Abstract
Purpose
This study assessed medication use and associated costs among 8- and 15-yearold children with autism spectrum disorders (ASD) identified by the South Carolina Autism and Developmental Disabilities Monitoring (SCADDM) Network.
Methods
All Medicaid-eligible SCADDM-identified children with ASD from surveillance years 2006 and 2007 were included (n=263). Children were classified as ASD cases when documented behaviors consistent with the DSM-IV-TR criteria for autistic disorder, Asperger disorder, or pervasive developmental disorder- not otherwise specified (PDD-NOS) were present in health and education evaluation records. Medication and cost data were obtained by linking population-based and Medicaid data.
Results
All 263 SCADDM-identified children had Medicaid data available; 56% (n=147) had a prescription of any type, 40% (n=105) used psychotropic medication, and 20% (n=52) used multiple psychotropic classes over the study period. Common combinations were (1) attention deficit hyperactivity disorder (ADHD) medications and an antihypertensive, antidepressant or antipsychotic; and (2) antidepressants and an antipsychotic. Multiple psychotropic classes were more common among older children. Both the overall distribution of the number of prescription claims and medication costs varied significantly by age.
Conclusions
Results confirm that medication use in ASD, alone or in combination, is common, costly, and may increase with age.
Keywords: Autism, Psychotropic Medication, Medicaid, Public Health Surveillance
Introduction
National survey and administrative data suggest that children with autism spectrum disorders (ASD) have high rates of prescription drug use. Between 30% and 60% of youth with ASD are prescribed psychotropic medication for managing aggression, irritability, hyperactivity, and other problem behaviors [1–5]. In addition, these children have substantially higher medical costs than children without ASD [6], particularly in regards to medication expenditures [7].
Though combination psychopharmacotherapy in ASD has not been well researched, the use of multiple psychotropic drug classes may be increasing [4, 8, 9]. Data from the Autism Treatment Network [10] and the Autism Society of North Carolina [11] suggest that nearly half of children with ASD who use psychotropic medication are prescribed two or more major drug classes. Since the early 2000s, multi-drug class use in ASD has been common [12], though specific drug-class combinations are not often reported. In 2001, Mandell et al. [8] reported that 20% of children with ASD in a Medicaid-eligible population who were taking psychotropic medication were taking three or more different classes; specific drug combinations were not reported [8]. Similarly, Esbensen et al. [12] found that the percentage of adolescents with ASD who were taking three or more psychotropics rose from 12% to 19% between approximately 2000 and 2004. Again, no specific class combinations were reported.
There may be an association between multiple medication use and intellectual disability in ASD. A survey of more than 5,000 parents [4] found that 15.4% of children with ASD and intellectual disability were prescribed three or more medications, compared to 6.5% of children with ASD without intellectual disability [4]. However, another study showed that of individuals with high-functioning autism who took psychotropics, the majority took multiple classes [13]; most commonly atypical antipsychotics and antidepressants.
Previous research in this population has typically relied on parent-reported medication data and volunteer participation [1, 2, 4, 5, 11, 12, 13], or administrative data where cases are identified based on diagnostic codes [3, 8, 9]. Perhaps because of the long and difficult process of obtaining an accurate and formal diagnosis [14, 15], 30% to 50% of children who meet diagnostic criteria in the United States have never had a documented diagnosis of ASD [16, 17]. Therefore, it is reasonable to believe that previous studies may not have included many children with ASD. To the authors’ knowledge, this is the first medication utilization research study in ASD to combine benefits of both active surveillance for case ascertainment and administrative pharmacy records to obtain age-specific medication prevalence rates.
South Carolina is in a unique position to address these issues because of a population-based surveillance project that identifies children with ASD based on standardized diagnostic criteria obtained through multiple health and education sources, and does not require a previous diagnosis for case ascertainment [18]. Furthermore, most surveillance cases are Medicaid-eligible due to income, disability, or out of home placement. The purpose of this study is to combine data from the South Carolina Autism and Developmental Disabilities Monitoring (SCADDM) Network and a Medicaid claims database to assess medication use among children with ASD. Specifically, the aims were to describe prescription drug use and examine the associated costs among children with ASD using methods that do not rely on volunteer participation, parent recollection, or a formal diagnosis.
