Abstract
Background/Aims
Heat shock proteins (HSPs) protect rats from cerulein-induced acute pancreatitis (AP) by preventing the subcellular redistribution of cathepsin B and the activation of trypsinogen. Autophagy plays a critical role in the secretion of digestive enzymes and triggering of cerulein-induced AP via the colocalization of trypsinogen and lysosomes. Therefore, using a rat cerulein-induced AP model, we investigated whether HSPs prevent AP by regulating autophagy.
Methods
Twelve hours after fed standard laboratory chow and water, the experimental groups (cerulein, water-immersion [WI]-cerulein and heat-shock [HS]-cerulein) and the control groups (control, WI, and HS) received one intraperitoneal injection of cerulein (50 µg/kg) or saline, respectively. All of the rats were sacrificed at 6 hours after injection. The severity of the AP was assessed based on the serum amylase level and the histological and electron microscopy findings. Western blotting was also performed for HSP60/70 and LC3B-II.
Results
WI and HS induced HSP60 and HSP70, respectively. The induced HSP60/70 effectively prevented the development of cerulein-induced AP. Autophagy developed in the rats with cerulein-induced AP and was documented by the expression of LC3-II and electron microscopy findings. The WI-stressed rats and HS-treated rats did not develop cerulein-induced autophagy.
Conclusions
HSPs exert protective effects against cerulein-induced AP in rats by inhibiting autophagy.
Keywords: Acute pancreatitis, Autophagy, Heat shock protein
INTRODUCTION
The normal pancreas prevents autodigestion by packaging protease in a precursor form, by the synthesis of protease inhibitors and by lowering the intracellular calcium concentrations to decrease the trypsin activity. However, in the early stage of acute pancreatitis (AP), autodigestion is provoked by inappropriate activation of proteolytic enzymes within the acinar cells. These early events are followed by the generation of proinflammatory mediators that promote an extra-acinar inflammatory response and in extreme cases these proinflammatory mediators promote a systemic inflammatory response syndrome.1,2
Heat shock proteins (HSPs) are protective mechanisms of living organisms that ensure survival under stressful conditions such as infection, inflammation, starvation, water immersion, heat, heavy metals, and oxidative stress.3-5 HSPs are classified into 6 major families according to their molecular weight (HSP100, HSP90, HSP70, HSP60, HSP40, and small HSPs).3 HSPs function as molecular chaperones and so they play important roles in protein synthesis, folding, intracellular and transmembrane transport.3,6-8 Previous studies have reported that water-immersion (WI) and heat-shock (HS) induced HSP60 and HSP70, respectively and these induced HSPs protected the rats from cerulein-induced AP by preventing the subcellular redistribution of cathepsin B as well as preventing the activation of intrapancreatic trypsinogen.1,4,9-12 However, the mechanism by which HSP60/70 inhibits trypsinogen activation is still uncertain.
Autophagy is highly preserved process that plays a part in development, differentiation and homeostasis by maintaining the synthesis, degradation and recycling of cellular constituents through the lysosomes.13-16 There are 3 types of autophagy:17 macro-autophagy, micro-autophagy, and chaperone-mediated autophagy. The role of autophagy in disease has been linked to the prevention of some diseases such as neurodegeneration and cancer, and protection against infection. However, it may also contribute to the development of diseases in some situations.17,18 In the pancreas, it has been reported that autophagy play a critical role in secreting digestive enzymes and to trigger cerulein-induced AP in its early stage.13,19 The mechanism for AP induction by autophagy is colocalization of trypsinogen and lysosomes, and this is followed by trypsinogen activation.13,20
Therefore, we investigated whether HSPs regulate autophagy formation and prevent AP with employing a cerulein-induced AP rat model and by measuring the severity of AP and the LC3 immunoreactivity, which is a marker of autophagosomes, with or without preconditioning the HSPs.
MATERIALS AND METHODS
1. Materials
Six to 8 week old Sprague-Dawley rats weighing 150 to 200 g each were purchased from Orient Bio (Seongnam, Korea). They were housed in cages in a climate controlled room with an ambient temperature of 23℃ and a 12 hour light/dark cycle; they were fed standard laboratory chow and water and they randomly assigned to the control or experimental groups. All the experiments were approved by the Korea University Ethics Committee for Animal Experiments. Cerulein was purchased from Sigma (St. Louis, MO, USA). The antibodies against HSP60/70 and LC3B were purchased from Cell Signaling (Danvers, MA, USA) and Nanotools (Wetzlar, Germany), respectively.
