Abstract
Background/Aims
Complementary medicines, including herbal preparations and nutritional supplements, are widely used without prescriptions. As a result, there has been growing interest in the risk of hepatotoxicity with these agents. It is difficult to determine causal relationships between these herbal preparations and hepatotoxicity. We report on 25 patients diagnosed with toxic hepatitis following ingestion of Polygonum multiflorum Thunb.
Methods
Twenty-five patients (median age, 48 years [24 to 65 years]; M:F=18:7) with suspected P. multiflorum Thunb-induced liver injury were admitted to our hospital between 2007 and 2009. We analyzed clinical and histological data, including the types and the duration of P. multiflorum Thunb intake and the duration of hospital care. We also determined the type of liver injury using the R ratio (serum activity of ALT/serum activity of ALP).
Results
The types of complementary medicine used included tea (n=16), liquor (n=5), tea and liquor (n=2), powder (n=1), and honeyed pudding (n=1). The most common presenting sign was jaundice (76%), and 18 patients (72%) had evidence of hepatocellular liver injury. Histological findings were consistent with acute hepatitis in all cases (n=10) for which liver biopsy was performed. Twenty-three patients (91.6%) recovered with conservative management, 1 patient (4%) had a liver transplant, and 1 patient (4%) died of hepatic failure.
Conclusions
In our cases, we found that P. multiflorum Thunb could be hepatotoxic and could lead to severe drug-induced liver injury, and even death.
Keywords: Drug induced liver injury, Polygonum multiflorum Thunb, Hepatotoxicity
INTRODUCTION
Drug induced liver injury (DILI) is an adverse drug reaction associated with commonly used drugs, mostly nonsteroidal anti-inflammatory drugs, paracetamol, and antimicrobial agents.1 In some reports, more than a 1,000 drugs of the modern pharmacopoeia can induce liver injury with different clinical presentations.2,3 Diagnosis of DILI is difficult and data on the incidence of DILI cases are extremely variable due to a lack of internationally accepted criteria of DILI.4-6 DILI associated with herbal preparations is not uncommon in Western and Asian societies.7-11 DILI caused by a single plant taken as a nutritional supplement has been occasionally reported. Whereas, it has not been reported to be caused by herbal preparations consisting of multiple plants.12,13 Many people understand that natural botanicals are beneficial but not harmful. However, natural botanicals can be also causes of DILI,13 because herbal medicine induced liver injury is caused by its metabolites as well as natural botanicals.
Polygonum multiflorum Thunb is one of Chinese herbs, which has generated a lot of interests. It is the root of P. multiplorum, a member of Polygonaceae. The root tuber of this plant was recorded in herbal Kaibao Bencao published by the imperial court of the Song Dynasty (973-974 A.D.).14 P. multiflorum Thunb is consumed either in its raw form, or as an extract after processing. It is generally referred to as Shou Wu Pian or Ho-Shou-Wu. The most well-known products associated with hepatotoxicity of P. multiflorum are Shou-Wu-Pian and Shen-Min, which are usually consumed as an anti-aging product, or as a tonic for dizziness with tinnitus. They also appear to be efficacious in the treatment of premature greying of hair, lumbago, spermatorrhea, leucorrhea and constipation.15,16 Thus far, 6 cases of acute hepatitis induced by P. multiflorum Thunb, a main constituent of Sho-Wu-Pian,14,15,17-19 have been reported worldwide, including one case in Korea.20
We describe 25 cases of hepatitis caused by P. multiflorum Thunb with analyzing the results of their clinical course, laboratory and histological findings.