Methods
South Carolina Autism and Developmental Disabilities Monitoring program (SC ADDM) has partnered with the Centers for Disease Control and Prevention (CDC)’s Autism and Developmental Disabilities Monitoring Network (ADDM) since 2000 to monitor the prevalence of ASD among 8-year old children across multiple birth-year cohorts [16, 17, 18]. Modeled after the CDC’s Metropolitan Atlanta Developmental Disabilities Surveillance Program (MADDSP) [16], SC ADDM is an active, population-based surveillance project that uses consistent methodology at multiple health and education sources to identify children with ASD, including those who meet diagnostic criteria but do not necessarily have a formal documented diagnosis [19]. South Carolina also recently completed a pilot surveillance study of 15-year old children using the same methodology that was used in previous 8-year old surveillance applied to a subregion of the original SCADDM surveillance area [20, 21] (Figure 1). This area was selected due to the similar demographic characteristics of the original SCADDM study area, while the assessment of 15-year olds allowed for an appropriate pilot study to evaluate the ADDM methodology in terms of the accuracy in case identification and classification among 8-year-old children in the target study areas [21] (both 8- and 15-year olds in the current study were born in 1992).
Figure 1.

South Carolina Autism and Developmental Disabilities Monitoring (SCADDM) Network surveillance area (light grey) and subregion (dark grey).
Case Definition and Ascertainment
Details of the SCADDM Network methodology, surveillance area characteristics, case definition, case ascertainment, and results of 8-year surveillance have been published previously [18]. Briefly, the case ascertainment protocol is designed to capture both previously diagnosed and undiagnosed cases of ASD by thoroughly screening educational and medical records for children with a range of symptoms or classifications associated with ASD [19]. Children were classified as an ASD case when documented behaviors consistent with the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV, TR) [22] criteria for autistic disorder, Asperger disorder, or pervasive developmental disorder- not otherwise specified (PDD-NOS) were present in a comprehensive evaluation by qualified professionals.
The first phase of the two-part case ascertainment process involved verification of residency requirements to ensure at least one parent or guardian resided in the surveillance area during the study year, and screening of records for behavioral key words [19]. Surveillance data were obtained from area public schools, SC Department of Disabilities and Special Needs (DDSN) boards, and the Medical University of SC [18, 21]. The second phase was to determine case status through a systematic review of abstracted records by a trained clinician using an objective coding scheme based on the DSM-IV ASD criteria [19]. This study includes (1) all children with ASD who were identified in 2006 at 8-years old and who were Medicaid-eligible between January 1, 2005 and December 31, 2006; and (2) all children with ASD who were identified in 2007 at 15-years old and who were Medicaid-eligible between January 1, 2006 and December 31, 2007. Thus, the study period was a 2-year period of time including the surveillance year and one calendar year prior.
Data linkage
All confidentiality procedures were followed and appropriate regulatory approvals were granted for the collection of data under the category of public health surveillance. Protected Health Information (PHI) was collected initially to avoid duplication of cases across multiple data sources. Following application and approval through Medicaid’s internal review process, data linkages were made using unique identifiers common to both datasets; PHI was then removed, resulting in a de-identified database to be used solely for the purpose of case characterization. This dataset contained a two-year history of Medicaid-reimbursed claims for each child, including the details of all pharmacy claims and total associated costs.
Variables
Characteristics of children with case-defined ASD
Children were grouped such that Group 1 included 8-years old in 2006, and Group 2 included 15-years olds in 2007. Intellectual disability (IQ of ≤70) was recorded from each child’s SCADDM data, as was sex. To allow separation of psychotropic versus non-psychotropic use of anti-epileptic medication, an indicator variable was created based on the presence of epilepsy diagnosis in the Medicaid record by International Classification of Diseases, 9th Revision (ICD-9) codes 345.xx (1–9) any time from birth through December 31st of the surveillance year.
Prescription data
Medication use was determined using Medicaid claims data pertaining to the 2-year study period and was organized by class based on modified drug information found in the DRUGDEX® System by Micromedex® Healthcare Series [23]. Dichotomous variables indicated the presence of claims for non-psychotropic and psychotropic medications. Categories included anti-infectives, analgesics or anti-inflammatory agents, gastrointestinal agents, allergy or upper respiratory agents, and psychotropic medications. Specific psychotropic classes were antipsychotics (e.g., aripiprazole, and risperidone); antidepressants (e.g., citalopram and sertraline); ADHD medications (i.e., stimulants and atomoxetine); anticholinergics (e.g., benztropine and amantadine); mood stabilizers (e.g., carbamazepine and levetiracetam); antihypertensives (e.g., clonidine and guanfacine); anxiolytics (e.g., buspirone and lorazepam); and sedatives or hypnotics (e.g., chloral hydrate and ramelteon). For children with a history of epilepsy, mood stabilizers were classified as non-psychotropic. Antihypertensive medications were included because evidence suggests the use of these medications among children with ASD may reduce symptoms of hyperactivity, inattentiveness, impulsivity, and insomnia [24, 25, 26].