2. Induction of HSPs and cerulein-induced pancreatitis
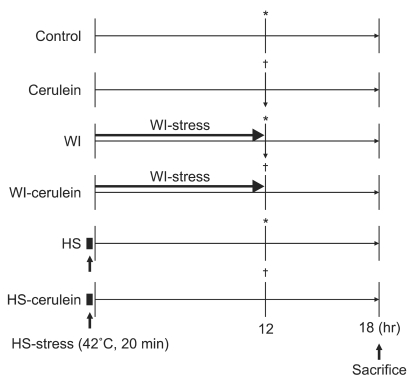
After 8 hours fasting except for water, the rats in the WI groups (WI and WI-cerulein) were vertically immersed in a bath (23℃) to the depth of the xyphoid process for 12 hours and then they were placed into a cage. The rats in the HS groups (HS and HS-cerulein) were vertically immersed in a waterbath (42℃) to the depth of the xyphoid process for 20 minutes and then they were placed into a cage. The experimental groups (cerulein, WIcerulein, and HS-cerulein) and the control groups (control, WI, and HS) received one intraperitoneal injection of cerulein (50 µg/kg) or saline, respectively, at 12 hours after the study onset. All the rats were sacrificed at 6 hours after cerulein or saline injection (Fig. 1).
Fig. 1.
Study protocol. All of the rats were fasted for 8 hours before the study. The control and cerulein rats (n=6, respectively) were fasted before saline or cerulein (50 µg/kg) injection, respectively, and were sacrificed at 6 hours after injection. The water-immersion (WI) and WI-cerulein rats (n=6, respectively) were pretreated with WI for 12 hours prior to saline or cerulein (50 µg/kg) injection, respectively, and were sacrificed at 6 hours after injection. The heat-shock (HS) and HS-cerulein rats (n=6, respectively) were pretreated with HS for 20 minutes. Twelve hours after the end of HS, rats were injected with saline or cerulein (50 µg/kg) and were sacrificed at 6 hours after injection. *,†Indicate saline or cerulein injection, respectively.
3. Preparation of the plasma and tissue samples
The rats were placed under deep anesthesia using ethyl ether (Duksan Pure Chemical Co., Ltd., Ansan, Korea) and a laparotomy was performed; the fresh pancreas was harvested immediately and stored at -70℃ for western blot analysis or it was fixed with 10% formaldehyde for paraffin sectioning. Intracardiac blood was collected and centrifuged at 3,000 rpm for 10 minutes at 4℃.
4. Characterization of pancreatitis
1) Serum amylase
The serum amylase was determined using an automatic biochemical analyzer (TBA200 FR Neo; Toshiba, Tokyo, Japan).
2) Histological evaluation
The removed pancreases that were assigned to histological evaluation were fixed in 4% formaldehyde solution overnight. The tissues were dehydrated and processed for embedding in paraffin wax (Tissue-Tek, Sakura, Japan) by a routine protocol. Five-µm-thick serial sections were then cut using a Leica RM 2155 rotary microtome (Leica Microsystems, Nussloch, Germany) and these were mounted on 3-amino-propyl-tri-ethoxy-silane (Sigma)-coated slides. The slides were examined for edema, vacuolization, inflammatory cell infiltration and necrosis by a pathologist who was unaware of their assignment to the groups.
3) Immunohistochemistry
The sections were dewaxed with xylene, then rehydrated and prepared for the Immunohistochemistry by routine methods. The endogenous peroxidase activity was blocked with 0.03% H2O2 for 15 minutes. The nonspecific binding was suppressed by incubation with 10% normal horse serum (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) for 1 hour at room temperature. Thereafter, incubation with the primary antibodies (anti-LC3B [Naonotools], rabbit anti-HSP60 [1:100, Cell Signaling], and rabbit anti-HSP70 [1:100, Cell Signaling]) was performed for 48 hours at 4℃. The primary antibody binding was visualized using an avidin-biotin-peroxidase kit (ImmPRESS reagent kit; Vector Laboratories, Burlingame, CA, USA) and this was employed according to the manufacturer's instructions. Labeling was visualized by incubating with 0.05% 3'3-diaminobenzidine (Sigma) and 0.01% H2O2 for 5 to 10 minutes. The necessary dilutions and thorough washes between the stages were performed using phosphate buffered saline pH 7.4, unless otherwise stated. The sections were dehydrated through a graded series of ethanol solutions; they were cleared with xylene and mounted with coverslip. Omission of incubation with the primary or secondary antibody served as a control for falsepositive immunoreactions. The immunolabelled images were directly captured using a C-4040Z digital camera (Olympus, Tokyo, Japan) and an Olympus BX-50 microscope (Olympus).
