Abstract
Metastasis of hepatocellular carcinoma (HCC) to the ovary is notably rare. We present a case of HCC metastasis to the ovary with a review of the literature, which includes only 7 reported cases. A 43-year-old hepatitis B virus carrier was admitted with a right ovarian cystic mass. She had been diagnosed with HCC 2 years prior, for which she underwent transarterial chemoembolization followed by right posterior sectionectomy. Eight months after the hepatectomy, the first intrahepatic recurrence was detected and treated with transarterial chemoembolization. An additional intrahepatic recurrence occurred 12 months after transarterial chemoembolization and was managed with left medial sectionectomy and intra-operative radiofrequency ablation. Over the following 3 months, the patient developed elevated alpha-fetoprotein, and positron emission tomography showed a cystic mass in the right side of the pelvic cavity with focal hypermetabolic activity, which suggested a site of recurrent HCC. An exploratory laparotomy was performed, and a soft, ovoid cystic mass was identified in the right ovary. There was no evidence of metastases in the liver, left ovary, or peritoneum. Because of the absence of tumor on the surface of the ovary and the lack of peritoneal seeding, the mode of metastasis was thought to be hematogenous. Therefore, a right salphingo-oophorectomy was performed. The pathological features showed metastatic HCC with clear resection margins. Although metastasis of HCC to the ovary is very rare, it should be suspected in a female patient with a lower abdominal mass and an elevated serum AFP level in the absence of other demonstrable metastases.
Keywords: Hepatocellular carcinoma, Metastasis, Ovary
INTRODUCTION
Hepatocellular carcinoma (HCC) spreads via the hematogenous route, the lymphatic route, or by direct invasion into adjacent organs.1 The common extrahepatic metastatic sites of HCC are the lungs, peritoneum, adrenal glands, and bone,2 although there are rare reports of metastases to the heart,3 nasal cavity,4 orbital cavity,5 skin,6 external auditory canals,7 and pharynx.8 The ovary is a rare site of metastasis for HCC, which was first reported in 1983.9 In the English literature, there have been 6 reported cases of metastatic HCC to the ovary and 4 cases reported in the Korean literature. We present a case of metastatic HCC to the ovary with a review of the literature.
CASE REPORT
A 43-year-old woman with a history of HCC secondary to chronic infection with hepatitis B virus (HBV) was admitted to the hospital for evaluation of a right ovarian cystic mass. She also had a family history of HBV in two of her siblings. She was diagnosed with HCC 2 years ago and underwent transarterial chemoembolization (TACE), followed by right posterior sectionectomy. Eight months after the hepatectomy, the first intrahepatic recurrence was detected, which was treated with TACE. An additional intrahepatic recurrence occurred 12 months after TACE, which was managed with left medial sectionectomy and intra-operative radiofrequency ablation.
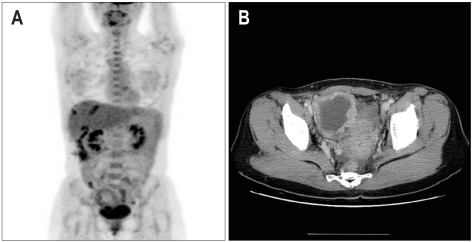
During the 3-month follow-up period after the second hepatectomy, the patient developed an elevated alpha-fetoprotein (AFP; 336,520 ng/µL [normal range, 0 to 7 ng/µL]). However, a liver dynamic computed tomography (CT) and chest CT showed no evidence of recurrent HCC. A positron emission tomography (PET) showed a hypermetabolic lesion (max SUV=4.5 in the right side of pelvic cavity), suggesting the site of recurrent HCC. Further examination of the abdominopelvic CT revealed a right ovarian cystic mass (Fig. 1).
Fig. 1.
(A) Fusion whole-body positron emission tomography scan shows a 6-cm cystic lesion in the right pelvic cavity and a more focal hypermetabolic lesion in the inferior portion. (B) Abdominopelvic computed tomography reveals a 7-cm heterogeneous mass in the right ovary, which suggested that metastatic hepatocellular carcinoma was more likely than primary ovarian cancer.