To determine multiple medication use, for each child the number of different psychotropic classes prescribed during the study period was counted, and two dichotomous variables indicated the presence of claims from (a) more than one, and (b) three or more different psychotropic classes. The exact dates of individual prescription claims could not be determined. Therefore, to quantify the variety of drug classes children were exposed to and the associated costs during the two-year period, continuous variables included the total number of all pharmacy claims stratified by non-psychotropics and psychotropics, as well as total pharmacy costs.
Data analysis
Our primary outcome measures were the proportion of children in each age group with any prescription claim, any psychotropic prescription, multiple psychotropic prescriptions, number of prescriptions, and total prescription costs during the 2-year time period. Categorical differences regarding variables of interest were tested for differences across age groups using chi-square tests or Fisher’s exact test (where appropriate). Several continuous outcomes of interest (e.g., the number and costs of prescriptions) were zero-inflated variables, meaning that a large fraction of participants reported a value of 0. These data are challenging to analyze and compare across groups due to the bimodal distribution, with one mode at 0 and another mode at a higher value. To display these distributions, kernel density estimates were used which essentially provide smoothed density plots. These are similar in interpretation to histograms in terms of their shape, but are smoothed over the range of the variable of interest and are a convenient way to display data which are zero-inflated. The function density in R was used to generate these estimates and plots. To compare these distributions, we used a two-part model and the associated two part test described by Lachenbruch [27, 28]. Briefly, the test compares both the proportion of zeros in each group (using a Pearson chi-square test statistic) and the distribution of the non-zero part (using a Kolmogorov-Smirnov test statistic). The resulting chi-square statistics from each part are then combined to generate one p-value testing that there is a difference in distribution across the groups compared. No adjustments were made for multiple comparisons, and results with a p-value less than 0.05 were considered significant. Statistical analyses were performed using SAS version 9.1 and R version 2.12.0.
Results
Characteristics of the study sample
One hundred percent of the 263 children identified by the SCADDM Network were Medicaid-eligible during the study period. There were 196 children who were aged 8, and 67 who were aged 15; fewer children were identified at age 15 because a smaller but geographically similar study region was utilized [20]. There were 223 males (85%) and 40 (15%) females. Overall, 12% had a history of epilepsy, and 43% were intellectually disabled. In the unadjusted analysis, neither epilepsy nor intellectual disability was associated with sex or age. We found that 39% of 15-year olds and 33% of 8-year olds had no record of a formal ASD diagnosis in their SCADDM records.
Medication use
Characteristics of medication use among children with case-defined ASD are shown in Table 1. Overall, 56% (n=147) of children with SCADDM-defined ASD had a prescription of any type filled during the study period; age was not statistically significantly associated with the presence of any prescription claim (59% at age 8 versus 46% at age 15, p=.07). There was no statistically significant difference across age groups for the use of gastrointestinal agents (17% and 12% of 8- and 15-year olds respectively (p=.30)); however, anti-infective agents were more common among 8- than 15-year olds (45% versus 25%, p<.01), as were analgesics or anti-inflammatory agents (24% versus 12%, p=.04), and allergy or upper respiratory medications (40% versus 18%, p=.001).
Table 1.
Characteristics of medication use among children with case-defined ASD.
| Variable | Total (N=263) | Age Group
|
p valuea | |
|---|---|---|---|---|
| 8 (n=196) | 15 (n=67) | |||
| Male | 223 (84.8%) | 168 (85.7%) | 55 (82.1%) | 0.478 |
| History of epilepsy | 31 (11.8%) | 21 (10.7%) | 10 (14.9%) | 0.356 |
| Intellectual disability | 113 (43.0%) | 86 (43.9%) | 27 (40.3%) | 0.609 |
| No previous ASD diagnosis | 91 (35.0%) | 65 (33.0%) | 26 (39.0%) | 0.703 |
|
| ||||
| Any medication prescription | 147 (55.9%) | 116 (59.2%) | 31 (46.3%) | 0.066 |
| Gastrointestinal agents | 42 (16.0%) | 34 (17.4%) | 8 (11.9%) | 0.297 |
| Anti-infective agents | 105 (39.9%) | 88 (44.9%) | 17 (25.4%) | 0.005 |
| Analgesic/anti- inflammatory | 55 (20.9%) | 47 (24.0%) | 8 (11.9%) | 0.037 |
| Allergy medications | 90 (34.2%) | 78 (39.8%) | 12 (17.9%) | 0.001 |
|
| ||||
| Any psychotropic medication | 105 (39.9%) | 78 (39.8%) | 27 (40.3%) | 0.944 |
| Multiple classes of psychotropic | 52 (19.7%) | 31 (15.8%) | 21 (31.3%) | 0.005 |
| Three or more classes | 24 (9.1%) | 12 (6.1%) | 12 (17.9%) | 0.004 |
| ADHD medicationb | 56 (21.3%) | 41 (20.9%) | 15 (22.4%) | 0.800 |
| Antipsychotic | 25 (9.5%) | 12 (6.1%) | 13 (19.4%) | 0.001 |
| Antidepressant | 24 (9.1%) | 9 (4.6%) | 15 (22.4%) | 0.0001 |
| SSRI | 19 (7.2%) | 6 (3.1%) | 13 (19.4%) | 0.0001 |
| TCA/other | 8 (3.0%) | 4 (2.0%) | 4 (6.0%) | 0.109 |
| Antihypertensive | 43 (16.3%) | 30 (15.3%) | 13 (19.4%) | 0.434 |
| Mood stabilizer | 10 (3.8%) | 4 (2.0%) | 6 (9.0%) | 0.020 |
| Anxiolytic | 17 (6.4%) | 9 (4.6%) | 3 (11.9%) | 0.035 |
| Sedative or hypnotic | 20 (7.6%) | 17 (8.7%) | 3 (4.5%) | 0.263 |
| Anticholinergic | 11 (4.2%) | 8 (4.1%) | 3 (4.5%) | 1.000 |
ADHD- attention deficit hyperactivity disorder; SSRI- selective serotonin reuptake inhibitor; TCA/other- tricyclic and other antidepressants.