5. Western blot analysis
The removed pancreas was homogenized in three volumes of ice-cold lysis buffer (50 mM Hepes-NaOH [pH 7.5], 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA) that contained a protease inhibitor cocktail tablet (Roche, Mannheim, Germany).
The supernatant was harvested after centrifugation (13,000 rpm for 20 minutes at 4℃). The protein concentrations were determined using the dye-binding microassay kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer's instructions. For the immunoblot assay, the supernatant samples containing 20 µg of protein each were loaded into individual lanes of 12% SDS-polyacrylamide gels, they were electrophoresed and then transferred onto polyvinylidene fluoride membranes. After electroblotting, the membranes were blocked with 5% skim milk in Tris buffer saline containing 0.05% Tween-20 (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.1% Tween-20) at room temperature for 1 hour. The membranes were sequentially probed with anti-LC3B (1:1,000, Nanotools, Wetzlar, Germany), rabbit anti-HSP60 (1:100, Cell Signaling), rabbit anti-HSP70 (1:100, Cell Signaling) and anti-β-actin (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA). The binding of a primary antibody was quantitated by using horse-radish peroxidase-conjugated secondary antibody at a dilution 1:4,000 (anti-rabbit IgG-horse-radish peroxidase; Amersham Biosciences Inc., Oslo, Norway). The blotted proteins were developed using an enhanced chemiluminescence detection system (iNtRON Biotech, Seongnam, Korea). According to the molecular weight, the LC3 protein was divided into 2 forms: LC3-I and LC3-II. The cytosolic LC3-I protein (18 kDa) is conjugated to the highly lipophilic phosphatidylethanolamine (PE) moiety to generate LC3-II (16 kDa). The PE group promotes integration of LC3-II into lipid membranes at the phagophores and autophagosomes. LC3 is expressed as three isoforms in mammalian cells (LC3A, LC3B, and LC3C), but only LC3B is correlated with the extent of autophagosome formation. So, we used anti-LC3B antibody for western blot analysis.
6. Electron microscopy
The pancreas tissue was cut into small pieces (~1 mm3) and these were fixed in 2% paraformaldehyde containing 2.5% glutaldehyde buffered by 0.1 M cacodylate (pH 7.2) at 4℃ for 4 hours; the pieces were further fixed with 1% OsO4 solution buffered by 0.1 M cacodylate (pH 7.2) at 4℃ for 2 hours, then they were dehydrated and embedded in Epon812/DDSA (dodecenyl scuccinio anhydride) according to a standard procedure. Ultrathin sections were stained with uranyl-magnesium acetate and lead citrate and these were observed under a transmission electron microscope (H-7500; Hitachi, Tokyo, Japan).
7. Statistical analysis
The results of the serum analysis are expressed as mean±standard error (SEM). The experimental data from the different groups were compared by analysis of variance (ANOVA). A p-value of less than 0.05 was considered to indicate a significant difference.
RESULTS
1. Serum amylase
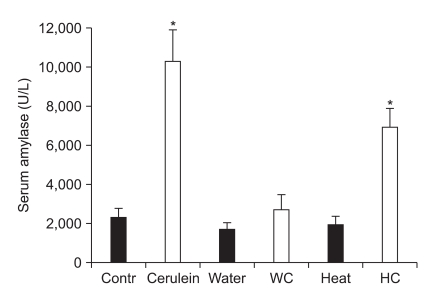
WI, HS, and WI-cerulein didn't affect the basal serum amylase level. However, the serum amylase level in the cerulein and HS-cerulein groups was significantly higher than that of the control group (Fig. 2).
Fig. 2.
Serum amylase level among the groups after water-immersion (WI) or heat-shock (HS) pretreatment. The serum amylase level after WI or HS pretreatment was similar to that of the control. Cerulein injection after WI (WC) failed to increase the serum amylase level. However, cerulein injection after HS (HC) significantly elevated the serum amylase compared with control. The results are reported as the mean±SEM.
