, Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gann. 1968;59:251–258. [PubMed] [Google Scholar]
- 30.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 31.Luinetti O, Fiocca R, Villani L, Alberizzi P, Ranzani GN, Solcia E. Genetic pattern, histological structure, and cellular phenotype in early and advanced gastric cancers: evidence for structure-related genetic subsets and for loss of glandular structure during progression of some tumors. Hum Pathol. 1998;29:702–709. doi: 10.1016/s0046-8177(98)90279-9. [DOI] [PubMed] [Google Scholar]
- 32.Haruma K, Sumii K, Inoue K, Teshima H, Kajiyama G. Endoscopic therapy in patients with inoperable early gastric cancer. Am J Gastroenterol. 1990;85:522–526. [PubMed] [Google Scholar]
- 33.Mita T, Shimoda T. Risk factors for lymph node metastasis of submucosal invasive differentiated type gastric carcinoma: clinical significance of histological heterogeneity. J Gastroenterol. 2001;36:661–668. doi: 10.1007/s005350170028. [DOI] [PubMed] [Google Scholar]
- 34.Nasu J, Nishina T, Hirasaki S, et al. Predictive factors of lymph node metastasis in patients with undifferentiated early gastric cancers. J Clin Gastroenterol. 2006;40:412–415. doi: 10.1097/00004836-200605000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148–152. doi: 10.1007/s10120-009-0515-x. [DOI] [PubMed] [Google Scholar]
- 36.Park YD, Chung YJ, Chung HY, et al. Factors related to lymph node metastasis and the feasibility of endoscopic mucosal resection for treating poorly differentiated adenocarcinoma of the stomach. Endoscopy. 2008;40:7–10. doi: 10.1055/s-2007-966750. [DOI] [PubMed] [Google Scholar]
- 37.Kim HM, Pak KH, Chung MJ, et al. Early gastric cancer of signet ring cell carcinoma is more amenable to endoscopic treatment than is early gastric cancer of poorly differentiated tubular adenocarcinoma in select tumor conditions. Surg Endosc. 2011;25:3087–3093. doi: 10.1007/s00464-011-1674-5. [DOI] [PubMed] [Google Scholar]
- 38.Fukase K, Matsuda T, Suzuki M, et al. Evaluation of the efficacy of endoscopic treatment for gastric cancer considered in terms of long-term prognosis: a comparison with surgical treatment. Dig Endosc. 1994;6:241–247. [Google Scholar]
- 39.Kim JJ, Lee JH, Jung HY, et al. EMR for early gastric cancer in Korea: a multicenter retrospective study. Gastrointest Endosc. 2007;66:693–700. doi: 10.1016/j.gie.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 40.Ono H. Early gastric cancer: diagnosis, pathology, treatment techniques and treatment outcomes. Eur J Gastroenterol Hepatol. 2006;18:863–866. doi: 10.1097/00042737-200608000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Gotoda T. A large endoscopic resection by endoscopic submucosal dissection procedure for early gastric cancer. Clin Gastroenterol Hepatol. 2005;3(7 Suppl 1):S71–S73. doi: 10.1016/s1542-3565(05)00251-x. [DOI] [PubMed] [Google Scholar]
- 42.Yahagi N, Fujishiro M, Kakushima N, et al. Endoscopic submucosal dissection for early gastric cancer using the tip of an electrosurgical snare (thin type) Dig Endosc. 2004;16:34–38. [Google Scholar]
- 43.Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228–1235. doi: 10.1016/j.gie.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 44.Isomoto H, Shikuwa S, Yamaguchi N, et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331–336. doi: 10.1136/gut.2008.165381. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka M, Ono H, Hasuike N, Takizawa K. Endoscopic submucosal dissection of early gastric cancer. Digestion. 2008;(77 Suppl 1):23–28. doi: 10.1159/000111484. [DOI] [PubMed] [Google Scholar]
- 46.Jeon SW, Jung MK, Cho CM, et al. Predictors of immediate bleeding during endoscopic submucosal dissection in gastric lesions. Surg Endosc. 2009;23:1974–1979. doi: 10.1007/s00464-008-9988-7. [DOI] [PubMed] [Google Scholar]
- 47.Min BH, Lee JH, Kim JJ, et al. Clinical outcomes of endoscopic submucosal dissection (ESD) for treating early gastric cancer: comparison with endoscopic mucosal resection after circumferential precutting (EMR-P) Dig Liver Dis. 2009;41:201–209. doi: 10.1016/j.dld.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 48.Ida K, Katoh T, Nakjima T, Tsuboi Y, Kojima T, Okuda J. Outcome after using EMR according to standard guideline for endoscopic treatment of early gastric cancer. Stomach Intest. 2002;37:1137–1143. [Google Scholar]
- 49.Tsunada S, Ogata S, Ohyama T, et al. Endoscopic closure of perforations caused by EMR in the stomach by application of metallic clips. Gastrointest Endosc. 2003;57:948–951. doi: 10.1016/s0016-5107(03)70051-0. [DOI] [PubMed] [Google Scholar]
- 50.Minami S, Gotoda T, Ono H, Oda I, Hamanaka H. Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video) Gastrointest Endosc. 2006;63:596–601. doi: 10.1016/j.gie.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 51.Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1–11. doi: 10.1007/s10120-006-0408-1. [DOI] [PubMed] [Google Scholar]
- 52.Coda S, Oda I, Gotoda T, Yokoi C, Kikuchi T, Ono H. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy. 2009;41:421–426. doi: 10.1055/s-0029-1214642. [DOI] [PubMed] [Google Scholar]
- 53.Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490–4498. doi: 10.1200/JCO.2005.19.935. [DOI] [PubMed] [Google Scholar]