Chi square or Fisher’s exact test, 2-sided.
Includes stimulants and atomoxetine.
Psychotropic medication use
Forty percent (n=105) of all children used psychotropic medication during the study period, with no statistical difference between the age groups (p=.94). The most commonly prescribed medications were ADHD medication (21%), antihypertensives (16%), antipsychotics (10%), antidepressants (9%), sedatives or hypnotics (8%), anxiolytics (7%), mood stabilizers (4%), and anticholinergics (4%). Although the age differences for the use of any psychotropic, and the use of ADHD medications were not statistically significant, older children were prescribed significantly more antipsychotics, antidepressants, mood stabilizers, and anxiolytics.
Combination use among children prescribed psychotropic medication
Overall, 20% (n=52) used multiple psychotropic classes. Of the subgroup of 105 children who were prescribed any psychotropic, 50% were prescribed multiple classes (40% at age 8 and 78% at age 15, p<.001). Significantly more 15-year olds with psychotropics were prescribed three or more different classes compared to 8-year olds (44% versus 15%, p<.01). The most common combinations were (1) ADHD medications and an antihypertensive (23%), antidepressant (14%), antipsychotic (13%), or a sedative or hypnotic (9%); and (2) antidepressants and an antihypertensive (12%), or an antipsychotic (11%), as shown Table 2. Drug combinations including ADHD medications and antidepressants or antipsychotics, and antidepressants and antipsychotics were more common among older children. The only drug combination used more frequently among younger children was an ADHD medication and a sedative or hypnotic. There was no statistical significance regarding the difference between children with ASD and intellectual disability versus those with ASD without intellectual disability who were prescribed three or more psychotropic classes (11% versus 8%, p=.47).
Table 2.
The most common combinations of psychotropic medications among those with any psychotropic (n=105).
| Medication Combination | Total n=(105) | Age Group
|
p valuea | |
|---|---|---|---|---|
| 8 (n=78) | 15 (n=27) | |||
| Multiple classes of psychotropic medication | 52 (49.52%) | 31 (39.74%) | 21 (77.78%) | 0.001 |
| Three or more psychotropic classes | 24 (22.86%) | 12 (15.38%) | 12 (44.44%) | 0.002 |
| ADHDb and antihypertensive | 24 (22.86%) | 17 (21.79%) | 7 (25.93%) | 0.660 |
| ADHDb and antidepressant | 15 (14.29%) | 6 (7.69%) | 9 (33.33%) | 0.001 |
| ADHDb and antipsychotic | 14 (13.33%) | 7 (8.97%) | 7 (25.93%) | 0.026 |
| ADHDb and mood stabilizer | 7 (6.67%) | 2 (2.56%) | 5 (18.52%) | 0.004 |
| ADHDb and sedative or hypnotic | 9 (8.57%) | 8 (10.26%) | 1 (3.70%) | 0.295 |
| Antidepressant and antihypertensive | 13 (12.38%) | 6 (7.69%) | 7 (25.93%) | 0.013 |
| Antidepressant and antipsychotic | 11 (10.48%) | 4 (5.13%) | 7 (25.93%) | 0.002 |
| Antidepressant and mood stabilizer | 6 (5.71%) | 2 (2.56%) | 4 (14.81%) | 0.018 |
ADHD- attention deficit hyperactivity disorder.
Chi square or Fisher’s exact test, 2-sided, for differences across age groups.