Contr, control; Water or WI, water-immersion; Heat or HS, heatshock; WC, water-immersion cerulein; HC, heat-shock cerulein. *Indicates p<0.05 when compared with the control.
2. Histology
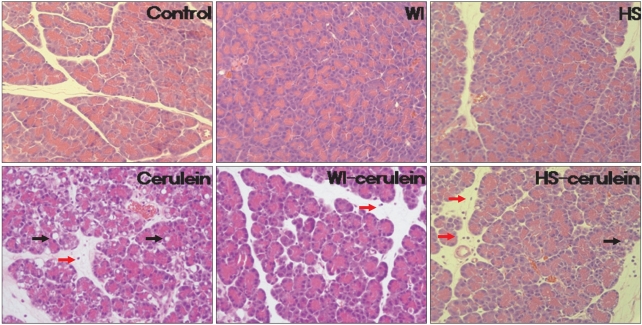
The histological findings are shown in Fig. 3. There was no evidence of pancreatitis in the control, WI, and HS groups. Interstitial edema, inflammatory cell infiltration, acinar cell vacuolization and necrosis were all noted in the cerulein group. Only mild edema and infiltration of inflammatory cell were observed in the WI-cerulein group. However, mild acinar cell vacuolization in addition to mild edema and infiltration of inflammatory cells were noted in the HS-cerulein group. Therefore, the severity of cerulein induced AP was more attenuated by WI than by HS preconditioning.
Fig. 3.
Histologic findings of the pancreas. There was no evidence of pancreatitis in the pancreas of the control, water-immersion (WI), and heatshock (HS) groups. Interstitial edema, acinar cell vacuolizations (black arrow) and infiltration of inflammatory cells (red arrow), which indicate the development of pancreatitis, were observed in the cerulein group. These findings were almost absent in the WI-cerulein group. However, in the HS-cerulein group, mild vacuole formation, edema and inflammatory cell infiltration were noted (H&E stain, ×200).
3. Immunohistochemical analysis
1) HSP60/70
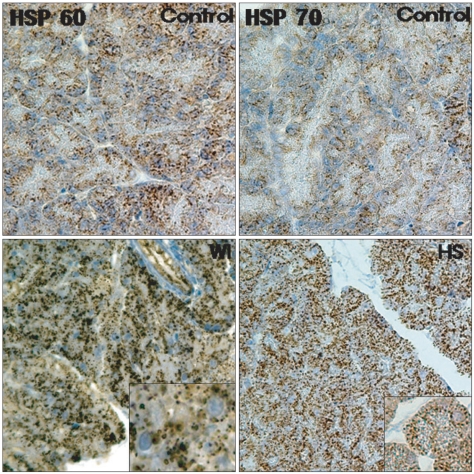
As shown in Fig. 4, the basal constitutive expression of HSP60 was noted in scattered locations within the pancreatic acinar cells in the control group. After preconditioning by WI, the expression of HSP60 within the acinar cells was markedly increased and this seemed to be confined to the luminal side of the perinuclear region. There was virtually no HSP70 expression in the pancreases from the control animals. In the HS group, there was profound upregulation of the HSP70 expression in the cytoplasm of the acinar cells.
Fig. 4.
Immunolocalization of heat shock protein 60/70 (HSP60/70) in the pancreas. The constitutive expression of HSP60 was visible in scattered locations in the acinar cells in the control group. The water-immersion (WI) group showed a markedly increased expression of HSP60 that was confined to the acinar cells. There was virtually no HSP70 expression in the control group. Heat-shock (HS) resulted in a significant up-regulation of HSP70 that was also confined to the acinar cells (H&E stain, ×200).