Since the metastasis was limited to the right ovary without evidence of widespread recurrence, she was recommended to undergo an exploratory laparotomy with removal of the right ovarian mass. At laparotomy, there was no evidence of metastases in the liver, left ovary, or peritoneum. A soft, ovoid, cystic mass was identified in the right ovary and the frozen section confirmed metastatic HCC. Because of the absence of tumor on the surface of the ovary and the lack of peritoneal seeding, the mode of metastasis was thought to be hematogenous. Therefore, a right salphingo-oophorectomy (SO) was performed.
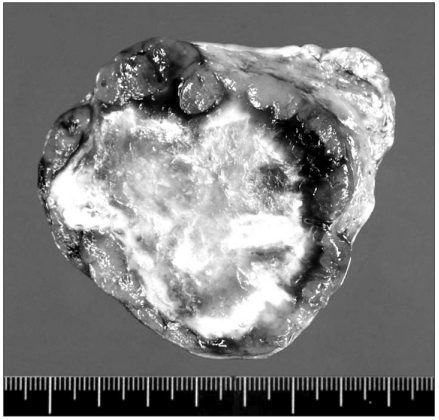
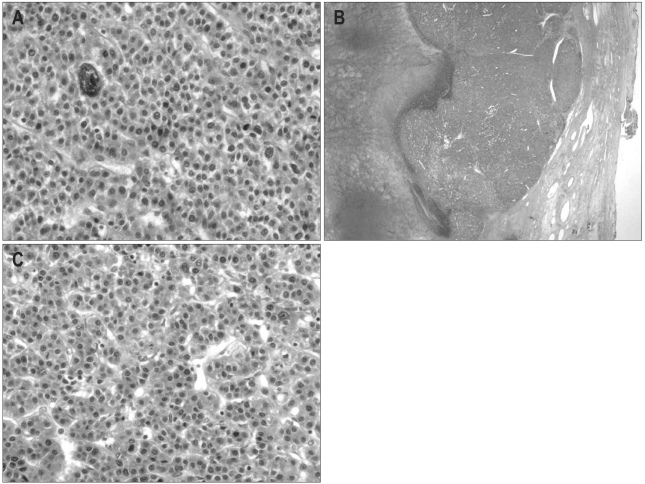
On the final pathologic examination, the right ovary showed a 7×6×5 cm sized tumor with a pink-yellow, soft, granular cut surface (Fig. 2). The tumor cells showed the typical trabecular pattern of HCC and had very similar features to the previously resected HCC mass. The tumor was compatible with metastatic HCC and there was no evidence of lymphovascular or perineural invasion. The ovarian capsule was intact and the fallopian tube had no tumor (Fig. 3).
Fig. 2.
The gross features of the right ovarian mass. It is a 7×6×5 cm tumor with a pink-yellow, soft, granular cut surface.
Fig. 3.
Microscopic features of primary hepatocellular carcinoma (HCC) (A) and HCC metastasis to the ovary (B, C). (A) Previously resected primary HCC. The typical trabecular pattern of HCC is shown (×400). (B) A hypercellular nodular mass on the ovary with focal hemorrhage and necrosis. The ovarian cortex is intact, and no normal ovarian cortex is identified (×10). (C) Polygonal tumor cells with vesicular nuclei and prominent nucleoli are separated by sinusoids. The HCC metastasized to the ovary is morphologically consistent with the features of primary HCC (×400).
DISCUSSION
HCC rarely metastasizes to the ovary. Moreover, metastatic HCC should be distinguished from a hepatoid yolk sac tumor and a primary hepatoid carcinoma of the ovary.10 Hepatoid carcinomas of the ovary and hepatoid yolk sac tumors are suspected when there is no clinical or operative evidence of HCC.
Hepatoid yolk sac tumors usually occur in females of reproductive age with germ cell neoplastic components or gonadal dysgenesis.10 Young et al.11 suggested that canaliculi are strongly suggestive of metastatic HCC rather than hepatoid yolk sac tumors.
Hepatoid carcinomas of the ovary have recently been described as a distinctive type of carcinoma that arises outside the liver, but to a considerable extent resembles HCC both histologically and immunohistochemically due to its staining for AFP.11 Hepatoid carcinomas of the ovary generally occur in older patients and the presence of focal staining for AFP is essential for a certain diagnosis of in a site as unusual as the ovary.10 Hepatoid carcinomas of the ovary can also present in the stomach, pancreas, lungs, kidneys, and urinary bladder.12 Because hepatoid carcinomas of the ovary are usually metastatic from the stomach and leave a lesion on the surface of the ovary, the existence of a hepatoid carcinoma outside the ovary and tumor growth on the surface of the ovary can elevate the possibility of a hepatoid carcinoma of the ovary.