MATERIALS AND METHODS
This study entailed collection and analyses of clinical information using questionnaires and laboratory results in 25 patients diagnosed with P. multiflorum Thunb-induced hepatitis, among patients diagnosed with toxic hepatitis in the Gastroenterology Department of the Gyeongsang National University Hospital between 2007 and 2009. The diagnosis was established on the basis of drug history before the symptomatic period, physical examination on admission and acute hepatitis according to laboratory investigations or histological findings. More than 2 well-experienced gastroenterologists in the field of toxic hepatitis were highly suspicious of DILI and excluded other possible causes of hepatitis such as HAV, HBV, HCV, HEV, EBV, CMV, HSV, autoimmune hepatitis, Wilson's disease and alcohol in our cases. When we initiated the study, there was a mass outbreak of DILI in the same region, which was thought to be possibly caused by P. multiflorum Thunb. Then we conducted a systematic questionnaire survey to assess the likelihood of P. multiflorum Thunb-induced liver injury with a good protocol. We were consistently meticulous in history-taking and assessment to rule out other possible causes of liver injury. Most of the patients were followed-up at least for 6 months after recovery from the disease. We conducted analyses based on sex, age, clinical manifestations on admission, laboratory data, duration of intake, the time of symptom onset, histological findings and clinical course. We also estimated the Roussel Uclaf Causality Assessment Method (RUCAM) scale to enhance the reliability of diagnosis of DILI. Sixty-four percents of the patients lived in single city, Sacheon and 15 cases (60%) of them occurred between May and November 2007.
RESULTS
The patient characteristics and outcomes were summarized in Table 1.
Table 1.
Patient Characteristics and Outcomes
CIOMS, Council for International Organizations of Medical Sciences; RUCAM, Roussel Uclaf Causality Assessment Method; ALP, alkaline phosphatase; ALT, alanine aminotransferase; TB, total bilirubin; PT, prothrombin time; INR, international normalized ratio; LT, liver transplantation.
*Warfarinized patient.
1. Gender and age
The patients consisted of 18 men and 7 women. Their age ranged between 24 and 65, with a median age of 48.
2. Clinical manifestations on admission
The main clinical manifestations leading to patient admissions were as follows: jaundice (19 cases), abdominal pain with nausea (2 cases), fatigue (2 cases), and myalgia (1 case), and no symptom (1 case). During the hospitalization 5 patients developed fever, arthralgia in 2 patients, eosinophilia in 1 patient and skin rash in 2 patients.
3. Type and duration of intake, and onset time of symptoms
Sixteen patients drank tea boiled with the root of the P. multiflorum Thunb, 5 patients drank liquor made of the plant, 2 patients drank tea and liquor soaked out from it, 1 patient took honey mixed with ground powder of its root, and 1 patient took the ground powder of its root. The median duration of intake was 30 days (range, 1 to 180 days). Toxic hepatitis developed in 1 patient who had ingested it for 1 day and another 1 patient for 2 days. The interval from intake to the onset of symptoms ranged between 1 and 120 days, with a mean of 31 days.
4. Laboratory findings
At the time of admission 24 patients showed markedly elevated alanine aminotransferase (ALT; >10×UNL) and 9 had remarkably elevated total bilirubin (>20 mg/dL). In patients who presented to our hospital via private hospitals, we included their initial laboratory data in private hospitals. Eighteen patients had hepatocelluar liver injury and the remainder of the patients including 1 alcoholics had mixed pattern liver injury according to laboratory data. Four patients had prolonged prothrombin time (international normalized ratio [INR] >1.2) and 2 of them had an INR of 9.56 and 14.98, respectively. Serologic markers were all negative: HBsAg(-), IgM Anti-HBc(-), IgM Anti-HAV(-), Anti-HCV(-), HCV-PCR(-), anti-CMV(-), EBV IgM(-), EBV IgG(-), FANA(-), anti-smooth muscle Ab(-), anti-mitochondrial Ab(-). Iron, TIBC, ceruloplasmin, α-1 antitrypsin and IgG were within the normal range. Based on these results we could exclude other causes of acute hepatitis. The mean score of Council of International Organizations of Medical Scientists (CIOMS) or RUCAM was 8 (range, 6-10).