2) LC3
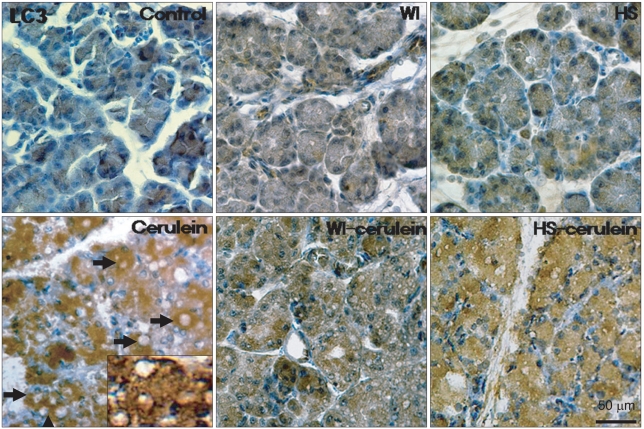
In the control group, there was a slight LC3 expression in the cytoplasm of the acinar cells, which may have been induced by starvation. In the cerulein group, LC3 was markedly expressed in the cytoplasm and abundant cytoplasmic vacuoles were noted. However, WI and HS themselves did not affect the LC3 expression as compared with that of the control group. Preconditioning with WI and HS markedly inhibited the cerulein induced LC3 expression and cytoplasmic vacuole formation, as is shown in Fig. 5.
Fig. 5.
Immunohistochemistry for LC3 expression in the pancreas. There was minimal LC3 expression in the control, water-immersion (WI), and heat-shock (HS) groups. Cerulein increased LC3 expression, which was confined to the acinar cells and was evident especially around the vacuoles. However, cerulein-induced LC3 expression and cytoplasmic vacuole formation were inhibited in the WI- and HS-cerulein groups (H&E stain, ×400).
4. Western blot analysis
1) HSP60 and LC3B
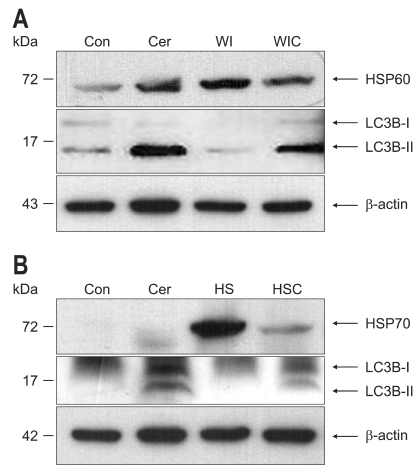
HSP60 was constitutively expressed in the rat pancreas, and WI markedly induced the expression of HSP60 compared with that of the control group (Fig. 6A). Cerulein markedly increased the expression of LC3B-II, which is a marker of autophagosomes. However, the LC3B-II expression was not increased in the WI cerulein group, which indicates that WI preconditioning suppressed the cerulein-induced autophagy formation.
Fig. 6.
Western blot analysis of heat shock protein 60/70 (HSP60/70) and LC3B in the pancreas. (A) HSP60 was constitutively expressed in the control group. Water-immersion (WI) preconditioning markedly induced the expression of HSP60. Cerulein greatly increased the expression of LC3B-II, which is a marker of autophagosomes. However, LC3B-II expression was not increased in the WI-cerulein group. (B) HSP70 was generally not expressed in the control group. Heat-shock (HS) preconditioning resulted in a markedly increased expression of HSP70. Cerulein increased LC3B-II expression. However, LC3B-II expression in the HS-cerulein group did not increase.
Con, control; Cer, cerulein; HSC, heat-shock cerulein; WIC, waterimmersion cerulein.
2) HSP70 and LC3B
HSP70 was virtually barely expressed in the control group, but the HS group showed the markedly increased expression of HSP70. Cerulein increased the LC3B-II expression. However, the LC3B-II expression in the HS-cerulein group was not increased, which indicates that HS preconditioning attenuated the cerulein-induced autophagy formation (Fig. 6B).
5. Electron microscopy
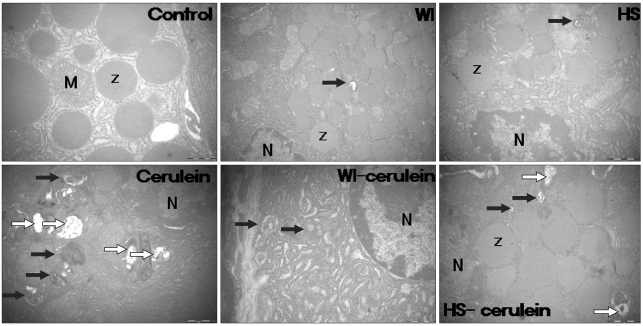
There were no cytoplasmic vacuoles in the control group, but some vacuoles were observed in the WI and HS groups. In the cerulein group, some vacuoles containing double-membrane organelles and large vacuoles with homogeneous electron-dense staining were found, which seemed to be autophagosomes and autolysosomes, respectively. Autophagosome formation was suppressed after preconditioning with WI, and this suppression was greater than that of preconditioning with HS (Fig. 7).