Includes stimulants and atomoxetine.
Variability of medication use
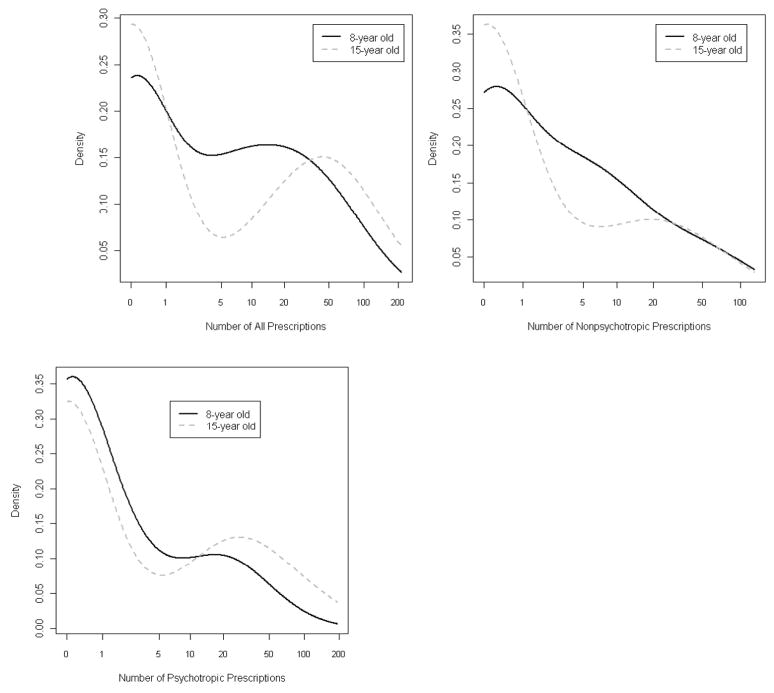
Due to the distribution of these data, the common ways of comparing the means or medians across groups to determine the probability that they share underlying parameters may provide misleading results. As described in the statistical methods section, our analysis approach accounted for both the large cluster of zeros in both groups as well as the skewed continuous distribution of the remaining outcomes to show that the overall distribution for the number of all prescriptions, non-psychotropic prescriptions, and psychotropic prescriptions varied significantly across age groups (p<.0001, p<.0001, and p=.001 respectively). To demonstrate this in the same figure, standard graphical displays such as boxplots are often not effective due to the large mode at zero. Instead, we used a density plot, which is essentially a smoothed histogram, to display the empirical data (Figure 2). Figure 2 provides scale-free estimates of the distribution of number of prescriptions for 8- and 15-year old children. Among those children with ASD who were prescribed medication, older children were prescribed a higher mean/median number of all prescriptions (53/46 versus 27/16), nonpsychotropics (17/6 versus 18/8), and psychotropic prescriptions (35/26 versus 10/2) compared to younger children.
Figure 2.
The distribution for all prescriptions, nonpsychotropic prescriptions, and psychotropic prescriptions varied significantly across age groups (p<.0001, p<.0001, and p=.001 respectively). Lines shown represent smoothed density plots generated in the R package using the function density. These are similar in interpretation to histograms, but are smoothed over the range of the number of prescriptions.
Prescription costs
Among those children who had any prescription, the mean/median cost of all prescriptions was $1380/$340 for Year 1 and $1670/$550 for Year 2. Over the 2-year study period, mean/median prescription costs for 15-year olds were $6480/$1880, and $2130/$800 for 8-year olds. Figure 3 demonstrates the significant difference in the distribution of costs between the age groups, stratified by those with and those without psychotropics.
Figure 3.
The overall distribution of medication costs varied by age among those with any psychotropic (p=.01), and among those without psychotropics (p=.03). Lines shown represent smoothed density plots generated in the R package using the function density. These are similar in interpretation to histograms, but are smoothed over the range of costs and are scale-free.
Discussion
This study combined population-based surveillance data with Medicaid to show that the use of prescription medication and the associated costs may vary significantly by age among children with SCADDM-identified ASD (autistic disorder, Asperger disorder, and PDD-NOS). Younger children were more often prescribed anti-infective agents, analgesics or anti-inflammatory agents, and allergy medications, while older children were more often prescribed multiple psychotropic medications, antidepressants, antipsychotics, and combinations involving ADHD medications, antidepressants, antipsychotics, mood stabilizers, and anxiolytics. The only drug combination more frequently prescribed among younger children than older children was an ADHD medication with a sedative or hypnotic. Total pharmacy costs were particularly high among older children who were prescribed psychotropics.