5. Findings of liver biopsy
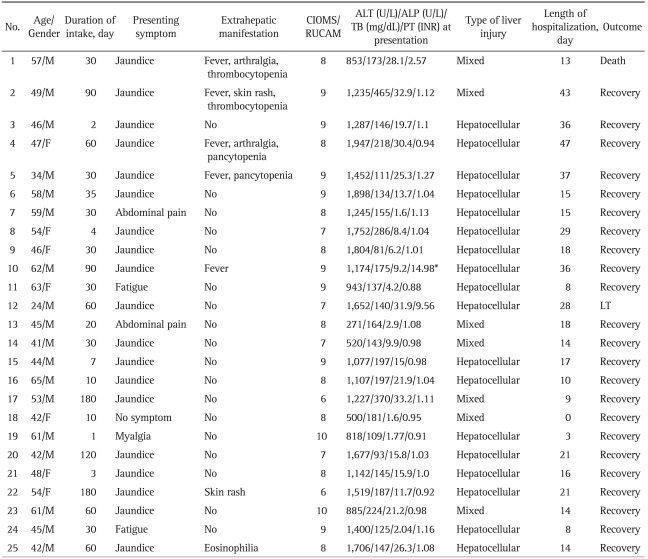
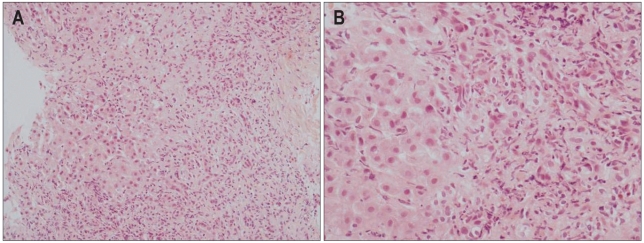
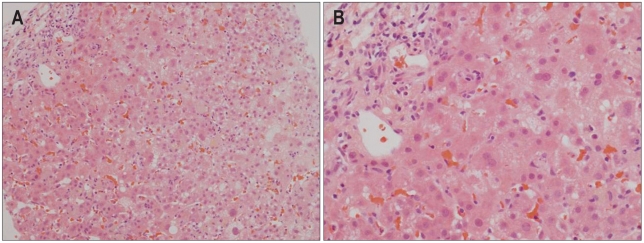
Liver biopsy was conducted in 9 individuals during hospital stay. One patient progressed to fulminant hepatitis and eventually received liver transplantation, which enabled us to obtain the liver specimen that demonstrated the extent of organ injury. Among the 13 individuals in whom liver biopsy was not performed due to rapid clinical improvement or outright refusal against the procedure, 1 patient died of rapid progressing hepatic failure. Of 2 patients with alcoholic liver disease, liver biopsy was conducted in one patient which demonstrated periportal and perivenular neutrophilic infiltration, fatty change, apoptotic body, bridging necrosis and fibrosis, indicative of toxic hepatitis mixed with alcoholic hepatitis (Fig. 1). The histological findings of the remaining 8 patients demonstrated inflammatory cell infiltration, necrosis of hepatic cells and mild to moderate cholestasis consistent with drug or toxin induced acute hepatitis (Fig. 2). The patient who received liver transplantation showed diffuse necrosis of hepatic cells (Fig. 3).
Fig. 1.
The liver biopsy of an alcoholic patient shows focal mild fatty change, the infiltration of many neutrophils and occasional eosinophils, apoptotic bodies with bridging necrosis and fibrosis (A, H&E stain, ×200; B, H&E stain, ×400).
Fig. 2.
Microscopic examination shows lobular portal inflammation with cholestasis and hepatocyte damage (A, H&E stain, ×200; B, H&E stain, ×400).
Fig. 3.
(A) The cut surface of the explanted liver shows slight nodularity and a brownish-tan color change. (B) Microscopic findings demonstrate massive hepatocellular necrosis (H&E stain, ×100).
6. Clinical course
Patients ceased taking P. multiflorum Thunb upon hospital admission. Twenty three patients showed gradual improvement of liver function, although one patient had progressed to fulminant hepatitis and subsequently received liver transplantation. Another one patient with underlying alcoholic liver cirrhosis died of acute hepatic failure with rapid clinical deterioration. The mean hospital stay of patients was 20 days.