In the case presented herein, the patient had a history of HCC and there was no evidence of hepatoid carcinomas in other abdominal organs. Furthermore, the pathologic features suggested HCC without any features of haptoid yolk sac tumors, such as germ cells or gonadal dysgenesis. There was also no tumor on the ovarian capsule or peritoneal cavity, thus the tumor was likely to have metastasized from the primary HCC by hematogenous spread.
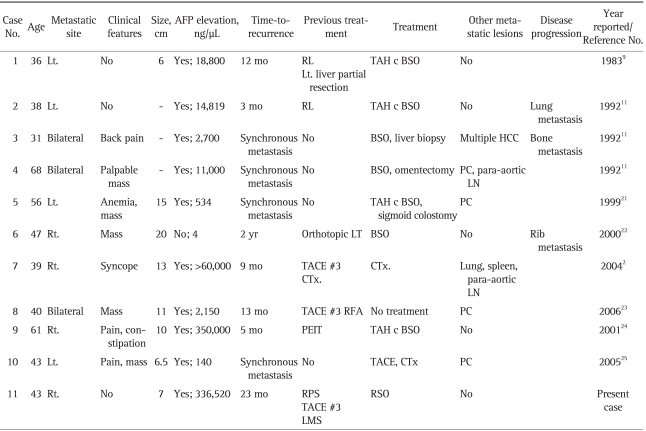
Ten cases of previously reported metastatic HCCs to the ovary are summarized and presented in Table 1. The mean age of the 10 cases and the current case was 45.6 years (range, 31 to 68 years). The number of right, left, and bilateral HCC ovarian metastases were 4, 4, and 3, respectively. In 7 patients, the ovarian metastases were noted during follow-up, of which 3 patients showed no clinical symptoms or signs (case no. 1, 2, and 8). The other 4 patients were diagnosed as synchronous ovarian metastases (case no. 3, 4, 5, and 10).
Table 1.
Summary of Seven Cases of Metastatic Hepatocellular Carcinoma Previously Reported and the Present Case
AFP, alpha-fetoprotein; RL, right hemihepatectomy; LT, liver transplantation; TACE, transarterial chemoembolization; CTx, chemotherapy; RPS, right posterior sectionectomy; LMS, left medial sectionectomy; TAH c BSO, total abdominal hysterectomy with bilateral salpingo-oophorectomy; HCC, hepatocellular carcinoma; PC, peritoneal carcinomatosis; PTI, percutaneous ethanol injection therapy.
All cases were treated surgically, except 3 cases (case no. 7, 8, and 10), which showed multiple lung, spleen, and para-aortic lymph node metastases (case no. 7) or peritoneal carcinomatosis (case no. 8 and 10). The surgical procedures performed included bilateral SO with or without transabdominal hysterectomy. In our case, we made the decision to perform a unilateral SO; the decision was based on the fact that there was no evidence of peritoneal spread or ovarian capsular involvement. In view of the likely hematogenous spread, a limited resection was performed. There was no clear oncologic evidence to support performing an extensive bilateral SO and hysterectomy with the increased risk of operative morbidity and hormonal consequences.
The recurrence of HCC can be detected by an elevated serum AFP or imaging findings.13 AFP is also known as an independent prognostic factor for HCC and a markedly elevated serum AFP level may reflect advanced HCC in terms of its large size or metastasis.14 In our review of the 11 cases of metastatic HCC to the ovary (Table 1), all cases showed elevated serum AFP levels, except one case (case no. 6). Amongst the reported cases of HCC metastatic to the ovary, the serum AFP levels declined after surgery and rose again when recurrence occurred (case no. 2 and 4). Therefore, the serum AFP can be a useful indicator of recurrent HCC in the ovary and should be closely observed. The serum AFP level did not correlate with the size of the metastatic ovarian HCC, which might be due to the small sample size and the incomplete data set (3 of 11 patients' data on tumor size were missing).