The 2-year rates of psychotropic medication use reported here are similar to claims-based and survey data regarding current and annual medication use in ASD [2, 4, 5, 6]. Our results that 40% of children who met SCADDM case definition for ASD used psychotropic medication support previous survey data by Rosenberg et al [4], who found that 35% of children with ASD took psychotropic medication.
Among Medicaid-eligible children with ASD identified by ICD9 codes, Mandell et al found an annual rate of 56% for the use of any psychotropic medication [8], which is slightly higher than our rate of 40%. However, the use of three or more different classes was similar (9% and 11% [8]).
In line with evidence that younger age may be associated with an increased risk of side effects and adverse events related to the use of SSRIs [29], we found that younger children were prescribed tricyclic and other (TCA/other) antidepressants equally as often as older children, though fewer younger children used SSRIs.
We confirmed that multiple psychotropic medication classes are often prescribed to children with ASD, and that this practice may be more prevalent among older children than in younger children. In these data, the prevalence of medication was not statistically significantly different across age groups. However, the overall distributions of the number of prescriptions filled differed significantly; perhaps the decision to try a medication may have been consistent, though older children were more likely to continue refilling or obtaining new prescriptions compared to younger children. Possibly, conditions more common in childhood that require acute treatment or non-psychotropic medication decrease with age, though target behaviors such as impulsivity or inattention remain present or increase in complexity. It is also possible that the age differences observed here could be explained by the small number of children in the multiple-drug analyses. It is interesting to note that the age differences observed correspond to a key transition in child development: from childhood into adolescence. These findings have important implications for mental health care providers, and should be considered by those who are involved in the mental health and behavioral care of children and adolescents with ASD particularly as they transition from one developmental period to another.
These findings should be interpreted carefully because of the difficulties in comparing results from different geographic regions, ages, time periods, and sources due to regional preferences, changes in diagnostic criteria, and emerging evidence-based interventions.
Strengths and limitations
This study has several limitations. Only the number and type of prescriptions filled among children who were Medicaid-eligible were characterized. This could result in an overrepresentation of a low-income population. However, although limited by the small sample size, 100% of surveillance cases were Medicaid-eligible. This speaks to both the generalizability of the current findings as well as the thoroughness and validity of the case-ascertainment methodology. One explanation is that South Carolina has a Katie Beckett waiver program (known as TEFRA) that provides Medicaid coverage for children with disabilities who would not otherwise be eligible.
Though small, our sample size permitted manageability of a complex multiple-dataset linkage with enough data to demonstrate the feasibility of using population-based surveillance data and external administrative sources to examine prescription utilization and costs among children with ASD.
The current study design did not allow for the analysis of medication adherence or reasons for medication initiation or discontinuation. Appreciating these factors could result in improved outcomes by identifying the overuse or underuse of medication. Although we did not include race or type of ASD as variables in the analysis, previous research that included seizure disorder and intellectual disability as covariates found no effect of race or type of ASD on medication use [4]. Lastly, over the counter medications such as omega-3 fatty acids and nutritional supplements [30, 31] were not included.
Despite the limitations, this study provides a more complete assessment of medication use for several reasons. First, the study population is likely to be more complete, as all cases were identified based on consistent diagnostic criteria (not considering formal diagnoses) [19]. The population-based case ascertainment method may lower the risk of inflated estimates of medication use due to a clinical sample. Capturing all psychotropic medications filled during the year identified and one year prior, rather than a cross-sectional design showed the variation of medication use and the variety of drug class exposure in this disorder. Finally, controlling for co-occurring epilepsy allowed the distinction between psychotropic and nonpsychotropic use of mood stabilizers in the analysis.
Clinical significance
The high rate, variability, and cost of medications highlight the complex nature of ASD. As medication usage becomes more common, concerns including drug-drug interactions, increased costs, and medication errors should be considered. Many children with complex needs undergo multifaceted and expensive medication regimens [32], though certainly instances arise where polypharmacy has been researched (for example, the use of stimulant augmentation with antihypertensives) [33]. As treatment guidelines are established, determining how factors such as Medicaid eligibility category, severity of disability, age of diagnosis, and regional preferences influence prescription practice should be done before the appropriateness of prescription rates can be assessed. Ultimately, understanding multi-drug regimes has implications for clinical, financial, and systems research.
Acknowledgments
This work was supported by Grant # TL1RR029881 from the National Center for Research Resources (NCRR), a part of the National Institutes of Health (NIH). SCADDM data were collected under the CDC Grant #CDC-RFA-DD06-601. Additional collaboration for this project came from the Commissioner of DHEC, who has designated the SCADDM team as a bona fide agent of the Health Department to conduct surveillance of autism spectrum disorders. Content is the responsibility of the authors and does not necessarily represent the views of the funding agencies. Special thanks to the SC Budget & Control Board, Office of Research & Statistics, and Heather Kirby and Wally Altman for providing exceptional technical assistance for this project.