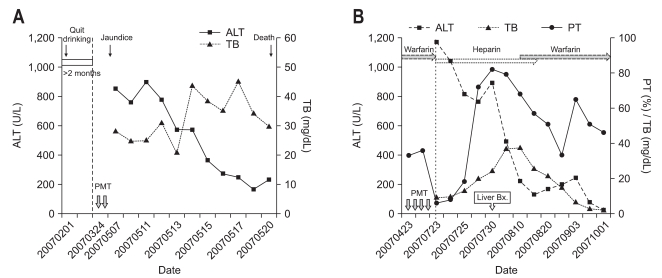
One patient (case 1) had taken one bottle of Soju (360 cc) daily for 20 years, which had about 20 percent alcohol content, who had ceased alcohol intake 3 months prior to hospitalization. He rapidly progressed to hepatic failure after hospitalization, and on the fourth day of admission brain computed tomography demonstrated hepatic encephalopathy and cerebral edema. He refused liver transplantation and then developed acute renal failure on the eighth day of admission and died on the 13th day of admission (Fig. 4A). As the patient had ceased alcohol intake 3 months prior to hospitalization before the intake of P. multiflorum Thunb, it is reasonable to conclude that the direct cause of liver injury was the plant, not the alcohol. It is also reasonable to assume that patients with underlying alcoholic liver cirrhosis have worse outcomes in drug induced liver injuries. We previously reported case 5,15 a 34-year-old man (case 5) who was diagnosed with reactivation of pulmonary tuberculosis in association with immunosuppression caused by P. multiflorum Thunb. A 62-year-old man (case 10) who was warfarinized due to cerebral infarction and atrial fibrillation had well-controlled INR in the range between 2 and 3 prior to hospitalization. A severely prolonged prothrombin time, an INR of 14.98, were noted on admission, it was rapidly improving after he stopped taking warfarin. This case showed the potential herb-drug interaction between the P. multiflorum Thunb and warfarin (Fig. 4B). A 24-year-old man (case 12), who had been healthy, drank tea boiled with P. multiflorum Thunb for approximately 1 month in addition to taking some folk remedies was admitted with acute liver failure. He was subsequently transferred to the liver transplantation center for receiving a liver transplant. The patient in case 18 is the wife of the patient in case 15, had a history of P. multiflorum Thunb ingestion. She was recommended to undergo a liver function test and diagnosed with asymptomatic hepatitis. She stopped taking P. multiflorum Thunb, and then her liver function test improved.
Fig. 4.
Changes in clinical parameters and characteristic findings during the hospital course of case 12 (A) and case 15 (B).
ALT, alanine aminotransferase; TB, total bilirubin; PT, prothrombin time; PMT, Polygonum multiflorum Thunb.
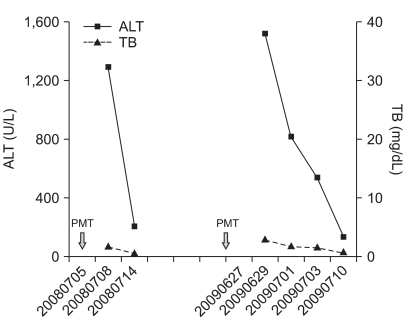
We put a premium on the patient in case 19, who developed liver injury by rechallenge of P. multiflorum Thunb (Fig. 5). In other words he had a past medical history of DILI associated with P. multiflorum Thunb 10 months previously. Even though he took a single dose of P. multiflorum Thunb this time, he presented with myalgia with markedly elevated serum transaminase. His liver function showed rapid improvement after ceasing P. multiflorum Thunb intake.
Fig. 5.
This figure shows 2-fold markedly elevated aminotransferase levels following exposure to the same substance and rapid recovery immediately after the patient stopped taking it (case 19).
ALT, alanine aminotransferase; TB, total bilirubin; PMT, Polygonum multiflorum Thunb.
DISCUSSION
DILI is defined as biochemical injury to the liver proven to be caused by intake of a drug within the previous three months. The diagnosis is established when other causes of the liver disease are excluded. DILI is characterized by the tendency to improve following discontinuation of the offending drug, which recurs after recommencement.21,22 However, the study of DILI is still difficult and controversial due to its lack of the reliability of serologic or histological tests to make a definite diagnosis. The articles recently reported showed unsatisfactory results regarding the reliability of RUCAM.23 A comparative study with respect to expert opinions and RUCAM revealed that the former was better in the diagnostic concordance.24 However, we do not have many researches to support this yet. Until now the most important method to make a diagnosis of DILI is to rule out other causes of liver injury for the patients with a history of medications or herbal medicines that have hepatotoxicity. Like the preceding diagnosis of DILI was based on the author's opinions and the RUCAM scale, and thus some controversy still exists in this study.