A fluorine-18 fluorodeoxyglucose (18F-FDG) PET has a potential role in the detection of tumor recurrence of HCC patients15-17 because glucose metabolism assessed by 18F-FDG PET is related to progression or aggressiveness of HCC.18,19 Park et al.20 reported that a 18F-FDG PET/CT scan has a relatively high sensitivity for the detection of extrahepatic metastases of HCC. In their data, the sensitivity of 18F-FDG PET/CT was 60.9% for primary HCC and 85.7% for metastatic HCC. Further, they concluded that large HCC (>5 cm) had high sensitivity (92.8%). In the present case, we also identified the site of recurrence using 18F-FDG PET. Therefore, PET is a useful diagnostic modality for detecting the recurrence of HCC.
The diagnosis of metastatic ovarian HCC is a diagnosis of exclusion. In patients with a rising AFP following removal of primary HCC, a careful clinical assessment and abdominopelvic CT should be the first line of investigation. In the absence of intrahepatic metastases, a chest CT and bone scan should be added, followed by a CT/MRI of the brain. A PET scan is a useful tool in the diagnosis of extrahepatic metastasis of HCC, as demonstrated in our case. The ovarian metastasis can be further examined using pelvic US and is finally confirmed at laparotomy.
In conclusion, although metastasis of HCC to the ovary is very rare, one should have a high index of suspicion in a female patient with a lower abdominal mass and an elevated serum AFP level in the absence of other demonstrable metastases. Rational use of investigations such as CT or PET should help to confirm the diagnosis of this unusual metastasis.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Hong SS, Kim TK, Sung KB, et al. Extrahepatic spread of hepatocellular carcinoma: a pictorial review. Eur Radiol. 2003;13:874–882. doi: 10.1007/s00330-002-1519-7. [DOI] [PubMed] [Google Scholar]
- 2.Kim TH, Cheung DY, Chung WB, et al. A case of metastatic hepatocellular carcinoma of the ovary. Korean J Gastroenterol. 2004;43:215–218. [PubMed] [Google Scholar]
- 3.Masci G, Magagnoli M, Grimaldi A, et al. Metastasis of hepatocellular carcinoma to the heart: a case report and review of the literature. Tumori. 2004;90:345–347. doi: 10.1177/030089160409000317. [DOI] [PubMed] [Google Scholar]
- 4.Frigy AF. Metastatic hepatocellular carcinoma of the nasal cavity. Arch Otolaryngol. 1984;110:624–627. [PubMed] [Google Scholar]
- 5.Font RL, Maturi RK, Small RG, Garcia-Rojas M. Hepatocellular carcinoma metastatic to the orbit. Arch Ophthalmol. 1998;116:942–945. doi: 10.1001/archopht.116.7.942. [DOI] [PubMed] [Google Scholar]
- 6.Yamanishi K, Kishimoto S, Hosokawa Y, Yamada K, Yasuno H. Cutaneous metastasis from hepatocellular carcinoma resembling granuloma teleangiectaticum. J Dermatol. 1989;16:500–504. doi: 10.1111/j.1346-8138.1989.tb01593.x. [DOI] [PubMed] [Google Scholar]
- 7.Yasumatsu R, Okura K, Sakiyama Y, et al. Metastatic hepatocellular carcinoma of the external auditory canal. World J Gastroenterol. 2007;13:6436–6438. doi: 10.3748/wjg.v13.i47.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oida Y, Ishii M, Dowaki S, et al. Hepatocellular carcinoma with metastasis to the pharynx: report of a case. Tokai J Exp Clin Med. 2005;30:31–34. [PubMed] [Google Scholar]
- 9.Oortman EH, Elliott JP. Hepatocellular carcinoma metastatic to the ovary: a case report. Am J Obstet Gynecol. 1983;146:715–717. doi: 10.1016/0002-9378(83)91020-7. [DOI] [PubMed] [Google Scholar]