Abbreviations
- ASD
autism spectrum disorder
- ID
Intellectual Disability
- CDC
Centers for Disease Control and Prevention
- SCADDM
South Carolina Autism Developmental Disabilities and Monitoring (Network)
- DSM-IV-TR
Diagnostic and Statistical Manual of Mental Disorder, Fourth Edition, Text Revision
- PDD-NOS
pervasive developmental disorder–not otherwise specified
- ICD-9
International Classification of Diseases, Ninth Revision
- ADHD
attention deficit hyperactivity disorder
- SSRI
selective serotonin reuptake inhibitor
- TCA/other
tricyclic and other antidepressant
- PHI
Protected Health Information
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aman MG, Lam KL, Van Bourgondien ME. Medication patterns in patients with autism: temporal, regional, and demographic influences. Journal of Child and Adolescent Psychopharmacology. 2005;15:116–126. doi: 10.1089/cap.2005.15.116. [DOI] [PubMed] [Google Scholar]
- 2.Green VA, Pituch KA, Itchon J, Choi A, O’Reilly M, Sigafoos J. Internet survey of treatments used by parents of children with autism. Research in Developmental Disabilities. 2006;27:70–84. doi: 10.1016/j.ridd.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Oswald DP, Sonenklar NA. Medication use among children with autism spectrum disorders. Journal of Child and Adolescent Psychopharmacology. 2007;17:348–355. doi: 10.1089/cap.2006.17303. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg R, Mandell D, Farmer J, Law J, Marvin A, Law P. Psychotropic medication use among children with autism spectrum disorders enrolled in a national registry, 2007–2008. Journal of Autism and Developmental Disorders. 2010;40:342–351. doi: 10.1007/s10803-009-0878-1. [DOI] [PubMed] [Google Scholar]
- 5.Witwer A, Lecavalier L. Treatment incidence and patterns in children and adolescents with autism spectrum disorders. Journal of Child and Adolescent Psychopharmacology. 2005;15:671–676. doi: 10.1089/cap.2005.15.671. [DOI] [PubMed] [Google Scholar]
- 6.Mandell DS, Cao J, Ittenbach RF, Pinto-Martin JA. Medicaid expenditures for children with autistic spectrum disorders: 1994 to 1999. Journal of Autism and Developmental Disorders. 2006 doi: 10.1007/s10803-006-0088-z. epub. April4, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Croen LA, Najjar DV, Ray T, Lotspeich L, Bernal P. A comparison of health care utilization and costs of children with and without autism spectrum disorders in a large group-model health plan. Pediatrics. 2006;118:1203–1211. doi: 10.1542/peds.2006-0127. [DOI] [PubMed] [Google Scholar]
- 8.Mandell DS, Morales KH, Marcus SC, Stahmer AC, Doshi J, Polsky DE. Psychotropic medication use among Medicaid-enrolled children with autism spectrum disorders. Pediatrics. 2008;121:e441–448. doi: 10.1542/peds.2007-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin DM, Feudtner C, Localio R, Mandell DS. State variation in psychotropic medication use by foster care children with autism spectrum disorder. Pediatrics. 2009;124:e305–e312. doi: 10.1542/peds.2008-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Academy of Pediatrics. Children with autism frequently receive psychotropic medications. Science Daily. Retrieved October 2010, from http://www.sciencedaily.com/releases/2010/05/100502080228.htm.