As interests in health grows, herbal preparations and plants become widely consumed by people all over the world, including Korea.25,26 Polygonaciae is generally known to be safe and not all who consume it develop liver injury. The patients enrolled in this study were symptomatic and were examined medically, except for one patient. In most cases of asymptomatic hepatitis following ingestion of P. multiflorum Thunb, as evidenced in case 18, the patients did not seek medical consultation. Considering those event of asymptomatic or mild hepatitis, the frequency of liver injury caused by P. multiflorum Thunb is expected to be high. The main constituents of Shou-Wu-Pain are polyphenols and anthraquinones - the latter is metabolized into anthrones and absorbed in the gastrointestinal tract, which can induce liver damage.13,15,17,27,28 To date, there are 6 case reports of P. multiflorum Thunb-induced hepatotoxicity14,15,17-20 although the mechanism of P. multiflorum Thunb-induced hepatotoxicity is still unclear. Most of the patients recovered their liver function with conservative management. Our patients consumed the root of P. multiflorum as the different forms including tea, liquor or powder. There existed a broad spectrum of their clinical course: from asymptomatic state to fulminant hepatitis and death. Unlike previous reports, our cases were different in that P. multiflorum Thunb was consumed in its natural form and not having been processed or blended with other ingredients. Therefore, the causality between P. multiflorum and hepatotoxicity was relatively clear. In this study we also checked the CIOMS/RUCAM research using causality assessment26 in all our patients, the results indicated that our patients were probable to definite DILI.
The patients had a history of having ingested P. multiflorum Thunb in various forms; tea boil with P. multiflorum Thunb, liquor made of P. multiflorum Thunb, honey soaked out with P. multiflorum Thunb, and the powder of dried P. multiflorum Thunb. However, it is assumed that the form of intake has no relation to the severity of hepatotoxicity. The type of liver injury on the basis of laboratory findings at the time of diagnosis was hepatocellular in 18 patients. The histological findings were consistent with toxic hepatitis in 7 out of 9 individuals in whom liver biopsy was performed, with the exception of 2 patients whose specimen demonstrated toxic hepatitis mixed with alcoholic hepatitis. Although only a few cases existed, given that the clinical course was aggravated by markedly prolonged prothrombin time following concurrent administration of P. multiflorum and warfarin, we cautiously suggest that P. multiflorum might interact with alcohol and/or warfarin. The potential for herb-drug interaction as well as DILI should also be considered.11
Given that liver injury was dose-independent and the time interval from P. multiflorum Thunb intake to clinical manifestation was short in some patients, there might be different opinions about their diagnosis. But, authors presume that the mechanism of P. multiflorum Thunb-induced liver injury is idiosyncratic liver injury even though we cannot assure you that our anticipation is absolutely true owing to the lack of data. The patient in case 19 had no history of previous chronic disease of other organs including the liver and had a history of hepatitis caused by the same substance one year before. He complained only myalgia without associated gastrointestinal symptoms just after another exposure to P. multiflorum Thunb and his liver function test showed hepatocelluar pattern with normal muscle enzyme. But, he made a rapid recovery after the discontinuation of P. multiflorum Thunb intake. Authors judged that this information supported the correlation between P. multiflorum Thunb and liver injury in case 19 which also carried an important thing as a rechallenge case.
Herbal preparations and dietary supplements are widely consumed in many countries under the assumption that they are natural and therefore safe.13,19,29 Consumers are unaware that they can potentially be hazardous to their health. As P. multiflorum Thunb is one of them, this study highlights the seriousness of its hepatotoxicity and reminds us of the fact that consumers tend to place undue emphasis on the perceived benefits of herbal preparations while neglecting their potential toxicities. P. multiflorum Thunb has been also favorably advertised on the Internet and the printed media, and being actively cultivated in lots of places.
Our study is worthy of notice for 3 main reasons. First, the patient in case 19 is all the more meaningful in that it can be a direct evidence for the causal relationship between liver injury and P. multiflorum Thunb. Second, even worse, many of our patients expressed their wish to continue consuming herbal preparations in the future. In other words, the repeated consumption of P. multiflorum Thunb is just as problematic as the liver injuries it caused. Third, the age of the enrolled patients ranged between 24 and 65 (median, 48), a relatively young generation. During hospital stay, the youngest patient received a liver transplant and another one eventually died from liver failure, which implies that there is a social context behind the use of P. multiflorum Thunb.