- 10.Ishikura H, Scully RE. Hepatoid carcinoma of the ovary: a newly described tumor. Cancer. 1987;60:2775–2784. doi: 10.1002/1097-0142(19871201)60:11<2775::aid-cncr2820601130>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 11.Young RH, Gersell DJ, Clement PB, Scully RE. Hepatocellular carcinoma metastatic to the ovary: a report of three cases discovered during life with discussion of the differential diagnosis of hepatoid tumors of the ovary. Hum Pathol. 1992;23:574–580. doi: 10.1016/0046-8177(92)90136-q. [DOI] [PubMed] [Google Scholar]
- 12.Kishimoto T, Nagai Y, Kato K, Ozaki D, Ishikura H. Hepatoid adenocarcinoma: a new clinicopathological entity and the hypotheses on carcinogenesis. Med Electron Microsc. 2000;33:57–63. doi: 10.1007/s007950070002. [DOI] [PubMed] [Google Scholar]
- 13.Zhou XD, Yu YQ, Tang ZY, et al. Surgical treatment of recurrent hepatocellular carcinoma. Hepatogastroenterology. 1993;40:333–336. [PubMed] [Google Scholar]
- 14.Wang CC, Iyer SG, Low JK, et al. Perioperative factors affecting long-term outcomes of 473 consecutive patients undergoing hepatectomy for hepatocellular carcinoma. Ann Surg Oncol. 2009;16:1832–1842. doi: 10.1245/s10434-009-0448-y. [DOI] [PubMed] [Google Scholar]
- 15.Yang SH, Suh KS, Lee HW, et al. The role of (18)F-FDG-PET imaging for the selection of liver transplantation candidates among hepatocellular carcinoma patients. Liver Transpl. 2006;12:1655–1660. doi: 10.1002/lt.20861. [DOI] [PubMed] [Google Scholar]
- 16.Lee JW, Paeng JC, Kang KW, et al. Prediction of tumor recurrence by 18F-FDG PET in liver transplantation for hepatocellular carcinoma. J Nucl Med. 2009;50:682–687. doi: 10.2967/jnumed.108.060574. [DOI] [PubMed] [Google Scholar]
- 17.Yim HJ, Yeon JE, Byun KS, Lee CH, Choi SY, Kim SK. Laparoscopic resection of HCC implanted in the peritoneal cavity: a case detected by PET after hepatic resection. Hepatogastroenterology. 2008;55:1549–1552. [PubMed] [Google Scholar]
- 18.Seo S, Hatano E, Higashi T, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts tumor differentiation, Pglycoprotein expression, and outcome after resection in hepatocellular carcinoma. Clin Cancer Res. 2007;13(2 Pt 1):427–433. doi: 10.1158/1078-0432.CCR-06-1357. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto Y, Nishiyama Y, Kameyama R, et al. Detection of hepatocellular carcinoma using 11C-choline PET: comparison with 18F-FDG PET. J Nucl Med. 2008;49:1245–1248. doi: 10.2967/jnumed.108.052639. [DOI] [PubMed] [Google Scholar]
- 20.Park JW, Kim JH, Kim SK, et al. A prospective evaluation of 18FFDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J Nucl Med. 2008;49:1912–1921. doi: 10.2967/jnumed.108.055087. [DOI] [PubMed] [Google Scholar]
- 21.de Groot ME, Dukel L, Chadha-Ajwani S, Metselaar HJ, Tilanus HW, Huikeshoven FJ. Massive solitary metastasis of hepatocellular carcinoma in the ovary two years after liver transplantation. Eur J Obstet Gynecol Reprod Biol. 2000;90:109–111. doi: 10.1016/s0301-2115(99)00212-2. [DOI] [PubMed] [Google Scholar]
- 22.Khunamornpong S, Siriaunkgul S, Chunduan A. Metastatic hepatocellular carcinoma of the ovary. Int J Gynaecol Obstet. 1999;64:189–191. doi: 10.1016/s0020-7292(98)00170-2. [DOI] [PubMed] [Google Scholar]
- 23.Lim TK, Uhm JE, Shin JA, et al. A case of hepatocellular carcinoma with ovarian metastasis. Korean J Med. 2006;71:573–576. [Google Scholar]
- 24.Park JH, Han CD, Huh CK, et al. Hepatocellular carcinoma metastatic to the ovary: one case report and review literature. Korean J Obstet Gynecol. 2001;44:1900–1904. [Google Scholar]
- 25.Kim MJ. A case of metastatic hepatocellular carcinoma of the ovary: an immunohistochemical study and literature review. Korean J Pathol. 2005;39:287–290. [Google Scholar]