- 11.Langworthy-Lam KS, Aman MG, Van Bourgondien ME. Prevalence and patterns of use of psychoactive medicines in individuals with autism in the autism society of North Carolina. Journal of Child and Adolescent Psychopharmacology. 2002;12:311–321. doi: 10.1089/104454602762599853. [DOI] [PubMed] [Google Scholar]
- 12.Esbensen A, Greenberg JS, Seltzer MM, Aman MG. A longitudinal investigation of psychotropic and non-psychotropic medication use among adolescents and adults with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39:1339–1349. doi: 10.1007/s10803-009-0750-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin A, Scahill L, Klin A, Volkmar F. Higher-functioning pervasive developmental disorders: rates and patterns of psychotropic drug use. Journal of American Academy of Child & Adolescent Psychiatry. 1999;38:923–931. doi: 10.1097/00004583-199907000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Mandell DS, Novak MM, Zubritsky CD. Factors associated with the age of diagnosis among children with autism spectrum disorders. Pediatrics. 2005;1 16(6):1480–1486. doi: 10.1542/peds.2005-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiggins LD, Baio J, Rice C. Examination of the time between first evaluation and first autism spectrum diagnosis in a population-based sample. J Dev Behav Pediatr. 2006;27(suppl 2):S79–S87. doi: 10.1097/00004703-200604002-00005. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders-autism and developmental disabilities monitoring network, United States, 2006. Morb Mortal Wkly Rep Surveillance Summaries. 2009;58:1–20. [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention, ADDM Network Surveillance Year 2002 Principal Investigators. Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, 14 sites, United States, 2002. Morb Mortal Wkly Rep Surveillance Summaries. 2007;56(SS01):12–28. [PubMed] [Google Scholar]
- 18.Nicholas J, Charles J, Carpenter L, King L, Jenner W, Spratt E. Prevalence and characteristics of children with autism-spectrum disorders. Annals of Epidemiology. 2008;18:130–136. doi: 10.1016/j.annepidem.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Avchen R, Wiggins L, Devine O, Van Naarden Braun K, Rice C, Hobson N, Schendel D, Yeargin-Allsop M. Evaluation of a records-review surveillance system used to determine the prevalence of autism spectrum disorders. J Autism Dev Disord. 2011;41:227–236. doi: 10.1007/s10803-010-1050-7. [DOI] [PubMed] [Google Scholar]
- 20.Nicholas J, Carpenter L, King L, Jenner W, Charles J. Autism spectrum disorders in preschool-aged children: Prevalence and comparison to a school-aged population. Annals of Epidemiology. 2009;19:808–14. doi: 10.1016/j.annepidem.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Nicholas J, Carpenter L, King L, Jenner W, Wahlquist A, Logan S, Charles J. Completeness of case ascertainment for surveillance of autism spectrum disorders using the Autism and Developmental Disabilities Monitoring Network methodology. 2011 doi: 10.1016/j.dhjo.2012.03.004. (submitted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and statistical manual of mental disorder. 4. Washington, DC, USA: American Psychiatric Association; text revision. [Google Scholar]
- 23.Micromedex® Healthcare Series [Internet database] Greenwood Village, CO: Thomson Healthcare; Updated periodically. [Google Scholar]
- 24.Arnsten A, Scahill L, Findling R. Alpha-2 adrenergic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: Emerging concepts from New Data. Journal of Child and Adolescent Psychopharmacology. 2007:393–406. doi: 10.1089/cap.2006.0098. [DOI] [PubMed] [Google Scholar]
- 25.Posey D, Puntney J, Sasher T, Kem D, McDougle C. Journal of Child and Adolescent Psychopharmacology. 2004 June;14(2):233–241. doi: 10.1089/1044546041649084. [DOI] [PubMed] [Google Scholar]
- 26.Arnsten A, Scahill L, Findling R. Alpha-2 adrenergic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: Emerging concepts from New Data. Journal of Child and Adolescent Psychopharmacology. 2007:393–406. doi: 10.1089/cap.2006.0098. [DOI] [PubMed] [Google Scholar]
- 27.Lachenbruch PA. Comparisons of two-part models with competitors. Statistics in Medicine. 2001;20(8):1215–34. doi: 10.1002/sim.790. [DOI] [PubMed] [Google Scholar]
- 28.Lachenbruch PA. Analysis of data with excess zeros. Statistical Methods in Medical Research. 2002;11(4):297–302. doi: 10.1191/0962280202sm289ra. [DOI] [PubMed] [Google Scholar]
- 29.Williams K, Wheeler DM, Silove N, Hazell P. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD) Cochrane Database Syst Rev. 2010;8:CD004677. doi: 10.1002/14651858.CD004677.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Levy SE, Hyman SL. Complementary and alternative medicine treatments for children with autism spectrum disorders. Child and Adolescent Psychiatric Clinics of North America. 2008;17:803–820. doi: 10.1016/j.chc.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meiri G, Bichovsky Y, Belmaker RH. Omega 3 fatty acid treatment in autism. Journal of Child and Adolescent Psychopharmacology. 2009;19:449–451. doi: 10.1089/cap.2008.0123. [DOI] [PubMed] [Google Scholar]
- 32.dosReis S, Zito JM, Safer DJ, Gardner JF, Puccia KB, Owens PL. Multiple psychotropic medication use for youths: a two-state comparison. Journal of Child and Adolescent Psychopharmacology. 2005;15:68–77. doi: 10.1089/cap.2005.15.68. [DOI] [PubMed] [Google Scholar]
- 33.Martin A, Van Hoof T, Stubbe D, Sherwin T, Scahill L. Multiple psychotropic pharmacotherapy among child and adolescent enrollees in Connecticut Medicaid Managed Care. Psychiatr Serv. 2003;54:72–77. doi: 10.1176/appi.ps.54.1.72. [DOI] [PubMed] [Google Scholar]