In summary, this study was derived from the author's experiences of managing liver injuries induced by P. multiflorum Thunb in 25 patients who followed various clinical courses, from an absence of symptom to death. Previous reports showed comparatively mild clinical courses with a handful of cases, while this study was based on a variety of cases pertaining to a single herb experienced in a single center. For this reason, this study is significant when it comes to establishing the causality between P. multiflorum Thunb and hepatotoxicity. To overcome diagnostic limitation, a new diagnostic guideline which minimizes the interobserver variability and has a good reliability urgently needs to be established. In conclusion, our study demonstrated an association between P. multiflorum Thunb and hepatotoxicity. As the increasing interests in botanical and nutritional supplements, we need to have systematic studies to identify the potential toxicities of those products and educate the general public from their harmful effects.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Verma S, Kaplowitz N. Diagnosis, management and prevention of drug-induced liver injury. Gut. 2009;58:1555–1564. doi: 10.1136/gut.2008.163675. [DOI] [PubMed] [Google Scholar]
- 2.Biour M, Ben Salem C, Chazouillères O, Grangé JD, Serfaty L, Poupon R. Drug-induced liver injury; fourteenth updated edition of the bibliographic database of liver injuries and related drugs. Gastroenterol Clin Biol. 2004;28:720–759. doi: 10.1016/s0399-8320(04)95062-2. [DOI] [PubMed] [Google Scholar]
- 3.Larrey D. Drug-induced liver diseases. J Hepatol. 2000;32(1 Suppl):77–88. doi: 10.1016/s0168-8278(00)80417-1. [DOI] [PubMed] [Google Scholar]
- 4.Meier Y, Cavallaro M, Roos M, et al. Incidence of drug-induced liver injury in medical inpatients. Eur J Clin Pharmacol. 2005;61:135–143. doi: 10.1007/s00228-004-0888-z. [DOI] [PubMed] [Google Scholar]
- 5.Bagheri H, Michel F, Lapeyre-Mestre M, et al. Detection and incidence of drug-induced liver injuries in hospital: a prospective analysis from laboratory signals. Br J Clin Pharmacol. 2000;50:479–484. doi: 10.1046/j.1365-2125.2000.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sgro C, Clinard F, Ouazir K, et al. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. 2002;36:451–455. doi: 10.1053/jhep.2002.34857. [DOI] [PubMed] [Google Scholar]
- 7.MacGregor FB, Abernethy VE, Dahabra S, Cobden I, Hayes PC. Hepatotoxicity of herbal remedies. BMJ. 1989;299:1156–1157. doi: 10.1136/bmj.299.6708.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woolf GM, Petrovic LM, Rojter SE, et al. Acute hepatitis associated with the Chinese herbal product jin bu huan. Ann Intern Med. 1994;121:729–735. doi: 10.7326/0003-4819-121-10-199411150-00001. [DOI] [PubMed] [Google Scholar]
- 9.Gordon DW, Rosenthal G, Hart J, Sirota R, Baker AL. Chaparral ingestion: the broadening spectrum of liver injury caused by herbal medications. JAMA. 1995;273:489–490. doi: 10.1001/jama.273.6.489. [DOI] [PubMed] [Google Scholar]
- 10.Chitturi S, Farrell GC. Herbal hepatotoxicity: an expanding but poorly defined problem. J Gastroenterol Hepatol. 2000;15:1093–1099. doi: 10.1046/j.1440-1746.2000.02349.x. [DOI] [PubMed] [Google Scholar]
- 11.Seeff LB. Herbal hepatotoxicity. Clin Liver Dis. 2007;11:577–596. doi: 10.1016/j.cld.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Stickel F, Egerer G, Seitz HK. Hepatotoxicity of botanicals. Public Health Nutr. 2000;3:113–124. doi: 10.1017/s1368980000000161. [DOI] [PubMed] [Google Scholar]