Fig. 7.
Electron microscopic assessment of autophagy formation. There were no cytoplasmic vacuoles in the control group, although some vacuoles were observed in the water-immersion (WI) and heat-shock (HS) groups. In the cerulein group, vacuoles containing double-membrane organelles and large vacuoles with homogeneous electron-dense staining were found; these vacuoles seemed to be autophagosomes (black arrow) and autolysosomes (white arrow), respectively. Autophagy induced by cerulein injection was suppressed after preconditioning with WI, and this suppression was greater than that observed after pretreatment with HS.
Z, zymogen; M, mitochondria; N, nucleus.
DISCUSSION
There are many causes of AP, but the mechanisms by which these conditions trigger pancreatic inflammation have not been identified. Obtaining pancreatic tissue in the early stage of AP is crucial for investigating the pathogenesis of AP. Unfortunately, human pancreas tissue is generally not available for research. For these reasons, various experimental animal models have been developed for investigating the pathophysiology of AP. The AP animal models developed should mimic clinical AP such as the provoked serum pancreatic enzymes and the remarkable histological changes.21 Although the cholecystokinin analogue cerulein induces a relatively mild manifestation of AP, the rapid induction, noninvasiveness, high repeatability, high applicability and the remarkable similarity to the histological changes in human AP have made the cerulein-induced AP animal model the most favored AP model.21 Previous studies about HSPs have shown the protective role of HSP60/70 against cerulein-induced AP, although there was some controversy about HSP70.22,23 In our study, HSP70 also showed protective effects against cerulein-induced AP. The mechanisms by which HSPs protect against cerulein-induced AP were commonly hypothesized that HSPs protect against pancreatitis by inactivating prematurely activated trypsinogen. However, in a recent study, Lee et al.1 reported that WI induced HSP60 prevents trypsinogen activation by interfering with the colocalization of lysosomal hydrolases and the digestive enzyme zymogen rather than by inhibiting the activated enzymes. Frossard et al.10 showed that both thermal and nonthermal stresses protect against pancreatitis by preventing intrapancreatic digestive enzyme activation, and that HSP70 may play a role in this protection. Bhagat et al.9 demonstrated that administration of antisense oligonucleotides to HSP70 reduced the thermal stress-induced HSP70 expression, it restored the ability of supramaximal cerulein administration to cause intrapancreatic trypsinogen activation and it abolished the protective effect of prior thermal stress against pancreatitis.
The mechanisms responsible for intracellular activation of trypsinogen to trypsin in AP have not been firmly demonstrated. There are 2 major hypotheses: the colocalization hypothesis,20 which explains that digestive enzymes become colocalized with lysosomal hydrolases such as cathepsin B and this activates trypsinogen in the cytoplasmic vacuoles of acinar cells, and the autoactivation hypothesis,24 which suggests that trypsinogen is autoactivated under low pH conditions in the presence of serine protease. The appearance of cytoplasmic vacuoles within the pancreatic acinar cells, which is an early feature of AP, was suggested as autophagic in origin by electron microscopy and immunohistochemical studies.25,26 Hashimoto et al.19 reported that conditional knockout mice lacking the Atg5 gene in acinar cells showed no AP except for mild edema in a restricted area after cerulein injection, and trypsinogen activation was greatly reduced in the absence of autophagy. These results suggest that autophagy exerts devastating effects in pancreatic acinar cells by activating trypsinogen to trypsin in the early stage of AP through delivering trypsinogen to the lysosomes.
Considering the conflicting role of HSPs and autophagy in trypsinogen activation, we hypothesized that HSPs might prevent cerulein-induced AP by inhibiting the autophagy formation. As expected, the induction of HSPs in acinar cells effectively prevented the development of AP. As shown in Fig. 3, the preexpression of HSPs prevents vacuole formation in the acinar cells in response to cerulein. Western blotting showed that the LC3B expression, which is a marker of autophagosomes, was clearly expressed in the cerulein group, but it was inhibited in the WI- and HS-cerulein groups. Interestingly, as shown in Fig. 7, electron microscopic examination revealed that the cerulein-induced autophagy formation after induction of HSP60 was inhibited to a greater degree than that observed after induction of the expression of HSP70. These results suggest that the expressions of HSPs may directly inhibit autophagosome formation, which results in prevention of trypsinogen activation during the development of cerulein-induced AP, and HSP60 shows a more complete preventive effect on AP as compared to that of HSP70.