- 13.Stickel F, Patsenker E, Schuppan D. Herbal hepatotoxicity. J Hepatol. 2005;43:901–910. doi: 10.1016/j.jhep.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 14.But PP, Tomlinson B, Lee KL. Hepatitis related to the Chinese medicine Shou-wu-pian manufactured from Polygonum multiflorum. Vet Hum Toxicol. 1996;38:280–282. [PubMed] [Google Scholar]
- 15.Park GJ, Mann SP, Ngu MC. Acute hepatitis induced by Shou-Wu-Pian, a herbal product derived from Polygonum multiflorum. J Gastroenterol Hepatol. 2001;16:115–117. doi: 10.1046/j.1440-1746.2001.02309.x. [DOI] [PubMed] [Google Scholar]
- 16.Laird AR, Ramchandani N, deGoma EM, Avula B, Khan IA, Gesundheit N. Acute hepatitis associated with the use of an herbal supplement (Polygonum multiflorum) mimicking iron-overload syndrome. J Clin Gastroenterol. 2008;42:861–862. doi: 10.1097/MCG.0b013e3181492515. [DOI] [PubMed] [Google Scholar]
- 17.Mazzanti G, Battinelli L, Daniele C, et al. New case of acute hepatitis following the consumption of Shou Wu Pian, a Chinese herbal product derived from Polygonum multiflorum. Ann Intern Med. 2004;140:W30. doi: 10.7326/0003-4819-140-7-200404060-00042-w3. [DOI] [PubMed] [Google Scholar]
- 18.Panis B, Wong DR, Hooymans PM, De Smet PA, Rosias PP. Recurrent toxic hepatitis in a Caucasian girl related to the use of Shou-Wu-Pian, a Chinese herbal preparation. J Pediatr Gastroenterol Nutr. 2005;41:256–258. doi: 10.1097/01.mpg.0000164699.41282.67. [DOI] [PubMed] [Google Scholar]
- 19.Cárdenas A, Restrepo JC, Sierra F, Correa G. Acute hepatitis due to shen-min: a herbal product derived from Polygonum multiflorum. J Clin Gastroenterol. 2006;40:629–632. doi: 10.1097/00004836-200608000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Cho HC, Min HJ, Ha CY, et al. Reactivation of pulmonary tuberculosis in a patient with Polygonum multiflorum Thunb-induced hepatitis. Gut Liver. 2009;3:52–56. doi: 10.5009/gnl.2009.3.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maria VA, Victorino RM. Development and validation of a clinical scale for the diagnosis of drug-induced hepatitis. Hepatology. 1997;26:664–669. doi: 10.1002/hep.510260319. [DOI] [PubMed] [Google Scholar]
- 22.Bénichou C. Criteria of drug-induced liver disorders: report of an international consensus meeting. J Hepatol. 1990;11:272–276. doi: 10.1016/0168-8278(90)90124-a. [DOI] [PubMed] [Google Scholar]
- 23.Rochon J, Protiva P, Seeff LB, et al. Reliability of the Roussel Uclaf Causality Assessment Method for assessing causality in druginduced liver injury. Hepatology. 2008;48:1175–1183. doi: 10.1002/hep.22442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rockey DC, Seeff LB, Rochon J, et al. Causality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment method. Hepatology. 2010;51:2117–2126. doi: 10.1002/hep.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States: prevalence, costs, and patterns of use. N Engl J Med. 1993;328:246–252. doi: 10.1056/NEJM199301283280406. [DOI] [PubMed] [Google Scholar]
- 26.Kessler RC, Davis RB, Foster DF, et al. Long-term trends in the use of complementary and alternative medical therapies in the United States. Ann Intern Med. 2001;135:262–268. doi: 10.7326/0003-4819-135-4-200108210-00011. [DOI] [PubMed] [Google Scholar]
- 27.de Witte P, Lemli L. The metabolism of anthranoid laxatives. Hepatogastroenterology. 1990;37:601–605. [PubMed] [Google Scholar]
- 28.Lucena MI, García-Cortés M, Cueto R, Lopez-Duran J, Andrade RJ. Assessment of drug-induced liver injury in clinical practice. Fundam Clin Pharmacol. 2008;22:141–158. doi: 10.1111/j.1472-8206.2008.00566.x. [DOI] [PubMed] [Google Scholar]
- 29.Ernst E. Risks of herbal medicinal products. Pharmacoepidemiol Drug Saf. 2004;13:767–771. doi: 10.1002/pds.1014. [DOI] [PubMed] [Google Scholar]