The location of HSPs and LC3 is crucial for directly explaining the preventive mechanism of HSPs against autophagy formation. As shown in Fig. 4, the expression of HSPs, regardless of the type, is scattered in the acinar cell cytoplasm and this is more intense around the nucleus where the Golgi and endoplasmic reticulum (ER) are located. An LC3 expression was also observed in the acinar cell cytoplasm, but it was mainly located mainly around vacuoles, as is shown in Fig. 5. We expected the colocalized expressions of HSP and LC3 in the very early form of vacuoles, which may tell us something about the intimate relationship of HSPs and the prevention of autophagosome. Unfortunately, it is hard to find very early vacuole lesions at the light microscopic level. The full blown injured acinar cells revealed large vacuoles that were stained for LC3, as was expected.
Although identifying the mechanism by which HSP60/70 inhibits autophagy formation in cerulein-induced AP is beyond the scope of our present experiment, plausible explanations for the mechanism and the difference between HSP60 and HSP70 are as follows. Arias et al.27 reported that HSP60 follows the increasing concentration gradient of proteins along the rough ER-Golgi granule secretory pathway in pancreatic acinar cells, and this protein is specifically concentrated in the ER crystals induced by DL-p-chlorophenylalanine methyl ester-treatment and plays an important role in regulating the processing and transport of digestive enzymes in that organ, whereas HSP70 is confined to the trans-Golgi network. Therefore, the up-regulated HSP60/70 in pancreatic acinar cells by WI or HS preconditioning may be involved in the autophagy related pathway which is best characterized by class I phosphatidylinositol 3-kinase and target of rapamycin,17 and this results in the inhibition of autophagy formation and HSP60 may be more directly related to inhibiting the pathway than HSP70 is. The relationship between HSPs and autophagy may be organ specific. In other organs, HSPs and autophagy may help each other for achieving homeostasis. Regardless of the mechanism by which HSPs inhibit autophagy formation, this study showed the temporal cause-effect relationship between the expression of HSPs and autophagy formation in a cerulein-induced AP animal model. In conclusion, the HSPs induced by WI or HS exerted protective effects against cerulein-induced AP in the rats by inhibiting autophagy formation. Further studies that will use conditional knockout of the HSP60/70 or autophagy related genes in rats are needed to elucidate the precise mechanism by which HSPs inhibit autophagy formation in cerulein-induced AP.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Lee HS, Bhagat L, Frossard JL, et al. Water immersion stress induces heat shock protein 60 expression and protects against pancreatitis in rats. Gastroenterology. 2000;119:220–229. doi: 10.1053/gast.2000.8551. [DOI] [PubMed] [Google Scholar]
- 2.Koopmann MC, Baumler MD, Boehler CJ, Chang FL, Ney DM, Groblewski GE. Total parenteral nutrition attenuates ceruleininduced pancreatitis in rats. Pancreas. 2010;39:377–384. doi: 10.1097/MPA.0b013e3181bb908e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rakonczay Z, Jr, Takács T, Boros I, Lonovics J. Heat shock proteins and the pancreas. J Cell Physiol. 2003;195:383–391. doi: 10.1002/jcp.10268. [DOI] [PubMed] [Google Scholar]
- 4.Hwang JH, Ryu JK, Yoon YB, et al. Spontaneous activation of pancreas trypsinogen in heat shock protein 70.1 knock-out mice. Pancreas. 2005;31:332–336. doi: 10.1097/01.mpa.0000183377.04295.c3. [DOI] [PubMed] [Google Scholar]
- 5.Saluja A, Dudeja V. Heat shock proteins in pancreatic diseases. J Gastroenterol Hepatol. 2008;23(Suppl 1):S42–S45. doi: 10.1111/j.1440-1746.2007.05272.x. [DOI] [PubMed] [Google Scholar]
- 6.Minowada G, Welch WJ. Clinical implications of the stress response. J Clin Invest. 1995;95:3–12. doi: 10.1172/JCI117655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72:1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- 8.Hightower LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- 9.Bhagat L, Singh VP, Song AM, et al. Thermal stress-induced HSP70 mediates protection against intrapancreatic trypsinogen activation and acute pancreatitis in rats. Gastroenterology. 2002;122:156–165. doi: 10.1053/gast.2002.30314. [DOI] [PubMed] [Google Scholar]
- 10.Frossard JL, Bhagat L, Lee HS, et al. Both thermal and nonthermal stress protect against caerulein induced pancreatitis and prevent trypsinogen activation in the pancreas. Gut. 2002;50:78–83. doi: 10.1136/gut.50.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhagat L, Singh VP, Hietaranta AJ, Agrawal S, Steer ML, Saluja AK. Heat shock protein 70 prevents secretagogue-induced cell injury in the pancreas by preventing intracellular trypsinogen activation. J Clin Invest. 2000;106:81–89. doi: 10.1172/JCI8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner AC, Weber H, Jonas L, et al. Hyperthermia induces heat shock protein expression and protection against cerulein-induced pancreatitis in rats. Gastroenterology. 1996;111:1333–1342. doi: 10.1053/gast.1996.v111.pm8898648. [DOI] [PubMed] [Google Scholar]
- 13.Ohmuraya M, Yamamura K. Autophagy and acute pancreatitis: a novel autophagy theory for trypsinogen activation. Autophagy. 2008;4:1060–1062. doi: 10.4161/auto.6825. [DOI] [PubMed] [Google Scholar]
- 14.Ohmuraya M, Hirota M, Araki M, et al. Autophagic cell death of pancreatic acinar cells in serine protease inhibitor Kazal type 3-deficient mice. Gastroenterology. 2005;129:696–705. doi: 10.1016/j.gastro.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 15.Barth S, Glick D, Macleod KF. Autophagy: assays and artifacts. J Pathol. 2010;221:117–124. doi: 10.1002/path.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuervo AM. Autophagy: in sickness and in health. Trends Cell Biol. 2004;14:70–77. doi: 10.1016/j.tcb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto D, Ohmuraya M, Hirota M, et al. Involvement of autophagy in trypsinogen activation within the pancreatic acinar cells. J Cell Biol. 2008;181:1065–1072. doi: 10.1083/jcb.200712156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Acker GJ, Perides G, Steer ML. Co-localization hypothesis: a mechanism for the intrapancreatic activation of digestive enzymes during the early phases of acute pancreatitis. World J Gastroenterol. 2006;12:1985–1990. doi: 10.3748/wjg.v12.i13.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan YC, Leung PS. Acute pancreatitis: animal models and recent advances in basic research. Pancreas. 2007;34:1–14. doi: 10.1097/01.mpa.0000246658.38375.04. [DOI] [PubMed] [Google Scholar]
- 22.Otaka M, Okuyama A, Otani S, et al. Differential induction of HSP60 and HSP72 by different stress situations in rats: correlation with cerulein-induced pancreatitis. Dig Dis Sci. 1997;42:1473–1479. doi: 10.1023/a:1018866727129. [DOI] [PubMed] [Google Scholar]
- 23.Rakonczay Z, Jr, Takács T, Iványi B, et al. The effects of hypo- and hyperthermic pretreatment on sodium taurocholate-induced acute pancreatitis in rats. Pancreas. 2002;24:83–89. doi: 10.1097/00006676-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Leach SD, Modlin IM, Scheele GA, Gorelick FS. Intracellular activation of digestive zymogens in rat pancreatic acini. Stimulation by high doses of cholecystokinin. J Clin Invest. 1991;87:362–366. doi: 10.1172/JCI114995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helin H, Mero M, Markkula H, Helin M. Pancreatic acinar ultrastructure in human acute pancreatitis. Virchows Arch A Pathol Anat Histol. 1980;387:259–270. doi: 10.1007/BF00454829. [DOI] [PubMed] [Google Scholar]
- 26.Adler G, Hahn C, Kern HF, Rao KN. Cerulein-induced pancreatitis in rats: increased lysosomal enzyme activity and autophagocytosis. Digestion. 1985;32:10–18. doi: 10.1159/000199210. [DOI] [PubMed] [Google Scholar]
- 27.Arias AE, Vélez-Granell CS, Torres-Ruíz JA, Bendayan M. Involvement of molecular chaperones in the aberrant aggregation of secretory proteins in pancreatic acinar cells. Exp Cell Res. 1994;215:1–8. doi: 10.1006/excr.1994.1306. [DOI] [PubMed] [Google Scholar]